Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Zhou | -- | 1747 | 2023-09-21 12:53:36 | | | |
| 2 | Catherine Yang | Meta information modification | 1747 | 2023-09-22 02:44:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhou, Y.; Pu, J.; Wu, Y. Lipid Metabolism in Influenza A Virus Infection. Encyclopedia. Available online: https://encyclopedia.pub/entry/49473 (accessed on 08 February 2026).
Zhou Y, Pu J, Wu Y. Lipid Metabolism in Influenza A Virus Infection. Encyclopedia. Available at: https://encyclopedia.pub/entry/49473. Accessed February 08, 2026.
Zhou, Yong, Juan Pu, Yuping Wu. "Lipid Metabolism in Influenza A Virus Infection" Encyclopedia, https://encyclopedia.pub/entry/49473 (accessed February 08, 2026).
Zhou, Y., Pu, J., & Wu, Y. (2023, September 21). Lipid Metabolism in Influenza A Virus Infection. In Encyclopedia. https://encyclopedia.pub/entry/49473
Zhou, Yong, et al. "Lipid Metabolism in Influenza A Virus Infection." Encyclopedia. Web. 21 September, 2023.
Copy Citation
Influenza A virus (IAV) is an important zoonotic pathogen that can cause disease in animals such as poultry and pigs, and it can cause infection and even death in humans, posing a serious threat to public health. IAV is an enveloped virus that relies on host cell metabolic systems, especially lipid metabolism systems, to complete its life cycle in host cells. On the other side, host cells regulate their metabolic processes to prevent IAV replication and maintain their normal physiological functions.
influenza A virus (IAV)
host cell
lipid metabolism
replication
1. Positive and Negative Regulation of FA Metabolism in IAV Infection
FA is closely related to the inflammatory and allergic responses of organisms and widely involved in the regulation of IAV infection. Studies have shown that compared with non-obese mice, obese mice will have more severe inflammatory reaction after IAV infection due to abnormal FA metabolism [1]. By analyzing the lipid metabolites of IAV-infected mice, Morita et al. also found that polyunsaturated fatty acids (PUFA) 12-hydroxyeicosatetraenoic acid (12-HETE), 15-hydroxyeicosatetraenoic acid (15-HETE), 17-hydroxy-4,7,10,13,15,19-docosahexaenoic acid (17-HDoHE) and omega-3 PUFA-derived lipid mediator protectin D1 (PD1) inhibited IAV infection after exogenous addition and reduced the lethality of IAV infection [2]. Further studies showed that PD1 specifically inhibited the nuclear export of IAV mRNA and suppressed viral replication [3]. Another in vitro study showed that lipoxygenin B4 (LXB4), derived from omega-6 PUFA arachidonic acid (AA), promoted the expression of B lymphocyte-induced maturation protein 1 (Blimp1) and X-box binding protein 1 (XBP1), the key transcription factors involved in plasma cell differentiation, thereby increasing the number of effector B cells and production of IgG, which promoted host resistance to IAV [4]. In addition, through the study on the basic metabolism of lung injury and multiple organ failure caused by human H7N9 virus infection, it was found that palmitic acid, a saturated fatty acid and erucic acid, omega-9 long chain monounsaturated fatty acid were negatively correlated with the severity of the disease, and could be used as key molecules for the prognosis of the disease [5]. In conclusion, FA such as PD1, LXB4, palmitic acid and erucic acid have negative regulatory effects on the infection process of IAV, which provides a scientific reference for the prevention and control of influenza and disease prognosis.
FA also have a positive regulatory effect on IAV infection that can exacerbate the severity of IAV infection. After PGE2 binds to its receptors EP2 and EP4, it can activate intracellular phosphatidylinositol 3-kinase (PI3K)-Akt and protein kinase A (PKA) signaling pathways, thereby inhibiting IRF3-mediated type I IFN activation and antigen presentation as well as T cell activation, exacerbating the damage caused by IAV infection [6][7][8][9]. In addition, phospholipase D (PLD), a key enzyme in FA metabolism, can promote IAV replication by inhibiting the expression of myxovirus resistance gene A (MxA) [10]. In 2010, PLD was identified as a host factor required for IAV replication [11], and PLD has potential use as an anti-influenza target drug [12].
2. Chol Plays a Major Role in Fusion and Release during the IAV Life Cycle
Chol is an indispensable substance in cells, not only participating in the formation of cell membranes but also serving as a precursor for the synthesis of a variety of bioactive substances. Additionally, Chol is an important component of the IAV envelope, accounting for approximately 11–12% of its total mass [13], and is very important for IAV fusion and release [14]. After binding to the sialic acid receptor, the successful entry of IAV into the cell further depends on fusion of the viral envelope with the intracellular body membrane [15], and viral envelope Chol plays a key role in this process. Pretreatment of IAV particles with different concentrations of methyl-β-cyclodextrin (soluble Chol) resulted in a dose-dependent reduction in the cellular infectivity of IAV via solubilization of Chol, but this inhibition was limited to the viral fusion process [16][17]. In addition to the critical role in the entry stage as the viral envelope Chol, cell membrane Chol is essential to ensure effective budding of progeny virions; the infectivity and stability of progeny virions released from cells deficient in cell membrane Chol are severely impaired because progeny virions acquire their envelope from the host cell membrane during budding, and Chol plays a key role in maintaining the IAV envelope structure [18][19][20]. In vivo, Chol homeostasis is regulated by Annexin A6 (ANXA6) [21][22][23]. Overexpression of ANXA6 decreased Chol levels in the cell membrane, thereby inhibiting IAV replication and propagation, and when siRNA was used to reduce the expression of ANXA6, the titer of the progeny IAV virions was increased [24]. In addition, ANXA6 inhibits IAV budding by directly interacting with influenza matrix protein 2 (M2) [25]. The CRAC motif of the M2 protein is a potential binding motif for Chol, but whether ANXA6 competes with progeny virions for binding to the CRAC motif is unknown [26]. ANXA6 may be a target for anti-influenza drugs by regulating Chol [21].
It is worth mentioning that Chol distributed on the cell membrane forms lipid rafts together with sphingolipids [27][28] Host lipid raft is selected by IAV as a host attachment factor for multivalent binding, and play a critical role in assembly and budding of progeny virions. The polyvalent binding of IAV HA protein with sialic acid receptor will cause the aggregation of lipid rafts, which leads to the concentration and aggregation of tyrosine protein kinase (RTK) and activation, and then causes the response of PI3K/Akt and other related signaling pathways, and promotes the endocytosis of IAV [29][30]. IAV also uses the “lipid raft” region on the host’s cell membrane as the virus budding site.
3. PL Metabolism Is Involved in Multiple Stages of IAV Infection
PL includes mainly glycerophospholipids and sphingomyelin (SM), which are the main components of biological membranes. Glycerophospholipids can be further divided into many categories depending on the substitution groups, for example, phosphatidylcholine (PC), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI) and cardiolipin (CL). As an enveloped virus, IAV needs to acquire an envelope from the host cell membrane during budding of progeny virions, which inevitably leads to changes in the PL content of the host cell. Alveolar type II (ATII) cells are the most important target cells of IAV; among the pulmonary surfactants synthesized by these cells, the PL content is as high as 35–50 mg/mL [31]. When infected by IAV, ATII cells undergo reprogramming of PL metabolism, and the cell surface levels of PC, PG and PE are reduced, while those of PS, PI and SM are increased [32].
Changes in glycerophospholipid levels affect IAV replication. On the one hand, glycosylation in the vicinity of cellular sialic acid receptors leads to blockade of virion binding to cellular receptors, but elevated PS levels can abolish this blockade and increase cellular susceptibility to enveloped viruses by up to 20-fold [33][34]. On the other hand, PI can exert anti-IAV effects by directly binding to IAV with high affinity, disrupting its adsorption to host cells and binding to sialic acid receptors [35]. In addition, PI can antagonize the activation of homologous ligands of Toll-like receptors (TLRs), central components of innate immunity, thereby inhibiting the transcription of proinflammatory genes, suppressing the cytokine storm induced by IAV infection and reducing damage to the organism [36][37]. This observation indicates that the reprogramming of PL metabolism is multifaceted and shows that during IAV infection, glycerophospholipids act not only as the body’s “guardian” against IAV but also as an “accomplice” of IAV.
SM is of interest due to its important intracellular signaling and regulatory roles [37], and its three metabolites of ceramide (Cer), sphingosine (Sph), and sphingosine-1-phosphate (S1P) are biologically active signaling molecules [38]. SM and Cer play roles in promoting and inhibiting IAV infection, respectively, and some related metabolic regulatory enzymes involved in SM metabolism have been reported to have related effects (Figure 1).
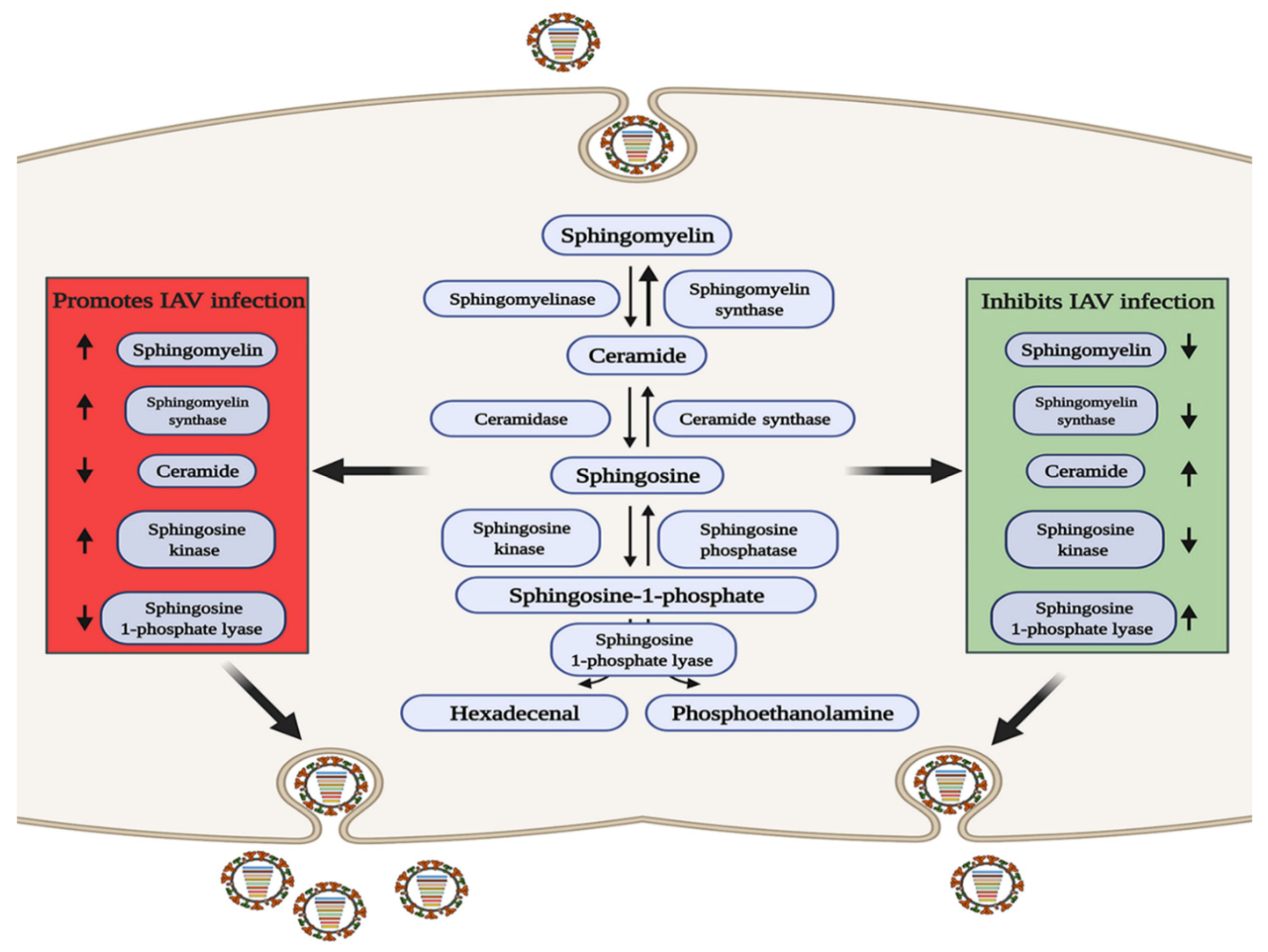
Figure 1. The role of sphingomyelin metabolism in IAV infection. In the red area, when the levels of sphingolipids or related metabolic enzymes are up-regulated or down regulated as shown by the arrow, IAV infection can be promoted through a variety of ways; conversely, in the green area, when the levels of sphingolipids or related metabolic enzymes are up-regulated or down regulated as shown by the arrow, IAV infection can be inhibited.
S1P is a terminal active metabolite of SM metabolism, and requires binding to different S1P receptor (S1PR1-S1PR5) to function. When S1PR1 is activated with CYM-5442, it can inhibit intercellular cell adhesion molecule-1 (ICAM1) production in a β-arrestin2-dependent manner, thereby reducing lung injury mediated by the strong immune response triggered by IAV infection, but it cannot reduce the viral titer [39][40][41], indicating that S1PR1 cannot reduce viral replication but can reduce pathological damage. Sphingosine kinase 1 (SPHK1) is the key enzyme that catalyzes the generation of S1P from Sph [42][43]. In infected cells, SPHK1 was shown to promote viral RNA synthesis by activating NF-κB and to maintain CRM1/RanBP3-mediated nuclear export of the viral ribonucleoprotein (vRNP) complex by activating ERK and AKT [44][45][46][47][48]. S1P lyase (SPL), which induces S1P degradation, has also been shown to participate in the host anti-IAV response. SPL induces rapid activation of ERK and STAT1 and enhances the activation of IKKε, thereby promoting the host immune response [49]. The above studies indicate that metabolites and enzymes in the SM metabolic pathway are widely involved in the regulation of IAV infection.
4. An Appropriate GL Concentration Is Important for IAV Infection
GLs are a class of amphiphilic lipid compounds containing glycosyl ligands that are widespread in living organisms, and glycosphingolipids (GSLs) are a main component of the GL family with multiple cellular roles. Some previous studies concluded that IAV must bind to cell surface GSLs to successfully infect cells [50][51], but other studies demonstrated that GSL is not required for IAV infection but acts as an adsorption stabilizer only during the entry stage of the IAV life cycle [52][53][54]. Glucosylceramide (GlcCer) is the main component of GSLs and can be converted into glucose and Cer under the catalytic activity of glucocerebrosidase (GBA) [55], while glucosylceramide synthase (GCS) catalyzes the synthesis of GlcCer from Cer. After using CRISPR-Cas9 to knock out GBA, the level of GlcCer was increased by approximately three- to four-fold, delaying the transport of IAV to late endosomes and inhibiting IAV infection [56]. In addition, when CRISPR/Cas9 was used to knock out the GCS gene, the level of GlcCer was reduced, and the infectivity of IAV was weakened [57]. The above findings suggest that either excessive or insufficient levels of GlcCer inhibit IAV replication, suggesting that an appropriate concentration of GlcCer is extremely important for maintaining optimal IAV infection ability without dose dependency.
References
- Milner, J.J.; Rebeles, J.; Dhungana, S.; Stewart, D.A.; Sumner, S.C.J.; Meyers, M.H.; Mancuso, P.; Beck, M.A. Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice. J. Immunol. 2015, 194, 4846–4859.
- Schultz, D.; Methling, K.; Rothe, M.; Lalk, M. Eicosanoid Profile of Influenza A Virus Infected Pigs. Metabolites 2019, 9, 130.
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125.
- Kim, N.; Lannan, K.L.; Thatcher, T.H.; Pollock, S.J.; Woeller, C.F.; Phipps, R.P. Lipoxin B-4 Enhances Human Memory B Cell Antibody Production via Upregulating Cyclooxygenase-2 Expression. J. Immunol. 2018, 201, 3343–3351.
- Sun, X.; Song, L.; Feng, S.; Li, L.; Yu, H.; Wang, Q.; Wang, X.; Hou, Z.; Li, X.; Li, Y.; et al. Fatty Acid Metabolism is Associated with Disease Severity After H7N9 Infection. EBioMedicine 2018, 33, 218–229.
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93.
- Smith, W.L. The eicosanoids and their biochemical-mechanisms of action. Biochem. J. 1989, 259, 315–324.
- Full, F.; Gack, M.U. Prostaglandin E-2: The Villain in the Host Response to Influenza Virus. Immunity 2014, 40, 453–454.
- Coulombe, F.; Jaworska, J.; Verway, M.; Tzelepis, F.; Massoud, A.; Gillard, J.; Wong, G.; Kobinger, G.; Xing, Z.; Couture, C.; et al. Targeted Prostaglandin E-2 Inhibition Enhances Antiviral Immunity through Induction of Type I Interferon and Apoptosis in Macrophages. Immunity 2014, 40, 554–568.
- Oguin, T.H.I.; Sharma, S.; Stuart, A.D.; Duan, S.; Scott, S.A.; Jones, C.K.; Daniels, J.S.; Lindsley, C.W.; Thomas, P.G.; Brown, H.A. Phospholipase D Facilitates Efficient Entry of Influenza Virus, Allowing Escape from Innate Immune Inhibition. J. Biol. Chem. 2014, 289, 25405–25417.
- Karlas, A.; Machuy, N.; Shin, Y.; Pleissner, K.; Artarini, A.; Heuer, D.; Becker, D.; Khalil, H.; Ogilvie, L.A.; Hess, S.; et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 2010, 463, 132–818.
- Behera, D.K.; Behera, P.M.; Acharya, L.; Dixit, A. Pharmacophore modelling, virtual screening and molecular docking studies on PLD1 inhibitors. SAR QSAR Environ. Res. 2017, 28, 991–1009.
- Lenard, J.; Compans, R.W. Mmbrabe structure of lipid-containing viruses. Biochim. Biophys. Acta 1974, 344, 51–94.
- Domanska, M.K.; Wrona, D.; Kasson, P.M. Multiphasic Effects of Cholesterol on Influenza Fusion Kinetics Reflect Multiple Mechanistic Roles. Biophys. J. 2013, 105, 1383–1387.
- Colman, P.M.; Lawrence, M.C. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 2003, 4, 309–319.
- Dunning, R.A.; Domanska, M.K.; Dryden, K.; Yeager, M.; Kasson, P.M. Effect of Cholesterol Depletion on HA Distribution in the Viral Membrane of Influenza. Biophys. J. 2015, 1081, 406A–407A.
- Liu, K.N.; Boxer, S.G. Target Membrane Cholesterol Modulates Single Influenza Virus Membrane Fusion Efficiency but Not Rate. Biophys. J. 2020, 118, 2426–2433.
- Bajimaya, S.; Frankl, T.; Hayashi, T.; Takimoto, T. Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses. Virology 2017, 510, 234–241.
- Goronzy, I.N.; Rawle, R.J.; Boxer, S.G.; Kasson, P.M. Cholesterol enhances influenza binding avidity by controlling nanoscale receptor clustering. Chem. Sci. 2018, 9, 2340–2347.
- Sieczkarski, S.B.; Whittaker, G.R. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 2002, 76, 10455–10464.
- Cubells, L.; de Muga, S.V.; Tebar, F.; Wood, P.; Evans, R.; Ingelmo-Torres, M.; Calvo, M.; Gaus, K.; Pol, A.; Grewal, T.; et al. Annexin A6-induced alterations in cholesterol transport and caveolin export from the golgi complex. Traffic 2007, 8, 1568–1589.
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461.
- De Diego, I.; Schwartz, F.; Siegfried, H.; Dauterstedt, P.; Heeren, J.; Beisiegel, U.; Enrich, C.; Grewal, T. Cholesterol modulates the membrane binding and intracellular distribution of annexin 6. J. Biol. Chem. 2002, 277, 32187–32194.
- Musiol, A.; Gran, S.; Ehrhardt, C.; Ludwig, S.; Grewal, T.; Gerke, V.; Rescher, U. Annexin A6-balanced late endosomal cholesterol controls influenza A replication and propagation. MBio 2013, 4, e00608-13.
- Ma, H.; Kien, F.; Maniere, M.; Zhang, Y.; Lagarde, N.; Tse, K.S.; Poon, L.L.M.; Nal, B. Human Annexin A6 Interacts with Influenza A Virus Protein M2 and Negatively Modulates Infection. J. Virol. 2012, 86, 1789–1801.
- Schroeder, C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell Biochem. 2010, 51, 77–108.
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224.
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572.
- Eierhoff, T.; Hrincius, E.R.; Rescher, U.; Ludwig, S.; Ehrhardt, C. The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells. PLoS Pathog. 2010, 6, e1001099.
- Verma, D.K.; Gupta, D.; Lal, S.K. Host Lipid Rafts Play a Major Role in Binding and Endocytosis of Influenza A Virus. Viruses 2018, 10, 650.
- Lewis, J.F.; Jobe, A.H. Surfactant and the adult respiratory-distress syndrome. Am. Rev. Respir. Dis. 1993, 147, 218–233.
- Woods, P.S.; Doolittle, L.M.; Rosas, L.E.; Joseph, L.M.; Calomeni, E.P.; Davis, I.C. Lethal H1N1 influenza A virus infection alters the murine alveolar type II cell surfactant lipidome. Am. J. Physiol.-Lung C 2016, 311, L1160–L1169.
- Coil, D.A.; Miller, A.D. Phosphatidylserine treatment relieves the block to retrovirus infection of cells expressing glycosylated virus receptors. Retrovirology 2005, 2, 49.
- Voelker, D.R.; Numata, M. Phospholipid regulation of innate immunity and respiratory viral infection. J. Biol. Chem. 2019, 294, 4282–4289.
- Numata, M.; Mitchell, J.R.; Tipper, J.L.; Brand, J.D.; Trombley, J.E.; Nagashima, Y.; Kandasamy, P.; Chu, H.W.; Harrod, K.S.; Voelker, D.R. Pulmonary surfactant lipids inhibit infections with the pandemic H1N1 influenza virus in several animal models. J. Biol. Chem. 2020, 295, 1704–1715.
- Numata, M.; Kandasamy, P.; Nagashima, Y.; Posey, J.; Hartshorn, K.; Woodland, D.; Voelker, D.R. Phosphatidylglycerol Suppresses Influenza A Virus Infection. Am. J. Respir. Cell Mol. Boil. 2012, 46, 479–487.
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191.
- Maceyka, M.; Payne, S.G.; Milstien, S.; Spiegel, S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta 2002, 1585, 193–201.
- Zhao, J.; Zhu, M.; Jiang, H.; Shen, S.; Su, X.; Shi, Y. Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: A potential therapy against pathogenic influenza virus. Sci. Rep. 2019, 9, 5272.
- Oldstone, M.B.; Rosen, H. Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine-1-phosphate agonist molecule. Curr. Top. Microbiol. Immunol. 2014, 378, 129–147.
- Jiang, H.; Shen, S.M.; Yin, J.; Zhang, P.P.; Shi, Y. Sphingosine 1-phosphate receptor 1 (S1PR1) agonist CYM5442 inhibits expression of intracellular adhesion molecule 1 (ICAM1) in endothelial cells infected with influenza A viruses. PLoS ONE 2017, 12, e175188.
- Gandy, K.A.O.; Obeid, L.M. Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handb. Exp. Pharmacol. 2013, 216, 275–303.
- Pitson, S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2011, 36, 97–107.
- Xia, C.; Seo, Y.; Studstill, C.J.; Vijayan, M.; Wolf, J.J.; Hahm, B. Transient inhibition of sphingosine kinases confers protection to influenza A virus infected mice. Antivir. Res. 2018, 158, 171–177.
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta 2006, 1758, 2016–2026.
- Seo, Y.; Pritzl, C.J.; Vijayan, M.; Bomb, K.; McClain, M.E.; Alexander, S.; Hahm, B. Sphingosine Kinase 1 Serves as a Pro-Viral Factor by Regulating Viral RNA Synthesis and Nuclear Export of Viral Ribonucleoprotein Complex upon Influenza Virus Infection. PLoS ONE 2013, 8, e75005.
- Carr, J.M.; Mahalingam, S.; Bonder, C.S.; Pitson, S.M. Sphingosine kinase 1 in viral infections. Rev. Med. Virol. 2013, 23, 73–84.
- Kumar, N.; Xin, Z.; Liang, Y.; Ly, H.; Liang, Y. NF-kappa B Signaling Differentially Regulates Influenza Virus RNA Synthesis. J. Virol. 2008, 82, 9880–9889.
- Seo, Y.; Blake, C.; Alexander, S.; Hahm, B. Sphingosine 1-Phosphate-Metabolizing Enzymes Control Influenza Virus Propagation and Viral Cytopathogenicity. J. Virol. 2010, 84, 8124–8131.
- Gambaryan, A.S.; Tuzikov, A.B.; Pazynina, G.V.; Webster, R.G.; Matrosovich, M.N.; Bovin, N.V. H5N1 chicken influenza viruses display a high binding affinity for Neu5Ac alpha 2-3Gal beta 1-4(6-HSO3) GlcNAc-containing receptors. Virology 2004, 326, 310–316.
- Hidari, K.I.P.J.; Shimada, S.; Suzuki, Y.; Suzuki, T. Binding kinetics of influenza viruses to sialic acid-containing carbohydrates. Glycoconj. J. 2007, 24, 583–590.
- Ablan, S.; Rawat, S.S.; Blumenthal, R.; Puri, A. Entry of influenza virus into a glycosphingolipid-deficient mouse skin fibroblast cell line. Arch. Virol. 2001, 146, 2227–2238.
- Chu, V.C.; Whittaker, G.R. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc. Natl. Acad. Sci. USA 2004, 101, 18153–18158.
- Kasson, P.M.; Pande, V.S. Structural basis for influence of viral glycans on ligand binding by influenza hemagglutinin. Biophys. J. 2008, 95, L48–L50.
- Ishibashi, Y.; Kohyama-Koganeya, A.; Hirabayashi, Y. New insights on glucosylated lipids: Metabolism and functions. Biochim. Biophys. Acta 2013, 1831, 1475–1485.
- Drews, K.; Calgi, M.P.; Harrison, W.C.; Drews, C.M.; Costa-Pinheiro, P.; Shaw, J.J.P.; Jobe, K.A.; Nelson, E.A.; Han, J.D.; Fox, T.; et al. Glucosylceramidase Maintains Influenza Virus Infection by Regulating Endocytosis. J. Virol. 2019, 93, e00017-19.
- Drews, K.; Calgi, M.P.; Harrison, W.C.; Drews, C.M.; Costa-Pinheiro, P.; Shaw, J.J.P.; Jobe, K.A.; Han, J.D.; Fox, T.E.; White, J.M.; et al. Glucosylceramide synthase maintains influenza virus entry and infection. PLoS ONE 2020, 15, e0228735.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
553
Revisions:
2 times
(View History)
Update Date:
22 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




