You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | jinsong xue | -- | 2833 | 2023-09-21 17:37:12 | | | |
| 2 | Catherine Yang | Meta information modification | 2833 | 2023-09-22 02:42:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xue, J.; Xiao, Q.; Zhang, M.; Li, D.; Wang, X.; Xue, J. Liver Toxicity Induced by Polybrominated Diphenyl Ethers. Encyclopedia. Available online: https://encyclopedia.pub/entry/49494 (accessed on 26 December 2025).
Xue J, Xiao Q, Zhang M, Li D, Wang X, Xue J. Liver Toxicity Induced by Polybrominated Diphenyl Ethers. Encyclopedia. Available at: https://encyclopedia.pub/entry/49494. Accessed December 26, 2025.
Xue, Jinsong, Qingqing Xiao, Min Zhang, Dan Li, Xiaofei Wang, Jinsong Xue. "Liver Toxicity Induced by Polybrominated Diphenyl Ethers" Encyclopedia, https://encyclopedia.pub/entry/49494 (accessed December 26, 2025).
Xue, J., Xiao, Q., Zhang, M., Li, D., Wang, X., & Xue, J. (2023, September 21). Liver Toxicity Induced by Polybrominated Diphenyl Ethers. In Encyclopedia. https://encyclopedia.pub/entry/49494
Xue, Jinsong, et al. "Liver Toxicity Induced by Polybrominated Diphenyl Ethers." Encyclopedia. Web. 21 September, 2023.
Copy Citation
Polybrominated diphenyl ethers (PBDEs) are a group of flame retardants used in plastics, textiles, polyurethane foam, and other materials. The liver is an essential organ for metabolic detoxification and is sensitive to environmental toxicants. Hence, the liver is susceptible to injury when exposed to xenobiotics. For instance, significant liver weight increase and cell swelling, coupled with an elevated expression of cytochrome P450 (CYP1A2, CYP3A1, and CYP2B1) enzymes and genes and hepatocytic fatty degeneration, have been reported in PBDEs.
polybrominated diphenyl ethers
exposures
toxic effects
toxic mechanisms
1. Oxidative Damage and Apoptosis
A study on zebrafish has shown that PBDE-47 and PBDE-153 exposure markedly increased catalase (CAT) and superoxide dismutase (SOD) activities [1]. Additionally, the upregulation of apoptotic-regulated genes, including cysteine-aspartic acid protease-3 (Caspase-3) and tumor protein 53 (P53), as well as downregulation of anti-apoptotic genes, including B-cell lymphoma 2 (Bcl2) were observed in zebrafish treatment with PBDE-47 and PBDE-153, indicating PBDEs may regulate hepatic oxidative stress, DNA damage and apoptosis [1]. In addition, PBDE-47 and PBDE-32 reduced cell viability, generated reactive oxygen species (ROS) and triggered apoptosis in human hepatocellular carcinoma cell line HepG2 cells [2][3]. Shao et al. have analyzed the response of primary human fetal liver hematopoietic stem cells (HSCs) to PBDE-47 induction. They found higher concentrations of PBDE-47 may elicit overt ROS generation and lipid peroxidation, whereas N-acetylcysteine (NAC) can alleviate oxidative damage induced by PBDE-47 [4]. Analogously, trout liver cells exposed to PBDE-47 displayed a significant reduction in cell viability. The enhanced 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence in the presence of PBDE-47 indicated liver cells may be sensitive to PBDE-47 via a mechanism involving oxidative stress [5]. Zhang et al. investigated a rescue strategy using troxerutin to ameliorate PBDE-47-induced hepatocyte apoptosis. Perturbation of proteasome functions leads to endoplasmic reticulum (ER) stress, which is associated with apoptosis. They found that troxerutin efficaciously mitigates mice’s liver apoptosis via modulating oxidative stress-mediated proteasome dysfunction. Furthermore, the downstream TNF receptor-associated factor 2 (TRAF2)/apoptosis signal-regulating kinase 1 (ASK1)/c-Jun N-terminal kinase (JNK) pathway was dramatically blocked by troxerutin in PBDE-47-treated mice livers [6]. Meanwhile, PBDE-47 promotes liver inflammation by inducing oxidative stress-triggered nicotinamide adenine dinucleotide (NAD+) depletion. Troxerutin may abate oxidative stress, preventing the NAD+-depletion-mediated loss of silent mating type information regulation 2 homolog 1 (Sirt1) and subsequent occurrence of inflammation [7]. In rat liver, PBDE-99 induced oxidative damage as evidenced by increased SOD activity and oxidized glutathione (GSSG) level, as well as decreased glutathione (GSH) level and CAT activity [8]. Likewise, PBDE-99 activated Caspases (i.e., Caspase-3 and Caspase-9) and generated toxic levels of ROS, thereby causing HepG2 cell apoptosis [9]. PBDE-209 and its quinone-type metabolite could induce an oxidative stress response, which activates ER stress and the autophagy-lysosomal system in hepatocytes [10][11]. Meanwhile, PBDE-209 disrupted calcium homeostasis, induced mitochondrial Ca2+ overload, and the subsequent cell apoptosis occurred [10][12]. Hu et al. have conducted several experiments to assess oxidative stress indicators. For example, increased ROS and lactate dehydrogenase (LDH) leakage have been observed in HepG2 cells dosed with PBDE-209 [13]. PBDE-209 could upregulate the activity of hepatic glutathione reductase (GR), and this elevation may compensate for cellular GSH depletion [14]. Interestingly, in 2013, samples of the kingfisher were collected from the e-waste recycling site and processed for biochemical analysis. The analysis showed that PBDEs, malondialdehyde (MDA) and ROS levels in kingfishers from e-waste sites were markedly increased compared with the normal group. Conversely, SOD and CAT activities in the liver from the exposed area were lower than in the reference group [15]. Transcriptional profiles of O.melastigma liver were analysed. The results discovered that PBDE-47 may activate phosphoinositide-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathway, which can modulate cell growth, proliferation, and survival [16]. The mechanisms are shown in Figure 1 and Figure 2.
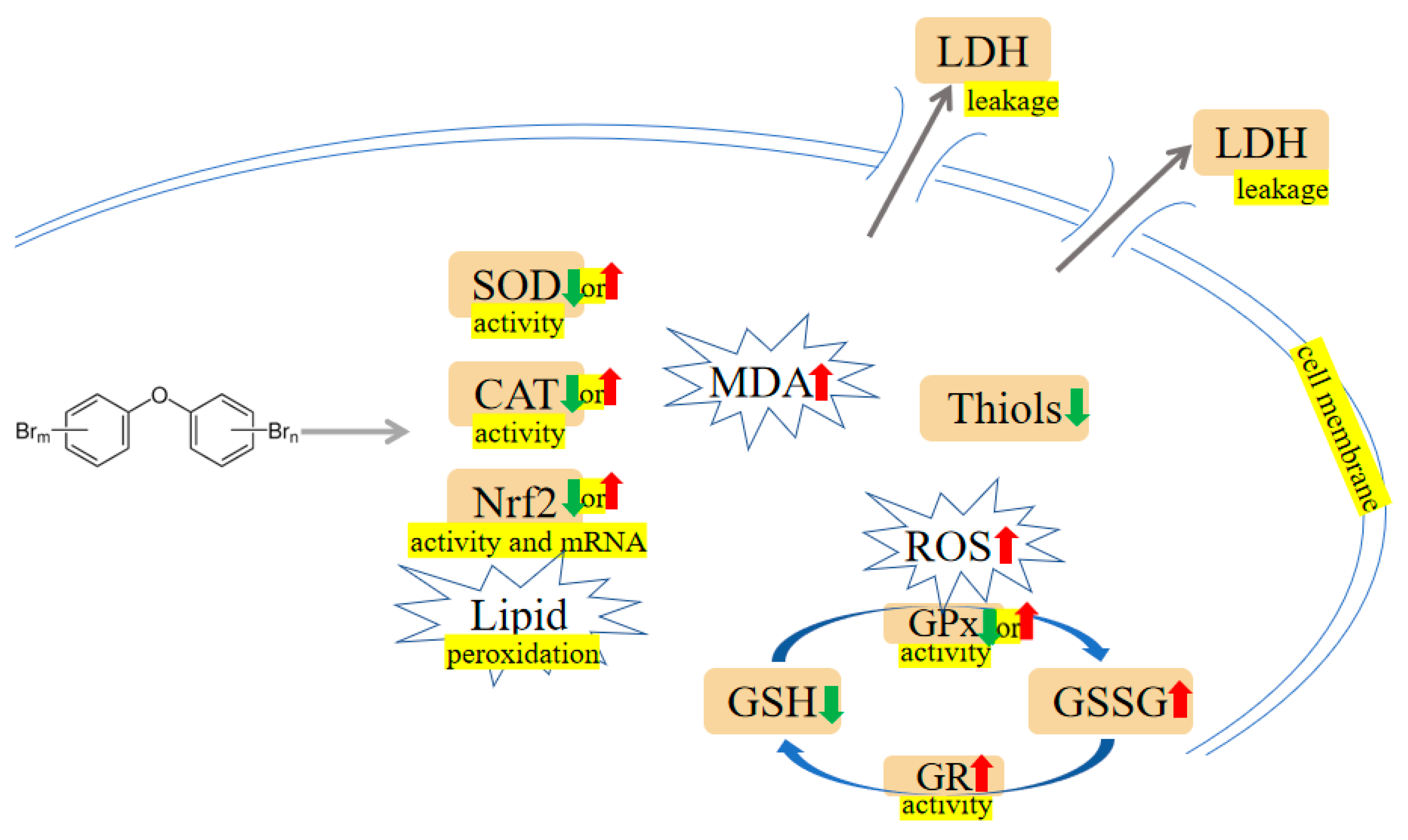
Figure 1. PBDEs-induced toxicity is associated with oxidative damage. PBDEs exposure can alter antioxidant enzyme activities, generate reactive oxygen species (ROS), increase malondialdehyde (MDA), and induce lactate dehydrogenase (LDH) leakage. Arrows indicate up (red colour), increased; down (green colour), decreased; up or down, increased or decreased (opposite research results exist). The yellow highlighted text is an explanation of the figure.
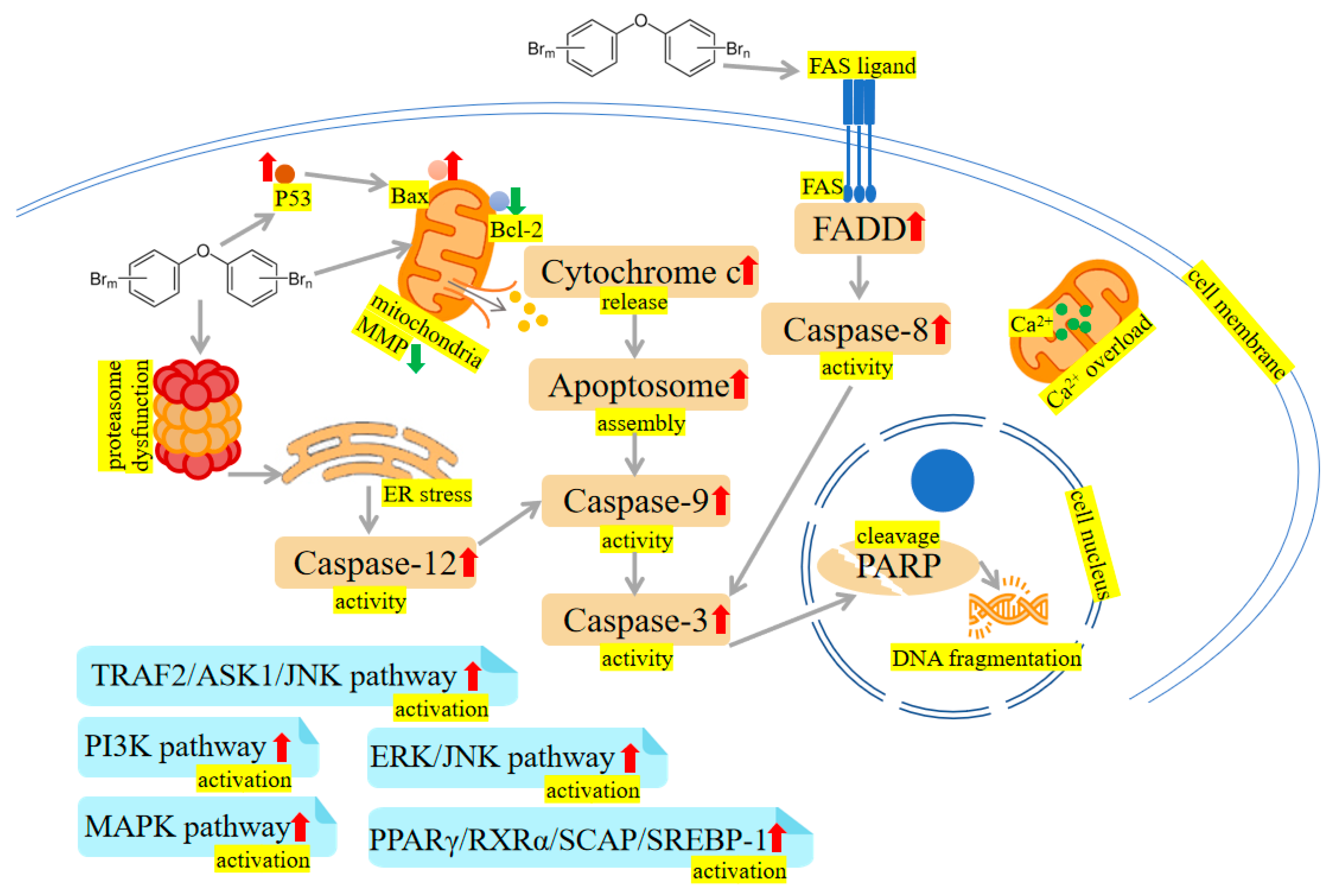
Figure 2. PBDEs-induced toxicity is associated with apoptosis. PBDEs exposure can reduce mitochondrial membrane potential (MMP), increase caspase activities, disrupt calcium homeostasis, induce endoplasmic reticulum (ER) stress and damage DNA. TNF receptor-associated factor 2 (TRAF2)/apoptosis signal-regulating kinase 1 (ASK1)/c-Jun N-terminal kinase (JNK) pathway, phosphoinositide-3-kinase (PI3K) pathway, extracellular signal-regulated kinase (ERK)/c-Jun N-terminal kinase (JNK) pathway, mitogen-activated protein kinase (MAPK) pathway and peroxisome proliferator-activated receptor γ (PPARγ)/retinoid X receptor α (RXRα)/sterol regulatory element-binding protein cleavage-activating protein (SCAP)/sterol regulatory element-binding protein-1 (SREBP-1) pathway are activated by PBDEs. Arrows indicate up (red colour), increased; down (green colour), decreased. The yellow highlighted text is an explanation of the figure.
2. Disturbance of Glucose and Lipid Metabolism
A growing body of evidence supports the idea that exposure to PBDEs is associated with metabolic dysfunction, with findings suggesting that these toxins may interfere with glucose and lipid metabolism. PBDE-47 and PBDE-153 have been reported to alter the blood-liver balance of lipids and disturb glucose metabolism in mice [17][18]. Moreover, to test if the aberrant metabolic phenotype is associated with altered liver epigenome, adult rats were exposed to PBDE-47, and functional analysis displayed that genes related to differentially methylated regions and differentially expressed miRNA were involved in lipid metabolism [19]. PBDE-71 has been found to reduce the activity of phosphoenolpyruvate carboxykinase, a key metabolic enzyme in hepatic glucose and lipid metabolism, and change the glucose: insulin ratio [20][21]. C57BL/6 mice that received PBDE-71 exhibited glucose intolerance, fasting hyperglycemia, retarded glucose clearance, and diminished thermogenic brown adipose tissue mass [22]. Zhu et al. reported PBDE-209 altered protein kinase A (PKA), phospho-PKA (p-PKA), adenosine 5′-monophosphate-activated protein kinase (AMPK), phospho-AMPK (p-AMPK), acetyl-CoA carboxylase (ACC), and fatty acid synthase (FAS) expression in rats’ liver and LO2 cells (human normal liver cells). Besides, protein kinase cyclic adenosine monophosphate (cAMP)-activated catalytic subunit α (PRKACA-1) hypermethylation induced by PBDE-209 was observed in LO2 cells. Further study revealed that hypermethylation may contribute to disturbance of glycolipid metabolism [23]. Casella et al. exposed HepG2 cells to PBDEs (i.e., PBDE-47, PBDE-99 and PBDE-209) at 1 nM. The following Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Set Enrichment Analysis (GSEA) analyses were carried out, and the results indicated that PBDE-47 perturbed the glucose metabolism and hypoxia pathway; the ternary mixtures containing PBDE-47, PBDE-99 and PBDE-209 influenced lipid metabolism and PI3K/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway. Meanwhile, PBDE-209 was reported to cause increased estrogen receptor α (ERα) and peroxisome proliferator-activated receptor α (PPARα) gene expression. These mechanism-based findings may reveal the potential relation between PBDEs and glycolipid metabolism [24]. PPARγ is an important nuclear receptor crucial in regulating lipid metabolism and glucose homeostasis [25]. Of interest, PBDE-47, a potential PPARγ ligand, could activate PPARγ [26][27]. PPARγ may push the adipocyte differentiation process forward by forming a positive-feedback loop with liver X receptor α (LXRα) [28]. It was reported that PPARγ activated by PBDE-47 may increase the expression of adipocyte-specific genes such as fatty acid binding protein 4 (Fabp4), lipoprotein lipase (Lpl), glucose transporter type 4 (Slc2a4), and adiponectin (Adipoq) [29]. Zhu et al. have found PBDE-209 led to histological impairment and lipid deposition, which were characterized by reduced glycogen and high-density lipoprotein (HDL) levels and increased low-density lipoprotein (LDL), glucose, triglyceride (TG) levels, and total cholesterol (CHOL) in mice livers. And besides they also found that LO2 cells’ survival declined after PBDE-209 treatment. Further exploration revealed that PBDE-209 impaired glucose homeostasis via preventing PI3K/AKT/Glucose transporter type 4 (GLUT4) signaling pathway and induced lipid metabolic abnormality by triggering mTOR/PPARγ/retinoid X receptor α (RXRα) signaling pathways [30][31]. The mTOR pathway activated by PBDE-209 is responsible for the induction of PPARγ expression. Subsequently, PPARγ increases lipogenesis by combining with RXRα to form dimers [30]. Intriguingly, PPARγ inhibitor antagonized the alterations to the expression of p-mTOR, PPARγ, and RXRα and hindered TG accumulation provoked by PBDE-209, suggesting PPARγ may participate in modulating glucolipid metabolism [30]. Rats orally administered with PBDE-209 have shown hyperglycemia as compared to control rats. The reduced GSH and SOD implied that oxidative damage may contribute to PBDE-209-induced hyperglycemia and the onset of diabetes [32]. PBDE-209 has been reported to hinder glucose absorption, increase the levels of total cholesterol (TC), TG, aspartate transaminase (AST), alanine aminotransferase (ALT), and MDA through insulin receptor substrate-1 (IRS-1)/GLUT4 and IRS-1/PI3K/AKT/Glycogen synthase kinase 3β (GSK-3β) pathways, eventually interfering with glucolipid metabolism in buffalo rat liver cells with insulin resistance (IR-BRL) [33]. The mechanisms are shown in Figure 3.
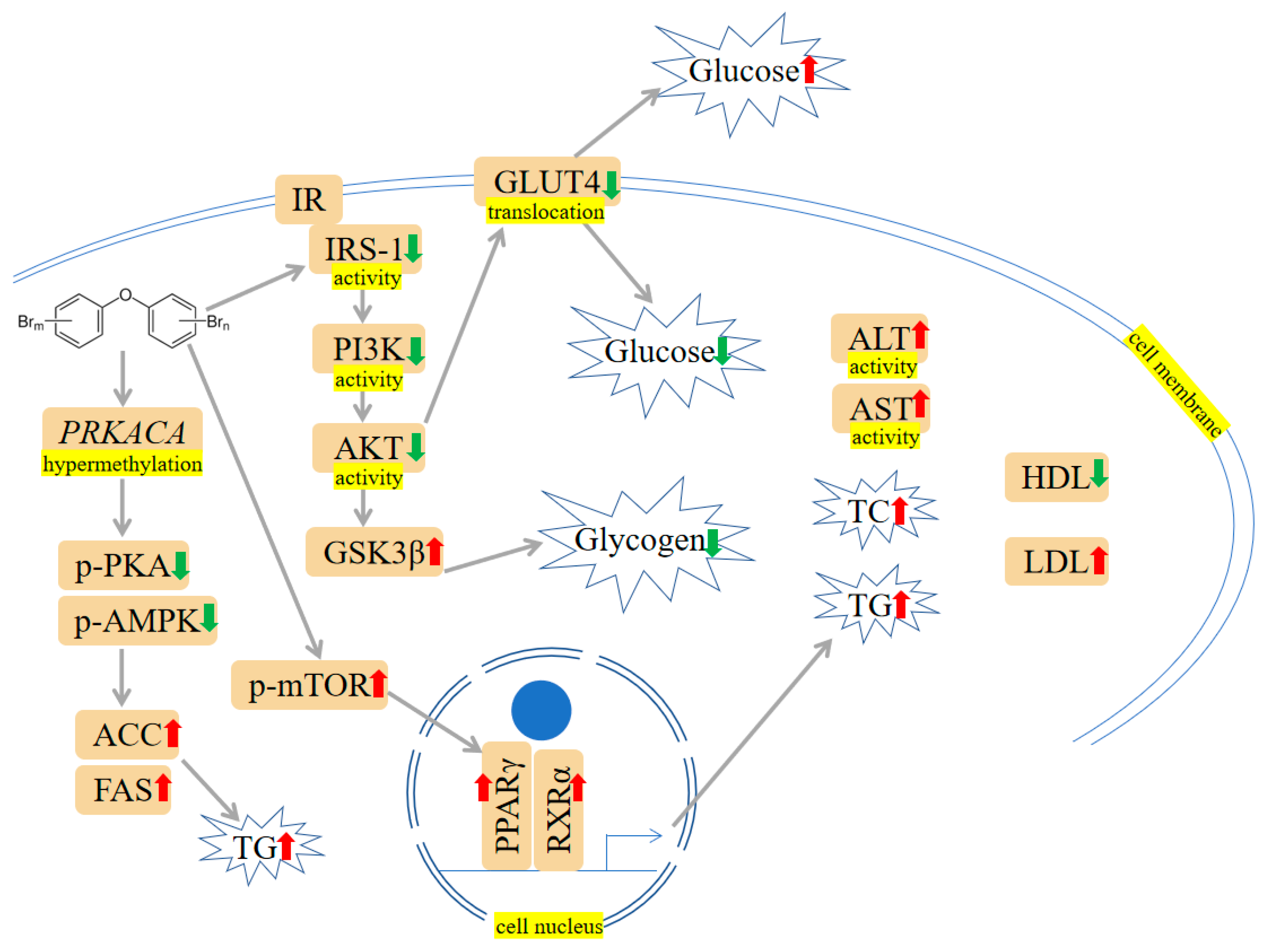
Figure 3. PBDEs-induced toxicity is associated with disturbances in glucose and lipid metabolism. PBDEs could increase glucose, total cholesterol (TC), triglyceride (TG), aspartate transaminase (ALT) activity, and aspartate transaminase (AST) activity. The PI3K/protein kinase B (AKT)/Glucose transporter type 4 (GLUT4) pathway is inhibited, and the mammalian target of rapamycin (mTOR)/PPARγ/RXRα pathway is elevated. Arrows indicate up (red colour), increased; down (green colour), decreased. The yellow highlighted text is an explanation of the figure.
3. Mitochondrial Damage
PBDE-47 increased miR-34a-5p level to trigger NAD+ insufficiency via targeting nicotinamide phosphoribosyltransferase (NAMPT) expression. Subsequently, Sirtuin 3 (Sirt3)/forkhead box O-3 α (FOXO3α)/PTEN-induced putative kinase1 (PINK1) pathway-associated mitophagy was inhibited, which results in mitochondrial dysfunction and oxidative damages in mouse livers [34]. Fetal liver HSCs with PBDE-47 treatment showed a loss of mitochondrial membrane potential (MMP) [4]. DNA damage and mitochondrial impairment were detectable in cells after exposure to PBDE-47 and PBDE-32 [2]. Pazin et al. have found that PBDE-47 or PBDE-99 can influence membrane potential, mitochondrial inner membrane, oxygen consumption, mitochondrial swelling, and calcium release, which results in adenosine triphosphate (ATP) exhaustion [35]. As the energy-producing organelles inside cells, mitochondria are essential in maintaining energy supplies. In isolated liver mitochondria, Pereira et al. observed that PBDE-153 can interact with the mitochondrial membrane and disrupt MMP, thus causing ATP deficiency [36]. Meanwhile, they have also investigated the effects of PBDE-209 on rat liver mitochondria. The results showed PBDE-209-induced matrix swelling and ATP depletion. This process may contribute to reduced HepG2 cell viability [37]. The mechanisms are shown in Figure 4.
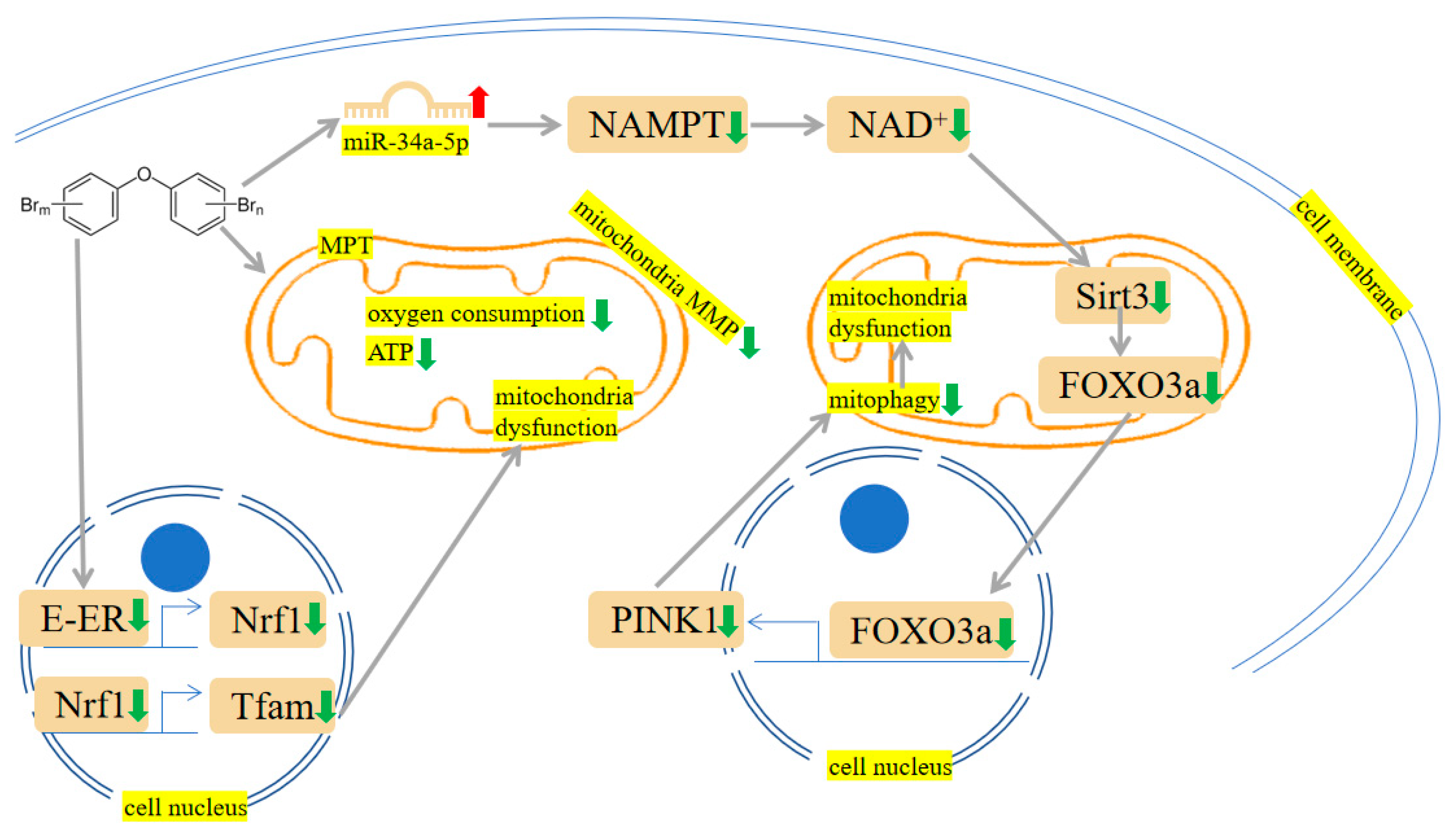
Figure 4. PBDEs-induced toxicity is associated with mitochondria damage. PBDEs exposure caused adenosine triphosphate (ATP) depletion, mitochondrial permeability transition (MPT) induction and mitochondria dysfunction. Sirtuin 3 (Sirt3)/forkhead box O-3 α (FOXO3α)/PINK1 pathway is suppressed by PBDE-209. Arrows indicate up (red colour), increased; down (green colour), decreased. The yellow highlighted text is an explanation of the figure.
4. Indirect Exposures
Indirect exposures occurred perinatally. For example, Dunnick et al. reported that PBDE-47 induced centrilobular hypertrophy and fatty change in pup livers on postnatal day (PND) 22. Liver transcriptomic changes were also measured, and the results showed that cytochrome p450 transcripts, nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway transcripts and ATP-binding cassette (ABC) membrane transport transcripts were upregulated. These alterations elicited lipids, oncogenes, and epigenetic changes, which can lead to liver damage and tumorigenesis [38]. Perinatal exposure to PBDE-99 can disrupt the nongenomic actions of thyroid hormone (TH), thereby reducing the activity of the PI3K/AKT pathway in rat pup livers and affecting cell survival [39].
5. Combined Exposures
Combined exposures produced a series of public health issues. It has been reported that PBDEs are tightly associated with the occurrence of obesity and NAFLD. Further exploration revealed that the combination of PBDE-47 and HFD treatment reduced carnitine palmitoyltransferase 1α (CPT1α) gene expression, inhibiting fatty acid oxidation. Besides, the expression of microsomal TG transfer protein was inhibited by PBDE-47, which led to dysfunction of TG metabolism [40]. Co-exposure of nanoplastics and PBDE-47 leads to changed liver colour and atrophied liver in zebrafish larvae. The liver degeneration or necrosis may be associated with reduced antioxidant glutathione peroxidase 1 (gpx1a) gene and increased CYP1A1 [41]. Using high-throughput sequencing approaches, Li et al. have proved that combined exposure of microplastics and PBDE-47 upregulated PPAR-related genes and reduced IL-17-associated genes [42]. Chen et al. have found that combined exposure to PBDE-209 and high fat resulted in elevated TG, MDA, and ROS levels in HepG2 cells, suggesting an increased lipid accumulation and oxidative stress. Similar to the in vitro results, mice receiving PBDE-209 and high fat showed elevated levels of sterol regulatory element-binding protein 1 (SREBP-1), stearoyl-CoA desaturase 1, and fatty acid synthase, thus promoting lipid deposition and NAFLD progression [43].
6. Others
Aside from those mentioned above, other effects and mechanisms of PBDEs on the liver are also worth mentioning. Crump et al. used an in vitro experiment to study the effects of PBDEs on cultured hepatocytes derived from embryonic chickens. They have found PBDE-71 diminished transthyretin (TTR), thyroid hormone–responsive spot 14-α (THRSP14-α), and liver fatty acid–binding protein (FABP) genes expression [44]. PBDE-71 can also induce hypomethylation at the T-Box Transcription Factor 3 (Tbx3) locus. As a transcription factor important in liver tumorigenesis, Tbx3 hypomethylation in mouse liver cells indicated that PBDE-71 may engage in liver carcinoma development [45]. To gain knowledge about the toxicological mechanisms of PBDEs, primary Atlantic salmon hepatocytes were exposed to these congeners alone or in combination (PBDE-47, PBDE-153 and PBDE-154). Levels of endoplasmic reticulum-responsive genes vitellogenin (VTG) and zona pellucida 3 (ZP3) become elevated [46]. Early life exposure to PBDE-99 can induce hepatic inflammation and increase acetate and succinate levels [47]. To elucidate the PBDEs-gut microbiome interactions in modulating hepatic long noncoding RNAs (lncRNAs) and protein-coding genes (PCGs), conventional and germ-free mice were orally dosed with PBDE-47 or PBDE-99. LncRNAs increased the translational efficiency of PCGs, and this process might be a compensatory mechanism in response to PBDEs exposure. Pathway analysis of PCGs paired with lncRNAs displayed that PBDE-47 regulated nucleic acid, retinol metabolism and circadian rhythm, whereas PBDE-99 regulated fatty acid metabolism in conventional mice. Likewise, in germ-free mice, glutathione conjugation and transcriptional regulation were regulated by PBDE-47. In addition, the xenobiotic-metabolizing CYP3A genes and the fatty acid-metabolizing CYP4 genes were modulated by PBDE-99 [48]. In Sueyoshi et al.’s study, human primary hepatocytes exposed to PBDE-47 exhibited upregulated CYP2B6 expression at both gene and protein levels. Because CYP2B6 is a constitutive androstane receptor (CAR) target gene, the changed expression pattern suggested a cause-and-effect relationship between PBDE-47 and CAR pathway [49]. It has been reported PBDEs modulated several processes linked to pregnane X receptor (PXR) and CAR (i.e., protein ubiqutination, PPARα-RXRα activation) [50]. A further study exploring potential underlying mechanisms revealed that PBDE-209 could incur liver morphological alteration, cause oxidative stress, and subsequently reduce PXR, CAR, and CYP3A expression [51]. The effects and mechanisms of liver toxicity induced by PBDEs are shown in Table 1.
Table 1. Effects and mechanisms of liver toxicity induced by PBDEs.
| Treatments | Effects and Mechanisms | References |
|---|---|---|
| PBDE-47 or -153, zebrafish | CAT activity↑, SOD activity↑, Caspase-3↑, P53↑, Bcl-2↓ | [1] |
| PBDE-47 or -32, HepG2 cells, trout liver cells |
Cell viability↓, ROS↑, apoptosis, DNA damage, mitochondrial impairment | [2][3][5] |
| PBDE-47, HSCs | ROS↑, lipid peroxidation, MMP↓ | [4] |
| PBDE-47, -99, -209, HepG2 cells | ERα↑, PPARα↑, intracellular lipid accumulation | [24] |
| PBDE-47, CAR and PXR null mice | CYP2B6↑, CYP2B6↑ | [49] |
| PBDE-47 or -99, isolated Wistar rat liver mitochondria | oxygen consumption↓, mitochondrial swelling, calcium release, ATP↓ | [35] |
| PBDE-47, CD-1 mice, ICR mice, C57 BL/6 mice | Proteasome dysfunction, TRAF2/ASK1/JNK pathway↑, NAD+ depletion, Sirt1↓, inflammation↑, abnormal insulin secretion, miR-34a-5p↑, Sirt3/FOXO3α/PINK1 pathway↓, mitochondrial dysfunction | [6][7][17][18][34] |
| PBDE-99, SD rats, HepG2 cells | SOD activity↑, CAT activity↓, GSSG↑, GSH↓, Caspase-3 activity↑, Caspase-9 activity↑, apoptosis | [8][9] |
| PBE-99, C57BL/6 mice | Inflammation, acetate↑, succinate↑ | [47] |
| PBDE-209, C57BL/6 mice, ICR mice, LO2 cells | ER stress↑, mitochondrial Ca2+ overload, apoptosis, ROS↑, PI3K/AKT/GLUT4 pathway↓, mTOR/PPARγ/RXRα pathway↑, Glucose↑, TG↑, HDL↓, liver and adipose structures damage | [10][30][31] |
| PBDE-209, SD rats | Hyperglycemia, GSH↓, SOD activity↓, liver weight↑, liver/body weight ratio↑, serum total bilirubin and indirect bilirubin↑, oxidative stress, PXR↓, CAR↓, CYP3A↓ | [32][51] |
| PBDE-209, IR-BRL cells | TC↑, TG↑, AST activity↑, ALT activity↑, MDA↑, IRS-1/PI3K/AKT/GSK-3β pathway↓, IRS-1/GLUT4↓ | [33] |
| PBDE quinone, LO2 cells | ER stress↑, autophagy-lysosomal system↑, ROS↑ | [11] |
| PBDE-209, SD rats, LO2 cells | PRKACA-1 hypermethylation, TG↑, Glucose↑, PI3K/AKT/GLUT4 pathway↓, mTOR/PPARγ/RXRα pathway↑ | [23][30] |
| PBDE-209, HepG2 cells, isolated mitochondria | Mitochondrial Ca2+ overload, apoptosis, ROS↑, LDH leakage, matrix swelling, ATP↓, cell viability↓ | [12][13][37] |
| PBDE-209, Carassius auratus | GR activity↑, GSH↓ | [14] |
| PBDEs in e-waste site, kingfisher (Alcedo atthis) | MDA↑, ROS↑, CAT activity↓, SOD activity↓ | [15] |
| PBDE-47, marine medaka (Oryzias melastigma) | PI3K pathway activity↑, MAPK pathway activity↑ | [16] |
| PBDE-71, Wistar rats | Glucose:insulin ratio↑ | [20][21] |
| PBDE-71, C57BL/6 mice | Glucose intolerance, fasting hyperglycemia, retarded glucose clearance, diminished thermogenic brown adipose tissue mass | [22] |
| PBDE-153, isolated rat liver mitochondria | MMP↓, ATP↓, ROS↑ | [36] |
| PBDE-47, Wistar Han rats, indirect exposure | Centrilobular hypertrophy, fatty change, cytochrome p450↑, Nrf2↑, lipid↑, oncogenes change, epigenetic change | [38] |
| PBDE-99, SD rats, indirect exposure | PIP3K/AKT pathway↓ | [39] |
| PBDE-47 and high-fat diet, HepG2 cells, C57BL/6J mice, combined exposure | CPT1α↓, fatty acid oxidation↓, microsomal triglyceride transfer protein↓, sterol regulatory element-binding protein 1↑, stearoyl-CoA desaturase 1↑, fatty acid synthase↑, lipid deposition, NAFLD, MDA↑, ROS↑, lipid accumulation | [40][43] |
| PBDE-47 and nanoplastics, zebrafish, combined exposure | Darker/browner liver colour, atrophied liver, liver degeneration or necrosis, gpx1a↓, CYP1A1↑, mortality↑, voluntary movements↑, hatching rate↑, heart rate↓ | [41] |
| PBDE-47 and microplastics, grouper (Epinephelus moara), combined exposure | PPAR-related genes↑, IL-17-related genes↓ | [42] |
| PBDE-71, hepatocytes derived from embryonic chickens | TTR↓, THRSP14-α↓, FABP↓ | [44] |
| PBDE-71, B6C3F1/N mice | Tbx3 hypomethylation | [45] |
| PBDE-47, PBDE-153 and PBDE-154 (alone or in combination), primary Atlantic salmon hepatocytes | VTG↑, ZP3↑ | [46] |
↑ represents upregulation, ↓ represents downregulation.
References
- Meng, S.; Chen, X.; Gyimah, E.; Xu, H.; Chen, J. Hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish following sub-chronic exposure to BDE-47 and BDE-153. Environ. Toxicol. 2020, 35, 1202–1211.
- Saquib, Q.; Siddiqui, M.A.; Ahmed, J.; Al-Salim, A.; Ansari, S.M.; Faisal, M.; Al-Khedhairy, A.A.; Musarrat, J.; AlWathnani, H.A.; Alatar, A.A.; et al. Hazards of low dose flame-retardants (BDE-47 and BDE-32): Influence on transcriptome regulation and cell death in human liver cells. J. Hazard. Mater. 2016, 308, 37–49.
- Tang, S.; Liu, H.; Yin, H.; Liu, X.; Peng, H.; Lu, G.; Dang, Z.; He, C. Effect of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) and its metabolites on cell viability, oxidative stress, and apoptosis of HepG2. Chemosphere 2018, 193, 978–988.
- Shao, J.; White, C.C.; Dabrowski, M.J.; Kavanagh, T.J.; Eckert, M.L.; Gallagher, E.P. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 101, 81–90.
- Shao, J.; Eckert, M.L.; Lee, L.E.; Gallagher, E.P. Comparative oxygen radical formation and toxicity of BDE 47 in rainbow trout cell lines. Mar. Environ. Res. 2008, 66, 7–8.
- Zhang, Z.F.; Shan, Q.; Zhuang, J.; Zhang, Y.Q.; Wang, X.; Fan, S.H.; Lu, J.; Wu, D.M.; Hu, B.; Zheng, Y.L. Troxerutin inhibits 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47)-induced hepatocyte apoptosis by restoring proteasome function. Toxicol. Lett. 2015, 233, 246–257.
- Zhang, Z.F.; Zhang, Y.Q.; Fan, S.H.; Zhuang, J.; Zheng, Y.L.; Lu, J.; Wu, D.M.; Shan, Q.; Hu, B. Troxerutin protects against 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD+-depletion. J. Hazard. Mater. 2015, 283, 98–109.
- Albina, M.L.; Alonso, V.; Linares, V.; Bellés, M.; Sirvent, J.J.; Domingo, J.L.; Sánchez, D.J. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology 2010, 271, 51–56.
- Souza, A.O.; Pereira, L.C.; Oliveira, D.P.; Dorta, D.J. BDE-99 congener induces cell death by apoptosis of human hepatoblastoma cell line-HepG2. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2013, 27, 580–587.
- Che, S.; Chen, S.; Li, S.; Ruan, Z. Decabromodiphenyl ether initiates mitochondria-dependent apoptosis by disrupting calcium homeostasis in mice livers. Chemosphere 2022, 291 Pt 1, 132767.
- Wang, Y.; Xu, L.; Peng, L.; Fang, C.; Qin, Q.; Lv, X.; Liu, Z.; Yang, B.; Song, E.; Song, Y. Polybrominated diphenyl ethers quinone-induced intracellular protein oxidative damage triggers ubiquitin-proteasome and autophagy-lysosomal system activation in LO2 cells. Chemosphere 2021, 275, 130034.
- Yuan, J.; Sun, X.; Che, S.; Zhang, L.; Ruan, Z.; Li, X.; Yang, J. AhR-mediated CYP1A1 and ROS overexpression are involved in hepatotoxicity of decabromodiphenyl ether (BDE-209). Toxicol. Lett. 2021, 352, 26–33.
- Hu, X.Z.; Xu, Y.; Hu, D.C.; Hui, Y.; Yang, F.X. Apoptosis induction on human hepatoma cells Hep G2 of decabrominated diphenyl ether (PBDE-209). Toxicol. Lett. 2007, 171, 19–28.
- Zhao, A.; Liu, H.; Zhang, A.; Wang, X.; Zhang, H.; Wang, H. Effect of BDE-209 on glutathione system in Carassius auratus. Environ. Toxicol. Pharmacol. 2011, 32, 35–39.
- Wu, J.P.; Peng, Y.; Zhi, H.; Wu, S.K.; Chen, X.Y.; Zeng, Y.H.; Luo, X.J.; Mai, B.X. Contaminant-related oxidative distress in common kingfisher (Alcedo atthis) breeding at an e-waste site in South China. Environ. Res. 2020, 182, 109079.
- Yu, W.K.; Shi, Y.F.; Fong, C.C.; Chen, Y.; van de Merwe, J.P.; Chan, A.K.; Wei, F.; Bo, J.; Ye, R.; Au, D.W.; et al. Gender-specific transcriptional profiling of marine medaka (Oryzias melastigma) liver upon BDE-47 exposure. Comp. Biochem. Physiol. Part D Genom. Proteom. 2013, 8, 255–262.
- Khalil, A.; Cevik, S.E.; Hung, S.; Kolla, S.; Roy, M.A.; Suvorov, A. Developmental Exposure to 2,2′,4,4′-Tetrabromodiphenyl Ether Permanently Alters Blood-Liver Balance of Lipids in Male Mice. Front. Endocrinol. 2018, 9, 548.
- Liu, Z.L.; Jiang, S.R.; Fan, Y.; Wang, J.S.; Wang, M.L.; Li, M.Y. 2,2′,4,4′,5,5′-Hexabromophenyl ether (BDE-153) causes abnormal insulin secretion and disorders of glucose and lipid metabolism in mice. J. Chin. Med. Assoc. JCMA 2023, 86, 388–398.
- Suvorov, A.; Naumov, V.; Shtratnikova, V.; Logacheva, M.; Shershebnev, A.; Wu, H.; Gerasimov, E.; Zheludkevich, A.; Pilsner, J.R.; Sergeyev, O. Rat liver epigenome programing by perinatal exposure to 2,2′,4′4′-tetrabromodiphenyl ether. Epigenomics 2020, 12, 235–249.
- Nash, J.T.; Szabo, D.T.; Carey, G.B. Polybrominated diphenyl ethers alter hepatic phosphoenolpyruvate carboxykinase enzyme kinetics in male Wistar rats: Implications for lipid and glucose metabolism. J. Toxicol. Environ. Health. Part A 2013, 76, 142–156.
- Cowens, K.R.; Simpson, S.; Thomas, W.K.; Carey, G.B. Polybrominated Diphenyl Ether (PBDE)-Induced Suppression of Phosphoenolpyruvate Carboxykinase (PEPCK) Decreases Hepatic Glyceroneogenesis and Disrupts Hepatic Lipid Homeostasis. J. Toxicol. Environ. Health. Part A 2015, 78, 1437–1449.
- Kozlova, E.V.; Chinthirla, B.D.; Pérez, P.A.; DiPatrizio, N.V.; Argueta, D.A.; Phillips, A.L.; Stapleton, H.M.; González, G.M.; Krum, J.M.; Carrillo, V.; et al. Maternal transfer of environmentally relevant polybrominated diphenyl ethers (PBDEs) produces a diabetic phenotype and disrupts glucoregulatory hormones and hepatic endocannabinoids in adult mouse female offspring. Sci. Rep. 2020, 10, 18102.
- Zhu, Y.; Jing, L.; Li, X.; Zhou, G.; Zhang, Y.; Sang, Y.; Gao, L.; Liu, S.; Shi, Z.; Sun, Z.; et al. Decabromodiphenyl ether-induced PRKACA hypermethylation contributed to glycolipid metabolism disorder via regulating PKA/AMPK pathway in rat and L-02 cells. Environ. Toxicol. Pharmacol. 2022, 90, 103808.
- Casella, M.; Lori, G.; Coppola, L.; La Rocca, C.; Tait, S. BDE-47, -99, -209 and Their Ternary Mixture Disrupt Glucose and Lipid Metabolism of Hepg2 Cells at Dietary Relevant Concentrations: Mechanistic Insight through Integrated Transcriptomics and Proteomics Analysis. Int. J. Mol. Sci. 2022, 23, 14465.
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49.
- Abrha, A.; Suvorov, A. Transcriptomic Analysis of Gonadal Adipose Tissue in Male Mice Exposed Perinatally to 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47). Toxics 2018, 6, 21.
- Fang, M.; Webster, T.F.; Ferguson, P.L.; Stapleton, H.M. Characterizing the peroxisome proliferator-activated receptor (PPARγ) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ. Health Perspect. 2015, 123, 166–172.
- Tung, E.W.; Boudreau, A.; Wade, M.G.; Atlas, E. Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells. PLoS ONE 2014, 9, e94583.
- Kamstra, J.H.; Hruba, E.; Blumberg, B.; Janesick, A.; Mandrup, S.; Hamers, T.; Legler, J. Transcriptional and epigenetic mechanisms underlying enhanced in vitro adipocyte differentiation by the brominated flame retardant BDE-47. Environ. Sci. Technol. 2014, 48, 4110–4119.
- Zhu, Y.; Jing, L.; Li, X.; Zheng, D.; Zhou, G.; Zhang, Y.; Sang, Y.; Shi, Z.; Sun, Z.; Zhou, X. Decabromodiphenyl ether disturbs hepatic glycolipid metabolism by regulating the PI3K/AKT/GLUT4 and mTOR/PPARγ/RXRα pathway in mice and L02 cells. Sci. Total Environ. 2021, 763, 142936.
- Zhu, Y.; Li, X.; Liu, J.; Zhou, G.; Yu, Y.; Jing, L.; Shi, Z.; Zhou, X.; Sun, Z. The effects of decabromodiphenyl ether on glycolipid metabolism and related signaling pathways in mice. Chemosphere 2019, 222, 849–855.
- Zhang, Z.; Sun, Z.Z.; Xiao, X.; Zhou, S.; Wang, X.C.; Gu, J.; Qiu, L.L.; Zhang, X.H.; Xu, Q.; Zhen, B.; et al. Mechanism of BDE209-induced impaired glucose homeostasis based on gene microarray analysis of adult rat liver. Arch. Toxicol. 2013, 87, 1557–1567.
- Mao, G.; Tang, J.; Liao, T.; Shi, X.; Dong, F.; Feng, W.; Chen, Y.; Zhao, T.; Wu, X.; Yang, L. Metabolism toxicity and susceptibility of decabromodiphenyl ether (BDE-209) exposure on BRL cells with insulin resistance. Environ. Sci. Pollut. Res. Int. 2022, 29, 91306–91324.
- Chen, F.; Feng, L.; Zheng, Y.L.; Lu, J.; Fan, S.H.; Shan, Q.; Zheng, G.H.; Wang, Y.J.; Wu, D.M.; Li, M.Q.; et al. 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) induces mitochondrial dysfunction and related liver injury via eliciting miR-34a-5p-mediated mitophagy impairment. Environ. Pollut. 2020, 258, 113693.
- Pazin, M.; Pereira, L.C.; Dorta, D.J. Toxicity of brominated flame retardants, BDE-47 and BDE-99 stems from impaired mitochondrial bioenergetics. Toxicol. Mech. Methods 2015, 25, 34–41.
- Pereira, L.C.; Cabral Miranda, L.F.; Franco-Bernardes, M.F.; Tasso, M.J.; Duarte, F.V.; Inácio Varela, A.T.; Rolo, A.P.; Marques Palmeira, C.M.; Dorta, D.J. Mitochondrial damage and apoptosis: Key features in BDE-153-induced hepatotoxicity. Chem.-Biol. Interact. 2018, 291, 192–201.
- Pereira, L.C.; Souza, A.O.; Tasso, M.J.; Oliveira, A.M.C.; Duarte, F.V.; Palmeira, C.M.; Dorta, D.J. Exposure to decabromodiphenyl ether (BDE-209) produces mitochondrial dysfunction in rat liver and cell death. J. Toxicol. Environ. Health. Part A 2017, 80, 1129–1144.
- Dunnick, J.K.; Shockley, K.R.; Pandiri, A.R.; Kissling, G.E.; Gerrish, K.E.; Ton, T.V.; Wilson, R.E.; Brar, S.S.; Brix, A.E.; Waidyanatha, S.; et al. PBDE-47 and PBDE mixture (DE-71) toxicities and liver transcriptomic changes at PND 22 after in utero/postnatal exposure in the rat. Arch. Toxicol. 2018, 92, 3415–3433.
- Blanco, J.; Mulero, M.; Domingo, J.L.; Sanchez, D.J. Perinatal exposure to BDE-99 causes decreased protein levels of cyclin D1 via GSK3β activation and increased ROS production in rat pup livers. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 137, 491–498.
- Yang, C.; Zhu, L.; Kang, Q.; Lee, H.K.; Li, D.; Chung, A.C.K.; Cai, Z. Chronic exposure to tetrabromodiphenyl ether (BDE-47) aggravates hepatic steatosis and liver fibrosis in diet-induced obese mice. J. Hazard. Mater. 2019, 378, 120766.
- Wang, Q.; Chen, G.; Tian, L.; Kong, C.; Gao, D.; Chen, Y.; Junaid, M.; Wang, J. Neuro- and hepato-toxicity of polystyrene nanoplastics and polybrominated diphenyl ethers on early life stages of zebrafish. Sci. Total Environ. 2023, 857 Pt 2, 159567.
- Li, H.; Li, Y.; Maryam, B.; Ji, Z.; Sun, J.; Liu, X. Polybrominated diphenyl ethers as hitchhikers on microplastics: Sorption behaviors and combined toxicities to Epinephelus moara. Aquat. Toxicol. 2022, 252, 106317.
- Chen, S.; Che, S.; Li, S.; Ruan, Z. The combined impact of decabromodiphenyl ether and high fat exposure on non-alcoholic fatty liver disease in vivo and in vitro. Toxicology 2021, 464, 153015.
- Crump, D.; Chiu, S.; Egloff, C.; Kennedy, S.W. Effects of hexabromocyclododecane and polybrominated diphenyl ethers on mRNA expression in chicken (Gallus domesticus) hepatocytes. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 106, 479–487.
- Shimbo, T.; Dunnick, J.K.; Brix, A.; Mav, D.; Shah, R.; Roberts, J.D.; Wade, P.A. DNA Methylation Changes in Tbx3 in a Mouse Model Exposed to Polybrominated Diphenyl Ethers. Int. J. Toxicol. 2017, 36, 229–238.
- Søfteland, L.; Petersen, K.; Stavrum, A.K.; Wu, T.; Olsvik, P.A. Hepatic in vitro toxicity assessment of PBDE congeners BDE47, BDE153 and BDE154 in Atlantic salmon (Salmo salar L.). Aquat. Toxicol. 2011, 105, 246–263.
- Lim, J.J.; Dutta, M.; Dempsey, J.L.; Lehmler, H.J.; MacDonald, J.; Bammler, T.; Walker, C.; Kavanagh, T.J.; Gu, H.; Mani, S.; et al. Neonatal Exposure to BPA, BDE-99, and PCB Produces Persistent Changes in Hepatic Transcriptome Associated With Gut Dysbiosis in Adult Mouse Livers. Toxicol. Sci. Off. J. Soc. Toxicol. 2021, 184, 83–103.
- Li, C.Y.; Cui, J.Y. Regulation of protein-coding gene and long noncoding RNA pairs in liver of conventional and germ-free mice following oral PBDE exposure. PLoS ONE 2018, 13, e0201387.
- Sueyoshi, T.; Li, L.; Wang, H.; Moore, R.; Kodavanti, P.R.; Lehmler, H.J.; Negishi, M.; Birnbaum, L.S. Flame retardant BDE-47 effectively activates nuclear receptor CAR in human primary hepatocytes. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 137, 292–302.
- Zhang, A.; Li, C.Y.; Kelly, E.J.; Sheppard, L.; Cui, J.Y. Transcriptomic profiling of PBDE-exposed HepaRG cells unveils critical lncRNA- PCG pairs involved in intermediary metabolism. PLoS ONE 2020, 15, e0224644.
- Sun, Y.; Wang, Y.; Liang, B.; Chen, T.; Zheng, D.; Zhao, X.; Jing, L.; Zhou, X.; Sun, Z.; Shi, Z. Hepatotoxicity of decabromodiphenyl ethane (DBDPE) and decabromodiphenyl ether (BDE-209) in 28-day exposed Sprague-Dawley rats. Sci. Total Environ. 2020, 705, 135783.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
670
Revisions:
2 times
(View History)
Update Date:
22 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




