Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Iram Siddiqui | -- | 8675 | 2023-09-21 12:43:17 | | | |
| 2 | Jason Zhu | Meta information modification | 8675 | 2023-10-19 04:38:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Siddiqui, I.; Kumar, S.; Tsai, Y.; Gautam, P.; Shahnawaz, ..; Kesavan, K.; Lin, J.; Khai, L.; Chou, K.; Choudhury, A.; et al. Chemical Structures and Characteristics of Blue Emitters. Encyclopedia. Available online: https://encyclopedia.pub/entry/49469 (accessed on 07 February 2026).
Siddiqui I, Kumar S, Tsai Y, Gautam P, Shahnawaz ., Kesavan K, et al. Chemical Structures and Characteristics of Blue Emitters. Encyclopedia. Available at: https://encyclopedia.pub/entry/49469. Accessed February 07, 2026.
Siddiqui, Iram, Sudhir Kumar, Yi-Fang Tsai, Prakalp Gautam, . Shahnawaz, Kiran Kesavan, Jin-Ting Lin, Luke Khai, Kuo-Hsien Chou, Abhijeet Choudhury, et al. "Chemical Structures and Characteristics of Blue Emitters" Encyclopedia, https://encyclopedia.pub/entry/49469 (accessed February 07, 2026).
Siddiqui, I., Kumar, S., Tsai, Y., Gautam, P., Shahnawaz, .., Kesavan, K., Lin, J., Khai, L., Chou, K., Choudhury, A., Grigalevicius, S., & Jou, J. (2023, September 21). Chemical Structures and Characteristics of Blue Emitters. In Encyclopedia. https://encyclopedia.pub/entry/49469
Siddiqui, Iram, et al. "Chemical Structures and Characteristics of Blue Emitters." Encyclopedia. Web. 21 September, 2023.
Copy Citation
Organic light-emitting diodes (OLEDs) have outperformed conventional display technologies in smartphones, smartwatches, tablets, and televisions while gradually growing to cover a sizable fraction of the solid-state lighting industry. Blue emission is a crucial chromatic component for realizing high-quality red, green, blue, and yellow (RGBY) and RGB white display technologies and solid-state lighting sources. For consumer products with desirable lifetimes and efficiency, deep blue emissions with much higher power efficiency and operation time are necessary prerequisites.
organic light-emitting diode (OLED)
blue and deep-blue OLEDs
high-efficiency
1. Fluorescent Type
Over the past two decades, researchers have developed various kinds of blue-fluorescent emitters, such as distyrylarylene (DSA), spiro-shaped, biaryl, silane, and polycyclic aromatic hydrocarbon (e.g., pyrene, anthracene, carbazole, fluorene, etc.) derivatives to enhance the performance of blue OLEDs. Among them, polycyclic aromatic hydrocarbon (PAH)-based small molecules, oligomers, starburst, tetrahedral, dendrimer derivatives, and long-chain polymers are extensively applicable as emitters and/or host materials in OLED devices. In this section, nine series of fluorescent-type blue-light emitting materials (Figure 1).
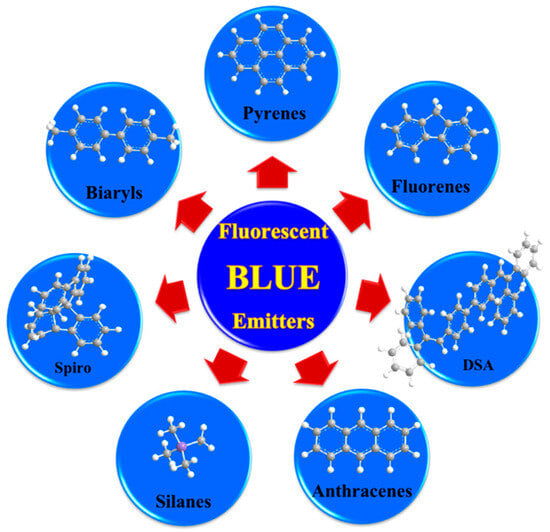
Figure 1. Numerous series of fluorescent blue emitters based on different core structure units.
In 1992, Hamada et al. reported a multi-layer blue OLED device employing an oxadiazole derivative, 1,3-bis(N,N-dimethylaminophenyl)-1,3,4-oxadiazole (OXD-8), as an emitter for the first time (Figure 2). It showed a maximum luminance of over 1000 cd/m2 and an emission peaking at 470 to 480 nm [1][2]. In the same year, Leising et al. reported an EQE of 0.05% for a single-layer blue OLED device using poly(p-phenylene) (PPP) [3][4].
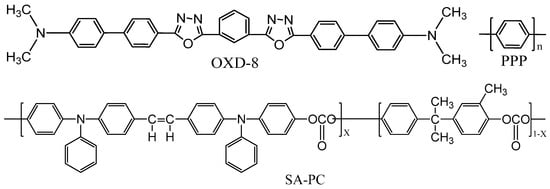
Figure 2. Chemical structures of some typical blue emitters, OXD-8, PPP, and SA-PC, for OLED devices.
Hosokawa et al. also observed a blue emission for a non-conjugated polymer-based emitter with an electron donor polycarbonate-containing styrylamine repeating unit (SA-PC) in a multilayered device. The power efficacy was 0.05 lm/W with a peak emission at 460 nm [5].
1.1. Distyrylarylene (DSA)
Over the last twenty years, researchers have extensively investigated the electroluminescent characteristics of DSA, DSA-ph, and their numerous derivatives as blue-light emitting materials in blue OLED devices [5][6][7][8][9]. In 1990, Hosokawa et al. measured light-emitting characteristics of DSA derivatives and reported that the exciplex formation or charge transfer complex with a hole-transporting layer drastically influenced device performance [6].
Researchers at Idemitsu Kosan Co. Ltd. reported that the formation of exciplex or charge transfer complexes can be avoided by introducing electron donor groups, such as ethyl, methoxy, diethylamine, phenylamine, and N-ethyl-carbazole, in DSA derivatives (Figure 3).
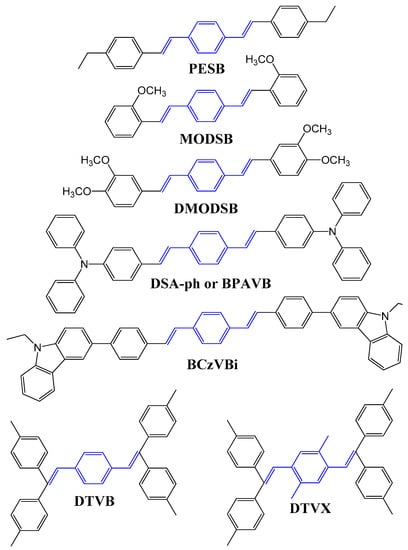
Figure 3. Molecular structures of typical DSA derivative-based blue emitters with electron donor groups.
Later in 1993, Tokailin et al. reported a series of nonplanar DSA derivatives with different DSA cores (Figure 4), namely, DTVB and DTVX, and different alkyl group substituents at the end of the molecule, namely, DPVBi, DTVBi, and DTBPVBi, as blue emitters. They also investigated the electroluminescence characteristics of these emitters in a multi-layer OLED device (ITO as anode; TDP as HTL; DPVBi or DTVBi or DTBPVBi as EML; Alq as ETL; Mg:Ag as cathode). The OLED device fabricated with a DPVBi emitter exhibited, at 1000 cd/m2, a power efficacy of 0.6 lm/W and maximum luminance of 2280 cd/m2, with blue emission peaking at 475 nm. The EL spectra did not exhibit any emission in the longer wavelength region, supporting that both DPVBi and DTVBi had entirely avoided the formation of exciplex or charge transfer complexes [10].
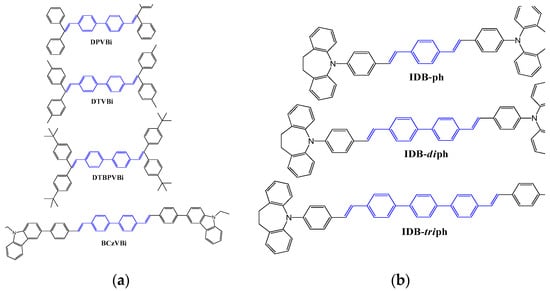
Figure 4. Molecular structures of non-planar (a) DSA diphenyl and (b) IDB phenyl, diphenyl, and triphenyl derivative-based blue emitters.
High device efficiencies and long operation lifetimes were realized in 1995 by doping a fluorescent styrylamine-based guest, BCzVB, into a DSA derivative, DPVBi, host material. Hosokawa et al., in 1995, utilized a DDPVBi as a blue host material and styrylamine (known as BCzVB) as a dopant [9]. Further improvement of this system was later published, in which the current efficacy reached 10.2 cd/A at 1.89 mA/cm2 with 1931 CIE coordinates of (0.17, 0.33) and a half-life of 20,000 h at an initial brightness (Lo) of 100 cd/m2. When oligoamine was used as a hole-injection layer for this device, the operational lifetime was further improved to 10,000 h with Lo = 500 cd/m2 [11]. It appears that the sky-blue emission was specifically designed by Idemitsu Kosan for the application of color-changing media (CCM) technology for full-color organic EL devices [12].
For a 3 wt% DSA-ph emitter doped with a 2-methyl-9,10-di(2-napthyl)anthracene (MADN) host, the device exhibited a half-decay lifetime of 46,000 h (LT50) at an initial brightness of 100 cd/m2. The resultant OLED also showed a much higher power efficacy of 5.5 lm/W and a current efficacy of 9.7 cd/A at 20 mA/cm2 with a sky-blue emission (0.16, 0.32) [11].
In 2007, Ho et al. reported a new series of emitters based on a seven-membered N-hetero-cyclic core structure of iminodibenzyl-distyrylarylene (IDB). In the distyrylarylene structure, a replacement of the amino group by an iminodibenzyl group improved the thermal properties (Tg varying between 119 and 137 °C) and slightly blue-shifted the emission with increasing phenyl units. The emission wavelength for DSA-ph is 458 nm, while it was 449, 447, and 443 nm for IDB-ph, IDB-diph, and IDB-triph, respectively [13].
In 2018, Wanshu Li et al. reported a wet-processed simple structure and high efficiency OLED using a DSA-Ph emitter doped in an MADN host.
1.2. Pyrenes
In the last fifteen years, numerous pyrene derivatives and hybrid-based blue-emitters were investigated in OLED devices due to their rigid planar structure [14][15], high thermal stability [16][17][18], high fluorescence quantum yield [19][20][21][22][23], long fluorescent lifetime [24][25], and effective carrier mobility [26][27].
However, the utilization of pyrene-based blue emitters was limited, owing to their strong tendency to form excimers and π-aggregates, which would cause red shifts and quenching of fluorescence, resulting in a lower solid-state PL quantum yield and quantum efficiency. Numerous efforts in the structural alteration of pyrene-based compounds have hence been made to enhance the PL quantum yield and diminish the aggregation characteristics.
In recent years, pyrene derivatives were extensively employed in efficient blue OLEDs, including polypyrenes [19][28][29], dipyrenylbenzene [30], alkynylpyrene [31][32], pyrene-functionalized calix[4]arenes [33], aryl-functionalized pyrenes [21][34][35][36][37], as well as oligothiophenes with pyrenyl side groups [38], pyrene-carbazole [17][20][31][39][40], pyrene-fluorene [41][42][43][44][45][46][47][48][49], pyrene-triphenylamine [27][50][51], and pyrene-anthracene [52] hybrids, due to their good emissive characteristics and hole mobility.
In 2011, Figueira-Duarte and Müllen comprehensively reviewed the synthesis techniques for numerous types of small molecular pyrene, oligo-pyrene, pyrene-dendrimer, and polypyrenes. They also reviewed the thermal, electrochemical, photophysical, and optical characteristics of all pyrene-based emitters and their electroluminescence characteristics in OLED devices [53].
In this part, the pyrene derivatives that were reported in the past few years. The Sonogashira, Sonogashira-Hogihara, Suzuki, Suzuki-Miyaura, Heck, and Yamamoto-coupling reactions have been extensively used to synthesize pyrene hybrid derivatives. The sky-blue emitter, 1-((9,9-diethyl-9H-fluorene-2yl)ethynyl)pyrene (PA-1) (Figure 5), was synthesized by the Sonogashira method via a cross-coupling reaction of 1-bromopyrene with 9,9-diethyl-2ethynyl-9H-fluorene by using a Pd(PPh3)2Cl2/PPh3/CuI catalytic system. The PA-1 exhibited a purplish-blue emission with CIE coordinates of (0.15, 0.06), a power efficacy of 1.2 lm/W, a current efficacy of 1.9 cd/A, and EQE of 3.5% at 100 cd/m2 as a 3 wt% sky-blue emitter doped into the polarity matching the CBP host [48].
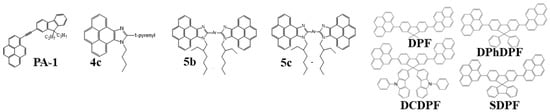
Figure 5. Molecular structures of pyrene-based blue emitters.
A series of pyrenoimidazole-based emitters that possess markedly high thermal characteristics were synthesized in three simple steps. Firstly, pyrene-4,5-dione was prepared via the oxidation of pyrene in the presence of ruthenium trichloride and sodium periodate. Subsequently, the pyrene-4,5-dione was reacted with the corresponding aldehyde in the presence of ammonium acetate to form sparingly soluble pyrenoimidazoles. Finally, the alkylation reaction was either executed under phase transfer catalysis to synthesize mono-pyrenoimidazoles or by employing the potassium carbonate/dimethylformamide (K2CO3/DMF) to synthesize bis-pyrenoimidazoles. These rigid pyrenoimidazole moiety-based hybrid compounds offered a very high thermal stability with decomposition temperatures from 462 to 512 °C.
With a CBP host doped with a 3 wt% mono-pyrenoimidazole, 9-butyl-10-(pyrene-1-yl)-9H-pyreno[4,5-d]imidazole (Figure 5), the resultant multi-layer OLED device showed, at 100 cd/m2, for example, a power efficacy of 1.2 lm/W, a current efficacy of 2.4 cd/A, and an external quantum efficiency (EQE) of 2.2% with CIE coordinates of (0.15, 0.32). The resultant device also exhibited a maximum luminance of 2740 cd/m2 [54].
It is well established that pyrene-based emitters, either molecular or polymeric, possess a planar or partial planar molecular structure, which tends to show excimer emissions and lower quantum efficiencies, resulting in an extensive bathochromic shift in emission spectra owing to the π–π stacking of pyrene chromophore units in the solid state.
In 2012, Hu and Yamato et al. synthesized a series of pyrene-based Y-shaped blue emitters using the Suzuki cross-coupling reaction of 7-tert-butyl-1,3-dibromopyrene with p-substituted phenylboronic acids. These emitters realized a low degree of π-stacking due to the steric effect of the tert-butyl group in the pyrene ring at the 7-position [19][55].
In the search for new light-emitting compounds, Promarak et al. synthesized a series of solution-processable pyrene-functionalized carbazole derivatives, N-dodecyl-3,6-di(pyren-1-yl)carbazole (CP2), N-dodecyl-1,3,6-tri(pyren-1-yl)carbazole (CP3) and N-dodecyl-1,3,6,8-tetra(pyren-1-yl)carbazole (CP4) (Figure 5), by using the Suzuki-coupling reaction [56].
These pyrene-carbazole hybrids showed a glass-transition temperature and decomposition temperature ranging from 70 to 170 °C and 375 to 407 °C, respectively. Among the four pyrene-moieties containing carbazole compounds, CP4, showed the highest thermal stability (Tg = 170 °C and Td = 407 °C).
However, these materials showed an undesirable donor-acceptor (DA) behavior because the carbazole central unit acts as a donor and the pyrene functional group acts as an acceptor. It is known in the literature that the DA molecular systems tend to extend π-conjugation and generate an intramolecular charge transfer (ICT) state, inducing an unwanted bathochromic shift in the search for a deep-blue emission.
An OLED device based on non-doped CP4 showed, by thermal evaporation deposition, for example, a maximum current efficacy of 6.7 cd/A and maximum luminance of 25,000 cd/m2 with CIE coordinates of (0.14, 0.26), and 2.5 cd/A and 9700 cd/m2 with CIE coordinates of (0.21, 0.28) via spin-coating [56].
1.3. Anthracenes
Adachi et al. reported a 9,10-diphenyl-anthracene (DPA) emitter with a 100% photoluminescence quantum yield (PLQY) [57]. The other major blue-doped emitter was developed by Shi et al. at Kodak in 2002 and utilized diphenylanthracene derivative 9,10-di(2-naphthyl)anthracene (ADN) (Figure 6) as a host and 2,5,8,11-tetra(t-butyl)-perylene (TBP) as a dopant to generate a blue emission of CIE (0.15, 0.23). The blue device showed a current efficacy of around 3.5 cd/A with a half-life of 4000 h at an initial light output of 700 cd/m2 [58].
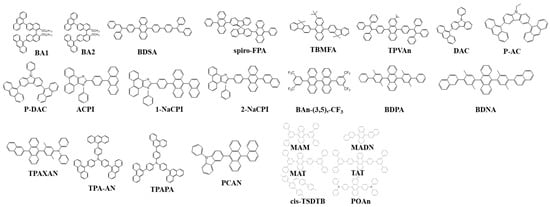
Figure 6. Molecular structures of anthracene-based blue emitters.
In 2007, Shih et al. developed a blue-emitting material 2-tert-butyl-9,10-bis[4-(1,2,2-triphenylvinyl)phenyl]anthracene (TPVAn) (Figure 6) that contained an anthracene core and two tetraphenylethylene end-capped groups. The material possessed a high Tg of 155 °C and was morphologically stable due to the presence of the sterically congested terminal groups. A bright saturated-blue emission with CIE coordinates (0.14, 0.12) along with a 5.3% EQE crossing the theoretical limit of fluorescent materials was reported, the best performance among the non-doped blue devices [59].
In 2008, three materials 9,10-Bis(3′,5′-diphenylphenyl)anthracene [MAM], 9-(3′,5′-diphenylphenyl)-10-(3‴,5‴-diphenylbiphenyl-4″-yl)anthracene [MAT], and 9,10-bis(3″,5″-diphenylbiphenyl-4′-yl)anthracene [TAT] (Figure 6) were designed and emitted in the range of 439–445 nm. MAM and MAT have a Tg higher than 64 °C, while TAT has above 150 °C. The Tg for TAT is twice as high as the commonly used host material DPVBi (64 °C) and slightly higher than MADN (120 °C). The CIE coordinates were (0.15, 0.08) with an EQE of 7.2% [60].
In 2010, Zheng et al. reported a series of non-doped devices with three newly designed emitters, 2-tert-butyl-9,10-bis(9,9-dimethylfluorenyl) anthracene (TBMFA), 2-tert-butyl-9,10-bis[4-(2-naphthyl)phenyl] anthracene (TBDNPA), and 2-tert-butyl-9,10-bis[4-(9,9-dimethylfluorenyl)phenyl] anthracene (TBMFPA) (Figure 6), using naphthalene or 9,9-dimethylfluorene side units. Due to the anthracene unit, the resultant devices showed a deep-blue emission. These materials also showed a high Tg ≥ 133 °C, due to the presence of sterically congested terminal groups. The TBDNPA-based device outperformed the others by showing a low turn-on voltage of 3.0 V with an initial brightness of 1 cd/m2. The device displayed an EQE of 5.2% at 8.4 mA/cm2 with CIEy < 0.1 [61].
In 2011, Kim et al. synthesized four molecules displaying blue emission using anthracene moieties. The material, 3-(anthracen-9-yl)-9-ethyl-9H-carbazole (AC), 3,6-di(anthracen-9-yl)-9-ethyl-9H-carbazole (DAC), 3-(anthracen-9-yl-)-9-phenyl-9H-carbazole (P-AC), and 3,6-di(anthracen-9-yl)-9-phenyl-9H-carbazole (P-DAC) (Figure 6), was also comprised of covalent carbazole units. Among these, the undoped device with P-DAC showed a near deep-blue emission at (0.16, 0.13) with a luminance efficacy of 3.1 cd/A and EQE of 2.8%. Moreover, P-DAC as a host material doped with a BDAVBi emitter displayed a 7.7 cd/A CE and 4.7% EQE with CIE coordinates of (0.15, 0.21) at 100 mA/cm2. A negligible efficiency roll-off is observed over a broad range of current density [62].
In 2012, Zhuang et al. reported a series of non-doped devices using emitters 2-(4-(anthracen-9-yl)phenyl)-1-phenyl-1H-phenanthro[9,10-d]imidazole (ACPI), 2-(4-(10-(naphthalen-1-yl)anthracen-9-yl)phenyl)-1-p-henyl-1H-phenanthro[9,10-d]imidazole (1-NaCPI), and 2-(4-(10-(naphthalen-2-yl)anthracen-9-yl)phenyl)-1-phenyl-1H-phenanthro[9,10-d]imidazole (2-NaCPI) (Figure 6). The materials possess good film-forming capability and a high Td (290–354 °C). Among them, ACPI displayed the highest CEmax, at 1.6 cd/A at a low turn-on voltage of 3.0 V corresponding to CIExy (0.16, 0.17). A negligible efficiency roll-off is observed at high current densities [63].
In 2019, a material (4-(diphenylamino)phenyl)anthracen-9-yl)pyren-1-yl)-N,N-diphenylaniline (p-TPA-AP-TPA) (Figure 6) was synthesized with a dual-core moiety of anthracene and pyrene with a triphenylamine (TPA) side group attached at a para position to control the emission in the blue region. Moreover, the bulky molecule yielded a twisted molecular structure that prevents intermolecular interactions. The PL emission was around 473 nm in the film with a high Tg of 194 °C. The device showed a low turn-on of 3.1 V with an EQE of 8.4% and a maximum luminance of 7100 cd/m2 at CIE of (0.14, 0.15) [64].
1.4. Fluorenes
Fluorenes were first reported in 2006 by Lai et al. for OLED applications. They designed oligofluorenes with a certain length to obtain a high quantum yield (ՓPL = 38–75%) for a pure-blue emission. The PL emission was observed around 400 nm. An emissive layer of 130 nm was spin-casted and displayed a peak brightness of 1300 cd/m2 at a turn-on voltage of 5.3 V with a low current density of 90 mA/cm2. The CIE coordinates of (0.15, 0.09) displayed a deep-blue emission with an EQE of 2.0% and CE of 2.1 cd/A. These hindered six-arm architectures (highly branched and dendritic structures) suppress aggregation and self-quenching while the triazatruxene core facilitates charge injection, transport, and combination. Moreover, the powerful microwave-enhanced synthesis diminished impurities and chain defects that may result in quenching or keto-defects [65].
In 2007, Lai et al. reported a kinked star-shaped fluorene/triazatruxene co-oligomer (C1, C2 and C3) (Figure 7) that can effectively suppress aggregation and crystallization. The HOMO level was well matched to that of the anode’s work function that significantly improved the carrier transportation. The single-layer EL showed almost no change in color even at a higher voltage. The device displayed a low turn-on voltage of 3.3 V, EQE of 2.2%, and maximum luminance of 2400 cd/m2 [66].
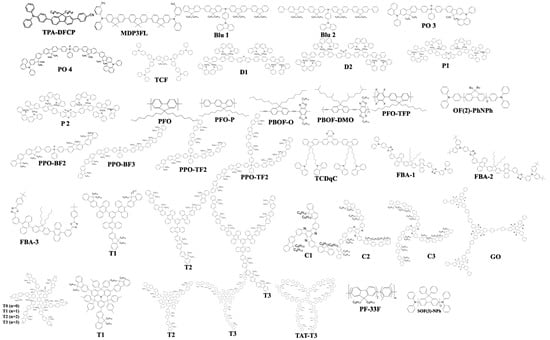
Figure 7. Molecular structures of fluorene-based blue emitters.
In 2008, Jou et al. reported 2,7-bis{2[phenyl(m-tolyl)amino]-9,9-dimethyl-fluorene-7-yl}-9,9-dimethyl—fluorene (MDP3FL) (Figure 7) blue emitter doped in a low-polarity host, 4,4′-bis(9-carbazolyl)-biphenyl (CBP). The neat film of MDP3FL exhibits an EQE of 1.9% at 100 cd/m2 displaying a CIE of (0.15, 0.12). When doped with a 10 wt% in CBP, a 5.1% EQE was observed for a deep-blue emission at CIE (0.14, 0.08) [67].
In 2009, Wang et al. reported a solution-processable fluorescent π-conjugated dendrimer G0 (Figure 7). The material displayed a CEmax of 5.3 cd/A with a deep-blue emission at (0.15, 0.09). The EQEmax was 4.2% while 3.6% at 1000 cd/m2 [68].
Figure 7 shows the fluorene derivatives extensively utilized in efficient blue OLEDs, such as fluorene-bridged anthracene, [69] oligofluorenes, [70][71][72][73][74] p-difluorophenylene [75], bis(4-diphenylaminophenyl)carbazole end-capped fluorene [76], phosphine oxide [77][78], perfluorinated polymer [79], poly(3,6-Dimethoxy-9,9-dialkylsilafluorene)s [80], and polycyclic [81].
In 2020, Liu et al. reported a blue dye TPA-DFCP with a twisted D–π–A configuration where triphenylamine (TPA), benzonitrile (CP), and 9,9-dioctylfluorene served as D, A, and π-conjugation units, respectively. TPA is a strong electron donor group and has a high HOMO level. Moreover, a certain twist angle is present in the molecular space of TPA to avoid molecular accumulation. The non-doped device showed a high EQE of 7.7% with CIE coordinates of (0.15, 0.07) displaying a deep-blue emission. Meanwhile, the doped device showed an EQEmax of 8.3% and a radiative exciton utilization energy (ηr) of 58% [82].
1.5. Biaryl
In 2005, Fisher et al. reported two materials: N,N′-diethyl-3,3′-bicarbazyl (DEC) and 1,4,5,8-N-pentamethylcarbazole (PMC) (Figure 8). Both the materials can be used as a hole-blocking layer and blue emitter. The device based on a DEC emitter doped in a DPVBi host displayed an EQE of 3.3%, CE of 4.7 cd/A, and PE of 1.3 lm/W at CIE coordinates of (0.15, 0.10) [83].
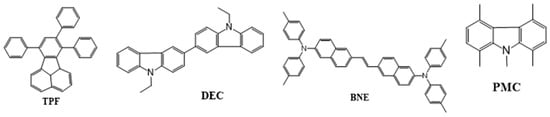
Figure 8. Molecular structures of biaryl-based blue emitters.
In 2006, Tseng demonstrated a device based on a 7,8,10-triphenylfluoranthene (TPF) emitter doped in a dipyrenylfluorene (DPF) host. With a 6 wt% of the TPF dopant, the device displayed a 3.3 lm/W PE and a 2.5% EQE at CIE coordinates of (0.16, 0.18) [84].
In 2007, Jou et al. reported three emitting materials, namely, trans-1,2-bis(6-(N,N-di-p-tolylamino)-naphthalene-2-yl)ethene (BNE), 2-(N,N-diphenylamino)-6-[4-(N,N-diphenylamino)styryl]naphthalene (DPASN), and di(triphenyl-amine)-1,4-divinylnaphthalene (DPVP) (Figure 8). Among them, BNE displayed the highest EQE, at 6.0%, and a PE of 12.5 lm/W at a low turn-on voltage of 3 V for a pan-blue emission at (0.19, 0.31) [85].
1.6. Spiro-Shaped
In 2010, Jeon et al. reported a series of spiro-based emitters, namely, N,N,N′,N′-tetraphenylspiro[fluorene-7,9′-benzofluorene] (SFBF)-5,9-diamine (BD-6DPA), N,N′-di-(2-naphthyl)-N,N′-diphenyl-SFBF-5,9-diamine (BD-6NPA), N,N′-diphenyl-N,N′-di-m-tolyl-SFBF-5,9-diamine (BD-6MDPA), and N,N′-diphenyl-N,N′-bis(4-(trimethylsilyl)phenyl)-SFBF-5,9-diamine (BD-6TMSA) (Figure 9), synthesized using an animation reaction. Among them, BD-6MDPA displayed the highest CE, at 9.1 cd/A at 6.5 V, and an EQE of 8.2% with coordinates of (0.13, 0.15). The device showed a full width at half maxima of 48 nm and an emission peaking at 463 nm [86].
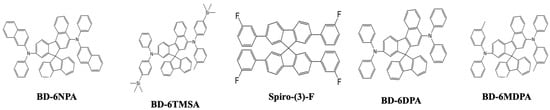
Figure 9. Molecular structures of spiro-based blue emitters.
In 2013, Hou’s group designed and synthesized a series of fluorinated 9,9′-spirobifluorene derivatives (SFs). The photophysical properties, energy levels, and thermal properties can be tuned using different substitutional groups of electron-withdrawing moieties, such as F and CF3. Among the series, a 2,2′,7,7′-tetrakis(3-fluorophenyl)spiro-9,9′-bifluorene (Spiro-(3)-F) (Figure 9) blue emitter displayed the best result in a non-doped stage with an emission at CIE (0.16, 0.12) peaking at 408 nm. These SFs also served as a host for blue-emitting dopants, especially Spiro-(3)-F, with a low turn-on voltage of 3.4 V showing a CE of 6.7 cd/A and an EQE of 5.0% [87].
1.7. Silanes
In 2001, Yu et al. reported for the first time a silane group as a blue emitter, tris(2,3-methyl-8-hydroxyquinoline) aluminum (III) (Alm23q3). The presence of two electron-donating methyl groups widened the HOMO/LUMO gap and blue-shifted the EL peaks to 470 nm. The resultant device showed a power efficacy of 0.6 lm/W at 300 cd/m2 [88].
In 2002, Chan et al. reported a molecular glass material (4-(5-(4-(diphenylamino)phenyl)-2-oxadiazolyl)phenyl)triphenylsilane (Ph3Si(PhTPAOXD)) (Figure 10) as a blue emitter. The material possessed a Tg of 85 °C, making it less vulnerable to heat and hence more stable. The device showed a maximum luminance of 19,000 cd/m2 with an EQE of 2.4% at CIE coordinates of (0.16, 0.18) [89].
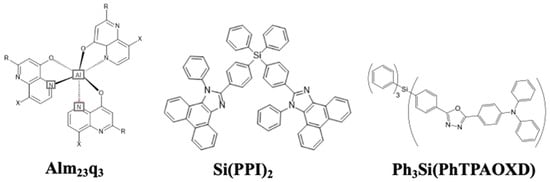
Figure 10. Molecular structures of silane-based blue emitters.
In 2015, Liu et al. reported an organosilane compound, bis(4-(1-phenylphenanthro[9,10-d]imidazol-2-yl)phenyl)diphenylsilane (Si(PPI)2) (Figure 10), as a bipolar host. The incorporation of one tetraphenylsilane and two PPI groups made the compound thermally stable with a high Td of 528 °C and a Tg of 178 °C. Moreover, when doped with a 10 wt% An(PPI)2 emitter, the device showed a PE of 8.0 lm/W and an EQE of 6.1% at CIExy (0.18, 0.17) [90].
1.8. Carbazole
In 2018, Jou et al. reported a carbazole-based deep-blue fluorescent emitter, 6-((9,9-dibutyl-7-((7-cyano-9-(2-ethylhexyl)-9H-carbazol-2-yl)ethynyl)-9H-fluoren-2-yl)ethynyl)-9-(2-ethylhexyl)-9H-carbazole-2-carbonitrile (JV55) (Figure 11), that showed a maximum EQE of 6.5% at CIE coordinates of (0.16, 0.06). The deep-blue emission realized a 101% color saturation according to the NTSC standard. The very high PLQY of 91%, effective host-guest energy transfer, efficient triplet energy utilization, and low doping concentration realized a low roll-off [91].
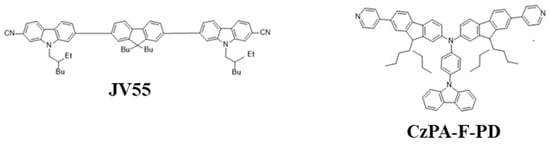
Figure 11. Molecular structures of carbazole-based blue emitters.
In 2020, Yang et al. reported a blue fluorescent material with a twisted A–π–D–π–A configuration, namely, CzPA-F-PD (Figure 11). This material exhibited dual fluorescent properties that emitted blue and red emissions under PL and EL processes when triggered by trifluoroacetic acid (TFA). The property had been reversed to its initial state on neutralizing TFA with triethylamine (TEA). It was observed that hydrogen protonation of TFA amplified the lone pair electrons around the nitrogen atoms in pyridine, enhancing the electron acceptor ability. This scheme also played a significant role during the dual fluorescent process. The material possessed a good exciton utilization efficiency of 67%, exceeding the upper limit of spin-statics for fluorescent materials [92].
1.9. Oxidiazole
In 2018, Wu et al. reported that triazine, oxadiazole, triazole, cyno-substituted benzene, and benzothiadiazole worked as acceptor units while carbazole, arylamine, phenothiazine, and their derivatives were utilized as donor moieties for a bipolar structure. Consequently, they utilized phenothiazine and 9,9-diphenyl-9,10-dihydroacridine as a donor unit and oxadiazole derivatives, 2,5-bis(4-bromophenyl)-1,3,4-oxadiazole and 2-(4-bromophenyl)-5-phenyl-1,3,4-oxadiazole, as acceptor units to synthesize a series of bipolar emitters, namely, 2DPAc-OXD, DPAc-OXD, 2PTZ-OXD, and PTZ-OXD (Figure 12). All the materials possessed a high decomposition temperature above 358 °C, with emissions in the PL range of 435–512 nm. For OLED applications, these materials showed a moderate turn-on voltage of around 4.2 V along with a highest EQE of 4.0% when using the material 2PTZ-OXD [93].
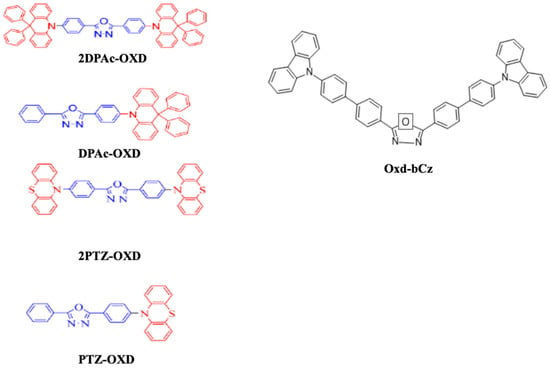
Figure 12. Molecular structures of oxadiazole-based blue emitters.
Later that year, Wang et al. reported a bipolar molecule, bis(4’-(9H-carbazol-9-yl)-[1,1-biphenyl]-4-yl)-1, 3, 4-oxadiazole (Oxd-bCz) (Figure 12), by incorporating electron-donor and electron-withdrawal moieties. The material emitted a purple-blue fluorescence at 431 nm when diluted in dichloromethane. From a device perspective, the device turned on at 3.6 V and displayed a maximum EQE of 5.6% at CIEy ~ 0.07 [94].
2. Phosphorescent Type
Developing phosphorescent emitters is essential as they have the potential to realize a high-efficiency OLED. In 1998, Forrest and Thompson et al. discovered a phosphorescent emitter to develop a nearly 100% internal quantum efficiency because of their ability to efficiently harvest both singlet and triplet excitons [95][96][97][98][99]. Since then, very high-efficiency phosphorescent green and red emitters have emerged. However, it was a great challenge to synthesize a highly energy-efficient phosphorescent blue emitter that exhibits a long lifetime with high color purity. Deep blue emitters should have a high triplet energy (~3 eV), a high photoluminance quantum yield (PLQY), and a short excited-state lifetime. To realize the above-mentioned features, considerable efforts have been made to develop a high performance deep-blue emitter.
On the basis of previous investigations, it is well established that complexes of heavy metals such as iridium (III), platinum (II), and ruthenium (II) have been utilized to synthesize promising blue emitters [100][101][102]. Among them, iridium complex-based phosphors have shown enormous potential to realize high-efficiency blue OLEDs. Moreover, to achieve a deep-blue emission, thermally and electrochemically stable fluorine-free cyclometalating ligands [101][103][104] were introduced either by using electron-withdrawing groups [105][106] or electron-deficient heterocycles [106][107]. Furthermore, to stabilize HOMO, ancillary ligands with strong ligand field strength, such as isocyanide or phosphine, were utilized [106][108].
2.1. Iridium (III) Complexes
In 2001, Adachi et al. reported a sky-blue phosphorescent emitter, FIrpic (Figure 13), with an EQE of 5.7% and CIE coordinates of (0.16, 0.29) [109]. In 2003, Holmes et al. synthesized a blue emitter, iridium(III) bis(2,4-difluorophenylpyridinato)tetrakis(1-pyrazolyl)borate (FIr6) (Figure 13), with an EQE of 12% at (0.16, 0.26) [110]. In the same year, Bazan’s group reported a series of anthracene-containing binaphthol emitters with CIE coordinates of (0.18, 0.21) without revealing device performance [100].
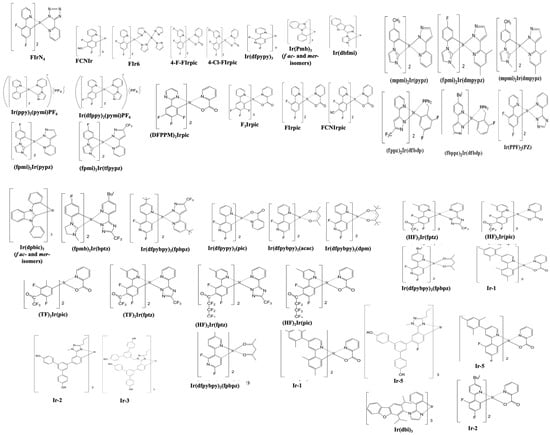
Figure 13. Molecular structures of Ir-complex-based blue emitters.
In 2005, Holmes reported fac- and mer-isomers of fluorine-free emitters tris(phenyl-methyl-benzimidazole)iridium(III) (f-Ir(pmb)3) and (m-Ir(pmb)3), respectively (Figure 13). When f-Ir(pmb)3 was doped into a p-bis(triphenylsilyly)benzene (UGH2) host, the device showed an EQE of 5.8% for a deep-blue emission with CIE coordinates of (0.17, 0.06) [101].
Moreover, other fluorine-free Ir complexes which resulted in a blue emission were also reported. They were mer-tris(Ndibenzofuranyl-N-methylimidazole) iridium(III) [Ir-(dbfmi)] (Figure 13) [103] and tris(1-cyanophenyl-3-methylimidazolin-2-ylidene-C,C2′) iridium(III) [Ir(cnpmic)] [104].
In 2009, Kang et al. introduced a blue emitter based on an iridium(III) complex of 2′,6′-difluoro-2,3′-bipyridine (fac-Ir(dfpypy)3) (Figure 13) with CIE coordinates of (0.14, 0.12) [111]. Meanwhile, the same group reported a comparatively more efficient device with an EQE of 10% at 1000 cd/m2 at (0.15, 0.24) by employing its modified complex, (dfpypy)2Ir(μ-Cl)2) [112].
In 2010, Kido et al. reported a halogen-free blue emitter, mer-tris(N-dibenzofuranyl-N′-methylimidazole)iridium(III) (Ir(dbfmi)) (Figure 13). By doping a 10 wt% Ir(dbfmi) into a 3,6-bis(diphenylphosphoryl)-9-phenylcarbazole (PO9) host, the resultant device showed a maximum EQE of 19% with CIExy (0.15, 0.19) [103]. In 2011, Hsieh et al. reported blue emitters of iridium bis(carbene) complexes, namely, Ir(mpmi)2(pypz), Ir(fpmi)2-(pypz), and Ir(fpmi)2(tfpypz) (Figure 13), that showed an EQE of 15, 14, and 7.6% and CIExy of (0.14, 0.27), (0.14, 0.18), and (0.14, 0.10), respectively [113].
In 2012, Fan et al. reported two phosphoryl/sulfonyl-substituted iridium complexes, POFIrpic and SOFIrpic (Figure 13), by introducing phosphoyl/sulfonyl moieties into the 5′-position of a phenyl ring in the structural frame of FIrpic. By doping a POFIrpic emitter into PVK:OXD-7 hosts, the solution-processable blue device showed CIE coordinates of (0.16, 0.29) and an EQE of 3.5% at 1000 cd/m2 [114]. In 2013, Fan et al. modified the FIrpic by introducing fluorine, chlorine, and bromine atoms to the 4-position of a pyridine ring. The 4-F-FIrpic-based device showed an EQE of 15% for a blue emission with CIExy (0.15, 0.28) [115]. Meanwhile, Kessler et al. synthesized a heteroleptic iridium complex based on a 4-(tert-butyl)-2′,6′-difluoro-2,3′-bipyridine ligand (FK306) (Figure 13) that showed an EQE of 13% at 1000 cd/m2 for a greenish-blue emission at (0.16, 0.25) [116]. In the same year, Yoon et al. reported a series of Ir(III) complexes using (4′-substituted-2′-pyridyl)-1,2,4-triazole ancillary ligands. By doping a 10 wt% of iridium complex of 5-(4′-methylpyridine-2′-yl)-3-trifluoromethyl-1,2,4-triazolate ancillary ligand (Ir5) (Figure 13) into a 4,4′-bis(9-carbazolyl)-2,2′-dimethylbiphenyl (CDBP) host, the resultant device showed a blue emission with CIE coordinates of (0.15, 0.18) [117].
Moreover, Lee et al. reported a series of near deep-blue iridium(III) complexes, (TF)2Ir(pic), (TF)2Ir(fptz), (HF)2Ir(pic), and (HF)2Ir(fptz) (Figure 13). They consisted of 2′,4″-difluororphenyl-3-methylpyridine with a trifluoromethyl carbonyl or heptafluoropropyl carbonyl group as the main ligand and a picolinate or a trifluoromethylated triazole as the ancillary ligand. Among them, (TF)2Ir(fptz) achieved an EQE of 7.4% at 100 cd/m2 for a blue emission with CIE coordinates of (0.14, 0.11) [118].
In 2014, Liu et al. reported a host molecule, 3,6-bis(diphenylphosphoryl)-9-(4′-(diphenylphosphoryl) phenyl)-carbazole (TPCz) (Figure 13). The solvent effect was studied by dissolving a mixture of TPCz and FIrpic (emitter) in various polar solvents. They thought that the higher polar halogen-free solvent isopropanol provided good solubility, helping resist the aggregation of FIrpic and hence render a more densely packed emissive layer. The efficiencies of 22 cd/A and 16 lm/W at (0.15, 0.32) were also reported [119].
In 2015, Sun et al. reported a series of 3,3′-bicarbazole (mCP)-functionalized tetraphenylsilane derivatives (SimCPx) (Figure 13). On changing the number of mCP subunits on the central Si atom, four molecules, namely, bis(3,5-di(9H-carbazol-9-yl)phenyl)diphenylsilane (SimCP2), tris(3,5-di(9H-carbazol-9-yl)phenyl)methylsilane (SimCP3-CH3), tris(3,5-di(9H-carbazol-9-yl)phenyl)phenylsilane (SimCP3-Ph), and tetrakis(3,5-di(9H-carbazol-9-yl)phenyl)silane (SimCP4), were synthesized as bipolar hosts. The derivatives possessed a wide bandgap and good solubility. Moreover, a high thermal and morphological stability was beneficial for the formation of amorphous and homogeneous film via wet processing. Among them, SimCP4 showed the best electron-transporting ability and hence, at a low driving voltage of 4.8 eV, achieved an EQEmax of 14%, CEmax of 28 cd/A, and PEmax of 14 lm/W corresponding to a CIExy (0.15, 0.35) by using a FIrpic emitter [120].
In 2016, Byeong et al. reported meta-linked diphenylether-based materials, 9-(3-(3-(9H-carbazol-9-yl)phenoxy)phenyl)-9H-pyrido[2,3-b]indole (CzDPEPI) and 9,90-(oxybis(3,1-phenylene))bis(9H-carbazole) (CzDPECz) (Figure 33). The presence of a pyridoindole moiety assisted in getting a better electron injection and hence a lower driving voltage. The CzDPEPI host possessed a high triplet energy and therefore was suitable for an Ir complex (Ir(dbi)3) emitter. The resultant device showed an EQEmax of 24% and PEmax of 39 lm/W at CIExy (0.19, 0.37) [121].
In 2017, Park et al. reported a series of bipolar host materials, namely, N-(3-(9H-carbazol-9-yl)phenyl)-N-(pyridin-2-yl)pyridin-2-amine (mCPPy), 5-(9H-carbazol-9-yl)-N1,N1,N3,N3-tetra(pyridin2-yl)benzene-1,3-diamine (CPDPy), and N-(3,5-di(9H-carbazol-9-yl)phenyl)-N-(pyridin2-yl)pyridin-2-amine (DCPPy) (Figure 13). The materials possessed a high bandgap (>3.5 eV) and a high triplet energy (>2.9 eV). The device composing CPDPy doped with FIrpic (emitter) showed a highest EQEmax of 22% and PEmax of 24 lm/W at CIE coordinates of (0.16, 0.31) [122].
In 2018, Kim et al. reported an emitter material, fac-tris(2,3-dimethylimidazo[1,2-f]phenanthridinato-k2C,N)iridium(III) (Figure 13), that had a facial (fac) geometry around the iridium atom. A multilayered device composed of 1,3-bis(N-carbazolyl)benzene as a host/hole-transporting layer with a 5 wt% emitter displayed a high EQEmax of 20% and PEmax of 32 lm/W with coordinates of (0.18, 0.34) [123].
In 2020, Yuan et al. reported a host material, 9,9-bis(9-phenyl-9H-carbazol-3-yl)-9H-thioxanthene,10,10-dioxide (BPhCz-ThX) (Figure 13), based on an insulating C(sp3) bridge-linked thioxanthone via a facile Friedel–Crafts-type substitution reaction. The material possessed a high thermal stability, good film-forming ability, a high triplet energy (3.05 eV), and a total yield of 63%. On using a FIrpic emitter, the device showed an EQEmax of 9.6%, CEmax of 21 cd/A, and PEmax of 8.5 lm/W at (0.19, 0.36) [124].
2.2. Platinum Complexes
Platinum (Pt) complexes are the second most appreciated organic dopants to realize a highly efficient deep-blue emitter. Pt-based phosphors appear to have the highest potential for EL applications due to their higher triplet quantum yield, relatively short triplet state lifetime, and tunable emission color [102][104][125]. Based on their structural features, these phosphors are classified into three main categories: (i) tetradentate ligands, (ii) tridentate ligands, and (iii) bidentate ligands.
In 2008, Yang et al. reported a blue device by utilizing a platinum(II) 1,3-difluoro-4,6-di(2-pyridinyl)benzene chloride (Pt-4) (Figure 14) emitter, and the resultant device exhibited CIE coordinates of (0.15, 0.26) with a maximum EQE of 16% [102].
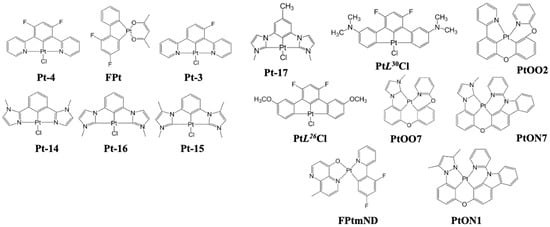
Figure 14. Molecular structure of platinum complex-based blue emitters.
In 2013, Turner et al. synthesized emitters based on cyclometalated tetradentate platinum complexes, a blue PtOO1, and a blue-green PtOO2 emitter (Figure 14). The device using PtOO2 exhibited an EQE of 8.7% with CIE coordinates of (0.21, 0.38) at 1000 cd/m2 [125]. In the same year, Hang et al. reported Pt complexes with tetradentate ligands. These complexes have a conventional cyclometalated fragment bridged with oxygen to an ancillary chelate, such as phenoxyl pyridine (POPy) or carbazolyl pyridine (CbPy). Doping 6 wt% emitters, Pt[pmi-O-POPy] (PtOO7), Pt[pmi-O-CbPy] (PtON7) and Pt[ppz-O-CbPy] (PtON1), into a 2,6-bis(N-carbazolyl)pyridine (26mCPy) host resulted in CIE coordinates of (0.15, 0.10), (0.15, 0.14), and (0.15, 0.13) and an EQE of 0.5, 15, and 21%, respectively, at 1000 cd/m2 [104].
In 2015, Liao et al. reported a series of Pt(II) metal complexes [Pt(Ln)], n = 1–5 based on tetradentate bis(pyridyl azolate) chelates. Among them, L5 (Figure 14) showed a high EQEmax of 15%, CEmax of 36 cd/A, and PEmax of 38 lm/W at CIE coordinates of (0.19, 0.34) [126].
In 2019, Klimes et al. reported a step-wise graded doping of platinum(II) 9-(pyridin-2-yl)-2-(9-(pyridin-2-yl)-9H-carbazol-2-yloxy)-9H-carbazole (PtNON) (Figure 14). The emissive layer was composed of a 20% PtNON:mCBP (10 nm)/10% PtNON:mCBP (4 nm)/2% TBPe:mCBP (2 nm)/10% PtNON:mCBP (4 nm)/6% PtNON:mCBP (10 nm). Consequently, the device showed an EQEmax of 18% and a lifetime of 709 h [LT70] at 1000 cd/m2 with CIExy (0.16, 0.28) [127].
2.3. Other Metal Complexes
Due to limited availability and high cost, some research efforts have been made to substitute heavy metal complexes with comparatively low-cost metals such as zinc (Zn), copper (Cu), etc. In 2004, Wang et al. synthesized a Zn(II) metal complex-based phosphorescent blue emitter using 4,4′-diphenyl-6,6′-dimethyl-2,2′-bipyrimidine ligands, [(Zn(pmbp)2(ClO4)2 and Zn(pmbp)Cl2]. However, no efficiencies or color coordinates were reported [128].
In 2008, Xu et al. reported a deep-blue emitting Zn(II) complex, Zn(Lc)2 (Lc− = 2-(1-(6-(9H-carbazol-9-yl)hexyl)-1H-benzo[d]imidazol-2-yl)phenolate) (Figure 15), based on a carbazole-functionalized N^O ligand. The material possessed a Td of 427 °C and an emission peak at 422 nm [129]. In 2013, Lee et al. synthesized two Zn complexes, bis(2-(oxazol-2-yl)phenolate)zinc (ZnOx2) and bis(2-(1-methyl-1h-imidazole-2-yl)phenolate)zinc (ZnIm2) host materials. The blue device based on a 3 wt% FIrpic (emitter) doped in ZnIm2 reached an EQE of 20% at 100 cd/m2 and 18% at 1000 nits [130].
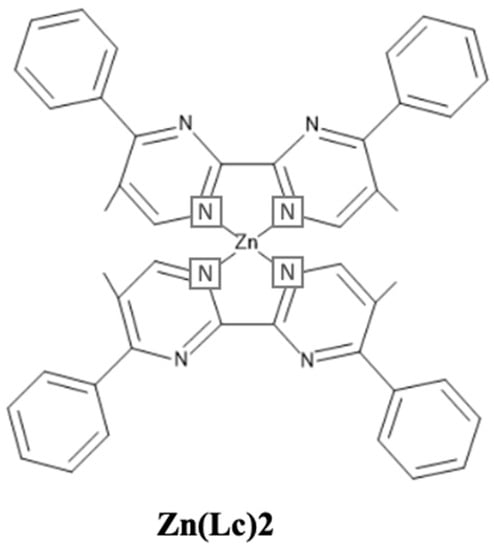
Figure 15. Molecular structure of Zn(Lc)2 blue emitter.
Suitable host materials were presented to aid the emission of phosphorescent-based blue emitters as reported by Chaskar et al [131] and Yook et al [132].
3. Thermally Activated Delayed Fluorescence (TADF) Type
High price and rare availability of heavy metals have limited the low-cost and long-term mass production of phosphorescent blue emitters. To resolve these problems, the search for a highly efficient deep-blue fluorescent emitter is still a subject of current interest, as they have a greatest possibility of a longer operational lifetime and a deep-blue emission at low cost with highly sustainable purely organic luminescent molecules [133].
In 2009, Adachi et al. designed and synthesized the first heavy metal-free TADF emitter [134]. Since then, numerous efforts have been made to realize efficient TADF-based blue emitters, especially with a smaller singlet-triplet energy difference (Δ𝐸𝑆𝑇) [134][135][136][137][138][139][140]. Presently, aromatic- and metal (such as Li, Al, Mg, Cu, and Zn)-based blue emitters have been found useful in achieving a small Δ𝐸𝑆𝑇.
3.1. Aromatic-based
Over the past few years, researchers have extensively designed and synthesized aromatic-based efficient TADF emitters by using abundant elements, such as carbon, hydrogen, oxygen, nitrogen, sulphur, etc. So far, a few molecular design strategies have been reported to achieve smaller Δ𝐸𝑆𝑇(<0.1 eV) values with a high PLQY (~100%). Most importantly, the delocalization of frontier molecular orbitals (highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels) in a donor–acceptor (D–A) type TADF emitter with a well-separated HOMO and LUMO levels can effectively realize a smaller Δ𝐸𝑆𝑇and a nearly 100% PLQY [141]. Moreover, increasing a steric hindrance between D–A units can also lead to the spatial separation of the frontier molecular orbitals.
In aromatic-based TADF emitters, the spatial overlap between the frontier molecular orbitals can be successfully controlled by choosing a suitable D–A pair. Moreover, it has been found that the donor–acceptor–donor (D–A–D) type emitters are more effective than those of the D–A type in achieving a higher PLQY and a strong spatial separation of HOMO LUMO levels [142][143]. Since 2009, several D–A- and D–A–D-type bipolar emitters have been reported. Among them, a few emitters demonstrated a nearly 100% internal quantum efficiency (IQE) and a 20% EQE in blue devices [141][144][145].
On the basis of D–A pairs, aromatic-based TADF blue emitters can be classified into seven sub-categories (Figure 16), which comprise diphenylamine, acridine, carbazole, and phenoxazines as electron-donor units and diphenylsulfone, thioxanthone, benzophenone, triazine, oxadiazole, triazole, and phthalonitrile as electron-acceptor units (Figure 17).
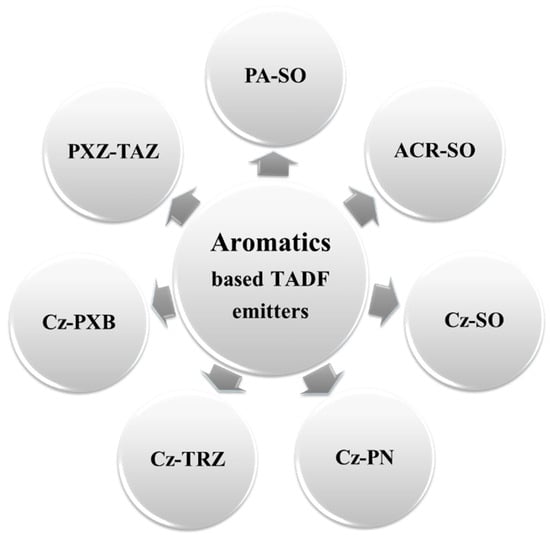
Figure 16. Types of aromatic TADF blue emitters based on different donor–acceptor pairs. All abbreviations are given as follows: PA (phenylamine), DPS (diphenylsulfone), ACR (acridine), Cz (carbazole), PN (phthalonitrile), PXB (phenoxaborin), PXZ (phenoxazines), TAZ (triazole), and TRZ (triazine).
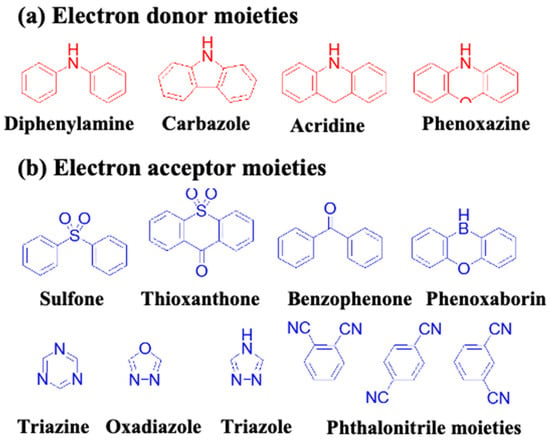
Figure 17. Molecular structures of (a) electron donor moities and (b) electron acceptor moities, frequently used in aromatic-based blue TADF emitters.
Diphenylamine and Diphenylsulfone (DPA-DPS) Derivatives
Diphenylamine moiety-containing aromatic compounds are well-established hole-transporting materials in OLED devices. In contrast, diphenylsulfone moiety-comprising materials are well studied as electron transporting materials. These moieties have also been used to design bipolar hosts [146][147][148][149]. In accordance with the molecular design strategy, in 2012, Adachi et al. synthesized a series of bipolar TADF emitters, bis[4-(diphenylamino)phenyl] sulfone (DPA-DPS) and bis{4-[bis(4-tert-butylphenyl)amine]phenyl} sulfone (DTPA-DPS) (Figure 18), using diphenylamine donor and diphenylsulfone acceptor moieties [150]. The DPA-DPS and DTPA-DPS emitters possessed a ΔEST of 0.54 and 0.45 eV and a PLQY of 57 and 65%, respectively. In a doped device with a bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) host, the DPA-DPS-based device exhibited an EQEmax of 2.9%, while the DTPA-DPS-based device showed an almost double EQEmax of 5.9%. The higher EQE of the DTPA-DPS emitter is attributed to a low ΔEST, which possessed an effective triplet to singlet-exciton upconversion.
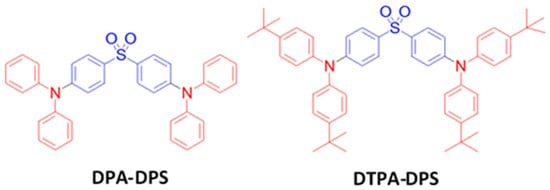
Figure 18. Molecular structures of DPA-DPS-based blue emitters.
Acridine and Diphenylsulfone (ACR-DPS) Derivatives
Acridine derivatives were utilized as hole-transporting and bipolar host materials in OLEDs [151][152][153][154][155][156][157][158]. Combinations of acridine-based electron-donor and diphenylsulfone-based electron-acceptor units have been widely studied for TADF type emitters. In 2014, Adachi’s group designed and synthesized an acridine donor and diphenylsulfone acceptor moiety-based 9,9-(dimethyl-9,10-dihydroacridin)diphenyl sulfone (DMAC-DPS) (Figure 19) emitter. The emitter possessed a ΔEST of 0.08 eV with an 80% PLQY and a 1.7 to 3 μs TADF decay time. By doping into a DPEPO host, the DMAC-DPS-based device exhibited an EQEmax of 20% for a greenish-blue emission at (0.16, 0.20). The resultant device performance was almost independent of the emitter doping concentration [144]. Moreover, the same group reported an EQEmax of 20% for a sky-blue emission with (0.16, 0.29) by using a non-doped DMAC-DPS emitter [145].
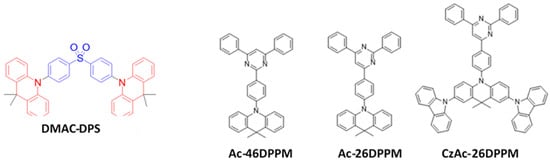
Figure 19. Molecular structures of ACR-DPS-based blue emitters.
In 2014, Nakanotani et al. achieved an EQEmax of 13% for a bluish-green emission with coordinates (0.17, 0.30) by using a 1 wt% fluorescent emitter, 2,5,8,11-tetra-tert-butylperylene, along with a 15 wt% of TADF emitter, 10-phenyl-10H, 10′H-spiro[acridine-9, 9′-anthracen]-10′-one (ACRSA), in a bis-(2-(diphenylphosphino)phenyl)ether oxide (DPEPO) host [159].
In 2017, Nakao reported three emitters, namely, Ac-46DPPM, Ac-26DPPM, and CzAc-26DPPM (Figure 39), with 2,4,6-triphenylpyrimidine as an acceptor and tri- or penta-substituted acridine as a donor unit. Among them, CzAc-26DPPM displayed an EQE of 15% and CE of 35 cd/A at 100 cd/m2 for a bluish-green emission with coordinates (0.21, 0.38) [160].
Carbazole and Diphenylsulfone (Cz-DPS) Derivatives
Carbazole-based polycylic aromatic hydrocarbon compounds are extensively studied in OLED devices due to their numerous superlative characteristics, such as a strong hole-transporting ability, high thermal stability, large optical band gap, high triplet energy, rational PLQY, and easy functionalization [161][162]. On the other hand, diphenyl sulfone derivatives are robust electron-transporting materials for OLED devices [150].
In 2012, Zhang et al. reported an EQEmax of 10% for a deep-blue emission with CIE coordinates of (0.15, 0.07) by doping a TADF blue emitter, bis[4-(3,6-di-tert-butylcarbazole)phenyl] sulfone (DTC-DPS) (Figure 20), into a DPEPO host. The resultant device realized an EQE over three times higher than that of a diphenylamine-based DPA-DPS and nearly two times higher than that of a tert-butyl diphenylamine-derivative DTPA-DPS. The DTC-DPS exhibiting a high EQE may be attributed to a relatively small ΔEST value of 0.32 eV and a high PLQY of 69% because of a considerably strong electron donating characteristic of carbazole units [150].
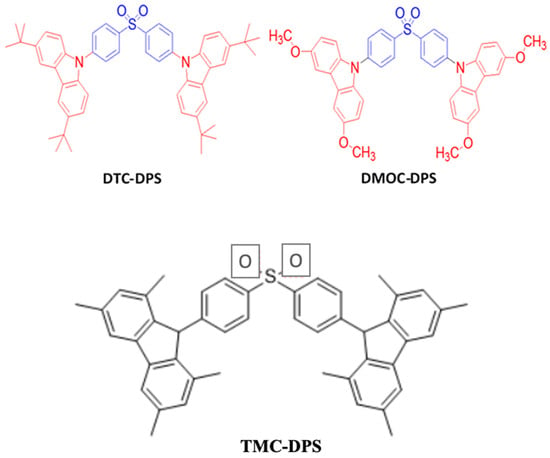
Figure 20. Molecular structures of Cz-DPS-based blue emitters.
In order to further decrease the ΔEST value, in 2014, Wu et al. designed a blue emitter, bis [4-(3,6-dimethoxycarbazole)phenyl]sulfone (DMOC-DPS) (Figure 20), by using a methoxy substituted carbazole with an electron-acceptor sulfone. The emitter realized a ΔEST of 0.21 eV with a 93 μs TADF decay time and a 56% PLQY. In a doped blue device with a DPEPO host, the DMOC-DPS exhibited an EQEmax of 15% with (0.16, 0.16) coordinates [163]. The reason why the DMOC-DPS resulted in an EQE much higher than that of the DTC-DPS may be attributed to a comparatively smaller ΔEST.
Furthermore, in 2016, Lee et al. reported a TADF emitter, TMC-DPS (Figure 20), with diphenyl sulfone (DPS) as an electron acceptor and 3,6-dimethoxycarbazole (DMOC) and 1,3,6,8-Tetramethyl-9H-carbazole (TMC) as electron donor units. The material possessed a low ΔEST of 0.09 eV, much lower than that of DMOC-DPS, due to a large dihedral angle that creates a small spatial overlap between the frontier molecular orbitals [164].
Carbazole and Triazine (Cz-TRZ) Derivatives
Nitrogen-containing heterocyclic compounds, such as triazine and 1,3,4-triazole, have been widely used as electron-accepting units in the molecular scaffold of electron-transporting materials because of their electron-deficient nature. To design a bipolar TADF emitter, strong hole-transporting carbazole moieties have been added onto transporting triazine moieties. Both moieties are extensively utilized to design an efficient bipolar host and blue light-emitting materials.
In 2013, Serevičius et al. reported a 6% EQEmax for a greenish-blue OLED with (0.23, 0.40) by using a 9-(4,6-diphenyl-1,3,5-triazin-2-yl)-9′-phenyl-3,3′-bicarbazole (CzT) (Figure 21) emitter [165]. In 2014, Hirata and co-workers reported a series of bipolar blue light-emitting materials (2a, 2b, and 2c) (Figure 21) by employing indolocarbazole and triazine moieties. The resultant device employing a 2a emitter doped in a DPEPO host realized a highest EQE of 21% for a sky-blue device with (0.19, 0.35) [141].
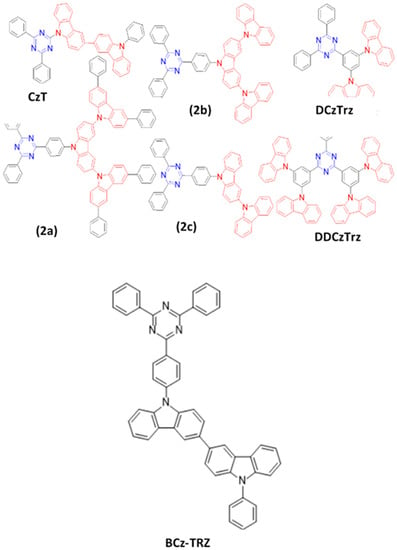
Figure 21. Molecular structures of Cz-TRZ-based blue emitters.
In 2015, Lee et al. reported a pair of bipolar TADF type blue emitters (DCzTRz and DDCzTRz) (Figure 21) with dicarbazolylbenzene donor and triazine acceptor moieties [166]. Both DCzTRz and DDCzTRz exhibited a ΔEST of 0.25 and 0.27 eV and a solid state PLQY of 43 and 66%, respectively. As both emitters were doped into a DPEPO host, the DCzTRz emitter-based device exhibited a maximum EQE of 18% with (0.15, 0.15), while a 19% EQE with (0.16, 0.22) was reported for the DDCzTRz-based device. Furthermore, owing to high thermal and electrical stability, the DDCzTRz-based device also demonstrated an operational lifetime (T80 = 52 h at an initial luminance of 500 cd/m2).
In 2020, Su et al. reported a 3,3′-bicarbazole/triphenyltriazine-derivative (BCz-TRZ) (Figure 21) emitter with a ΔEST of 0.37 eV by controlling the length of donor and acceptor moieties and increasing the D–A distance. The BCz-TRZ-based device exhibited a lifetime (LT50) of 495 h and an EQEmax of 20% at an emission wavelength of 486 nm [167].
Carbazole and Phthalonitrile (Cz-PN) Derivatives
Phthalonitrile-derivative compounds have attracted considerable attention because of their strong electron-withdrawing nature. Phthalonitrile-functionalized aromatic compounds are widely used as electron-transporting, charge-generation, and light-emitting materials in OLEDs. To achieve bipolarity in the molecular design of phthalonitrile-based aromatic compounds, such as bipolar hosts and TADF emitters, strong electron donor carbazole units are appended onto the strong electron donor phthalonitrile unit [168][169].
In 2012, Uoyama et al. designed a series of carbazole and phthalonitrile materials. Among them, 4,5-di(9H-carbazol-9-yl) phthalonitrile (CzTPN) (Figure 22) realized a sky-blue emission. The CzTPN exhibited a ΔEST of 0.29 eV with a 47% PLQY. In a doped device with a CBP host, the CzTPN emitter exhibited a maximum EQE of 8% for a sky-blue emission with an emission wavelength at 473 nm [168]. Furthermore, in 2013, Masui et al. achieved a maximum EQE of 13% with (0.17, 0.30) by doping the CzTPN emitter into an mCP host [170].
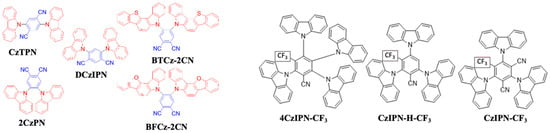
Figure 22. Molecular structures of Cz-PN-based blue emitters.
In 2015, Lee et al. reported a maximum EQE of 16% for a blue device with (0.17, 0.19) by doping a 15 wt% 4,6-di(9H-carbazol-9-yl)isophthalonitrile (DCzIPN) (Figure 22) in a mCP host [171]. In the same year, Lee et al. designed and synthesized two blue emitters, 4,5-bis(benzofuro[3,2-c]carbazol-5-yl)phthalonitrile (BTCz-2CN) and 4,5-bis(benzo[4,5]thieno [3,2-c]carbazol-5-yl)phthalonitrile (BFCz-2CN) (Figure 22), by employing benzofuro- and benzothieno-functionalized carbazole units with a phthalonitrile unit. BTCz-2CN and BFCz-2CN exhibited a ΔEST value of 0.13 and 0.17 eV with a 95 and 94% PLQY, respectively. The BTCz-2CN showed a decay lifetime of 2.6 μs, which is larger than that of the BFCz-2CN counterpart (1.98 μs). The short decay time of the BTCz-2CN and BFCz-2CN may be attributed to their small ΔEST. Moreover, BTCz-2CN and BFCz-2CN also showed a high Tg of 204 and 219 °C and Td of 397 and 434 °C, respectively. In doped devices with an mCP host, the BTCz-2CN- and BFCz-2CN-based devices resulted in a maximum EQE of 12.1 and 11.8% with a similar EL emission peaking at 486 nm [172].
In 2020, Yi et al. reported three molecules, namely, 4CzIPN-CF3, 3CzIPN-H-CF3, and 3CzIPN-CF3 (Figure 22). They were synthesized by lowering the electron-withdrawing ability of the acceptor group or by reducing the number of carbazole donors of the base molecule, 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN). Among them, the 4CzIPN-CF3- and 3CzIPN-H-CF3-based devices with a SimCP:oCF3-T2T co-host system displayed a blue-shifted emission with coordinates (0.21, 0.41) and (0.16, 0.19), respectively, as compared with a 4CzIPN emission (0.22, 0.48). The devices showed an EQEmax of 23 and 17%, and 21 and 15% at 1000 nits, respectively [173].
Acridine and Phenoxaborin (Cz-PXB) Derivatives
In 2015, Numata et al. synthesized a series of D–A-type emitters (1-4) (Figure 23) with acridine derivatives as donor units and a 10H-phenoxaborin-type acceptor [174]. The resultant maximum EQE ranged from 20 to 13%, while chromaticity coordinates for a sky-blue emission ranged from (0.14, 0.23) to deep-blue (0.15, 0.08) by doping a 20 wt% of 1–4 emitters into an 8-bis(diphenylphosphine oxide) dibenzofuran (PPF) host. Moreover, the resulting sky-blue device also demonstrated a maximum EQE of 22% when the doping concentration of emitter 1 was further increased to 50 wt% in the same PPF host. The high efficiencies may be attributed to three key factors. First, the ACR-PXB series emitters possessed a higher PLQY value ranging from 56 to 100%, a shorter decay time ranging from 1.6 to 4.02 μs, and a smaller ΔEST value ranging from 0 to 0.16 eV. Second, rational bipolar characteristics of the emitters may help to achieve an effective charge carrier injection balance in the emissive layer. Third, the high triplet energy (3.21 eV)-host material restricted any backward energy transfer and facilitated an effective host-to-guest energy transfer. As reported, the device performance was greatly influenced by the ΔEST and PLQY of the emitters. The PLQY increased from 56 to 100% for emitter 1 as ΔEST decreased from 0.16 to 0 eV [174].
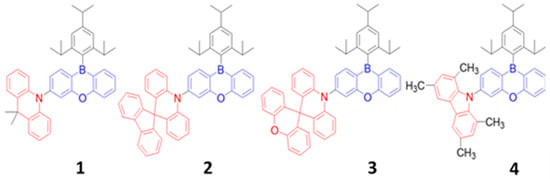
Figure 23. Molecular structures of Cz-PXB-based blue emitters.
Phenoxazines and Triazole (PXZ-TAZ) Derivatives
Bipolar compounds based on phenoxazine electron-donor and triazole electron-acceptor moieties have been widely investigated to design the bipolar host materials for OLEDs [refs]. In 2013, Adachi et al. reported two TADF blue emitters, 10-(4-(4,5-diphenyl-4H-1,2,4-triazol-3-yl)phenyl)-10H-phenoxazine (PXZ-TAZ) and 10,10′-((4-phenyl-4H-1,2,4-triazole-3,5-diyl)bis(4,1-phenylene))-2-bis(10H-phenoxazine) (2PXZ-TAZ) (Figure 44), by using 1,2,4-triazole core-based emitters with a 10H-phenoxazine group. The PXZ-TAZ and 2PXZ-TAZ emitters exhibited a PLQY of 13 and 15% in neat films. The PLQY was greatly enhanced from 15 to 52% as a 6 wt% 2PXZ-TAZ emitter was doped into a DPEPO host. The 2PXZ-TAZ also showed a maximum EQE of 6.4% with (0.16, 0.15) and an EL emission peaking at 456 nm [142].
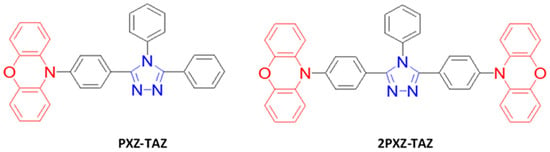
Figure 24. Molecular structures of PXZ-TAZ-based blue emitters.
Acridine and Triazine (ACR-TRZ) Derivatives
As described in prior sections, both phenoxazine and triazine moieties have been extensively utilized to design bipolar hosts and TADF emitters for OLEDs. In 2015, Tsai et al. designed and synthesized a DMAC-TRZ (Figure 25) emitter based on 9,9-dimethyl-9,10-dihydroacridine as a donor unit and 2,4,6-triphenyl-1,3,5-triazine as an acceptor unit. The DMAC-TRZ emitter showed a ΔEST of 0.05 eV and a delay lifetime of 3.6 μs with an 83% PLQY. Furthermore, the ΔEST and the delay lifetime were decreased to 0.046 eV and 1.9 μs, respectively, while the PLQY was increased to 90% as the DMAC-TRZ emitter was doped into a 9-(3-(9H-carbazol-9-yl)phenyl)-9H-carbazole-3-carbonitrile (mCPCN) host. At 100 nits, the resultant non-doped DMAC-TRZ-based device exhibited a maximum EQE of 20% with a bluish-green emission, while a doped emissive layer (at 8 wt% DMAC-TRZ and mCPCN)-based device showed a maximum EQE of 27% with a slightly blue shifted emission. These devices also exhibited relatively high power efficacy, at 46 and 66 lm/W, and current efficacy of 61 and 67 cd/A at 100 cd/m2, respectively, for non-doped and doped emissive layer-based devices [175].
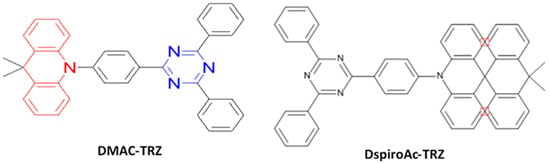
Figure 25. Molecular structures of ACR-TRZ-based blue emitters.
In 2020, Li et al. reported an emitter, DspiroAc-TRZ (Figure 25), with a tight and regular intermolecular packing composed of X- or L-shaped J-aggregates. PLQYs of 79 and 84% were obtained for the DspiroAc-TRZ crystal and nondoped film, respectively, due to the synergistic effect of the intermolecular packing mode and monomolecular structure. Thus, a very high EQEmax of 26% and CEmax of 56 cd/A were realized for a sky-blue emission with (0.17, 0.35) by using a host-free emissive layer [176].
3.2. Metal-Based TADF Emitters
Metal-based TADF blue emitters had also been reported to realize a very small ΔEST, which can effectively up-convert the entire fraction of triplet excitons (75%) into singlet excitons via ultra-fast reverse intersystem crossing (RISC) [177][178][179][180]. This prudent combination of ultra-fast RISC and a very small ΔEST would result in a 100% delayed fluorescence yield, [180][181][182][183] and significantly diminish the efficiency roll-off, especially at high luminance or current density [184].
The metal-based TADF emitters also exhibited a higher thermal stability than that of pure aromatic-based counterparts [184][185][186][187]. By virtue of these advantages, a considerable number of blue TADF emitters based on non-precious and abundant metals, such as lithium (Li), magnesium (Mg), copper (Cu), zinc (Zn), and tin (Sn), were reported (Figure 26) [188]. However, only a few of them were investigated in OLEDs [134][189][190][191][192][193][194][195].
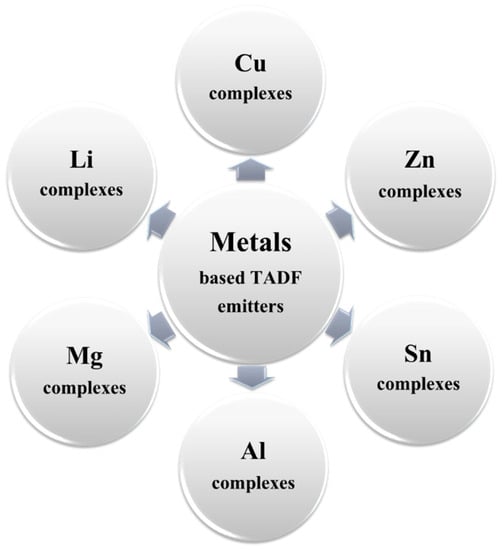
Figure 26. Types of non-precious metal-based TADF blue emitters.
Cu Complexes
Copper (Cu) is one of the highly abundant coinage metals which has been investigated in the design of high-electroluminescence (EL) active complexes for low-cost OLEDs. In order to realize a sustainable way out, Ma et al. designed and synthesized the first Cu complex-based emitter in 1999 [196][197]. Later, many Cu complex-based phosphorescent emitters were reported because of their ability to realize a high rate of intersystem crossing (ISC) (S1→T1) and triplet radiative decay (T1→S0) [180][198].
Due to a small ΔEST, a few Cu complexes showed effective RISC (T1→S1) and singlet radiative decay (S1→S0) rates [192][199]. These complexes exhibited a small ΔEST and a fast RISC because of low-lying metal-ligand charge transfer (MLCT) excited states, which played a crucial role in realizing an effective triplet up-conversion [189][190][191][192][193][194][195].
Over the past few years, several Cu-based TADF emitters have been utilized in OLEDs [200][201][202][203][204][205][206][207][208]. Among them, only a few emitters demonstrated a blue emission. In 2011, Yersin et al. designed and synthesized a series of Cu (I) complexes (Cu-4 to Cu-6) (Figure 27) [196]. The ΔEST and PLQY of these compounds ranged from 0.09 to 0.16 eV and 45 to 90%, respectively, in solid state films. The chromaticity coordinates of the Cu-4 to Cu-6 emitters ranged from (0.14, 0.11) to (0.16, 0.22). In the same year, Leitl et al. reported a design strategy to realize a strong TADF emission in another Cu complex [209]. They observed that a small ΔEST value can be obtained by selecting heteroleptic ligands with a low torsion angle. A Cu complex (Cu-7) with two N-heterocyclic ligands, 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene and di(2-pyridyl)dimethylborate), showed a blue emission peaking at 475 nm. The Cu-7 exhibited a solid state PLQY of 70% and a ΔEST of 0.09 eV, resulting in a TADF decay fraction of 62% with a weak phosphorescence decay fraction of 38% [209].
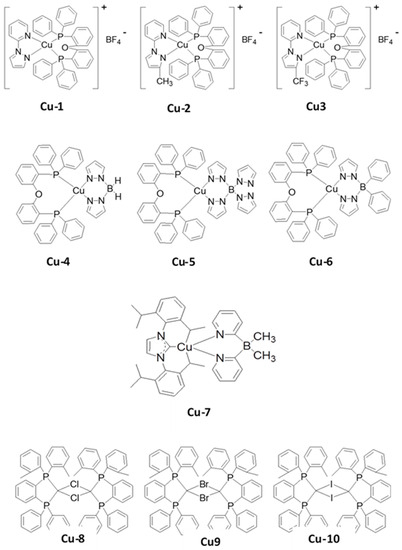
Figure 27. Molecular structures of copper (Cu)-based blue emitters.
In 2013, Chen et al. reported a series of Cu complex (Cu-1 to Cu-3) (Figure 27)-based emitters [210]. These complexes showed a constant ΔEST of 0.17 ± 0.01 eV with a PLQY ranging from 56 to 87%. Via wet-processing, the resultant maximum EQE ranged from 8.5 to 1.5%, with chromaticity coordinates ranging from greenish-blue (0.24, 0.36) to sky-blue (0.20, 0.30) as a 20 wt% of the emitters was doped into a hole-transporting host 26mCPy. The Cu-3 complex-based device also exhibited a maximum current efficiency of 24 cd/A.
In 2014, Yersin et al. reported a series of Cu complex-based emitters. Their structures were similar to the phosphorescent emitters reported earlier [209][211].
In 2015, Kang et al. reported a series of binuclear Cu halide complexes (Cu-8–Cu-10) (Figure 27) with different halogen molecules (Cl, Br, and I) [189]. These complexes exhibited a ΔEST value from 0.05 to 0.04 eV, a PLQY from 42 to 95%, and a decay time ranging from 4.9 to 5.9 μs with an emission wavelength ranging from blue (482 nm) to greenish blue (490 nm).
Zn Complexes
Over the past few years, several Zn complexes have been employed as EL-active emitters, hosts, and electron transporting materials in OLEDs [129][212][213][214][215][216][217][218][219][220]. However, Zn complexes exhibited a poorer device performance than that of Cu complex-based counterparts while displaying a higher stability against atmospheric moisture and air. Therefore, numerous efforts have been devoted to design Zn complex-based TADF emitters [194][221][222].
In order to devise a highly stable metal complex-based emitter, in 2015, Adachi’s group reported two different Zn complexes (Zn-1 and Zn-2) (Figure 28) with blue emission [184]. The Zn-1 and Zn-2 complexes demonstrated a PLQY of 78 and 58% and a ΔEST of 0.06 and 0.18 eV, respectively. In addition, the Zn-1 emitter also realized a low TADF decay time of 38 μs. In an mCBP host-based doped device, the Zn-1 complex showed a maximum EQE of 20% with a greenish-blue emission.
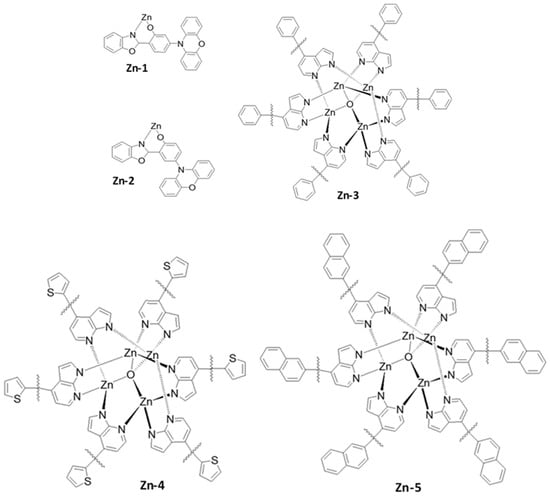
Figure 28. Molecular structures of zinc (Zn)-based blue emitters.
Moreover, Cheng et al. designed and synthesized a series of tetranuclear zinc(II) complexes of substituted 7-azaindoles. The decay time and PLQY of these complexes ranged from 0.015 to 0.008 μs and 96 to 71%, respectively, as a 5 wt% emitter was doped into a PMMA host. The PLQY values of these compounds were measured in solid state film. The resultant maximum EQE ranged from 5.6 to 3.3%, with chromaticity coordinates ranging from (0.16, 0.19) to (0.18, 0.26) as an 8 wt% of the emitters was doped into a mixture of PVK and OXD-7 hosts [223].
Other Metal Complexes
In 2015, Adachi et al. studied other metal (Li, Mg, Al, and Sn) complexes for OLED device applications [184]. The lithium (Li) complex-based TADF emitter (Li-1) exhibited a ΔEST of 0.08 eV with a total PLQY of 70%. In a doped device with an mCBP host, the Li-1 (Figure 29) realized a maximum EQE of 13% with a greenish-blue emission. Moreover, a magnesium-based (Mg-1) (Figure 29) complex achieved a ΔEST of 0.08 eV and a PLQY of 71%, including a 58% delayed quantum yield. In a device doped with an mCBP host, the Mg-1 complex-based device exhibited a maximum EQE of 17% for a greenish-blue emission.
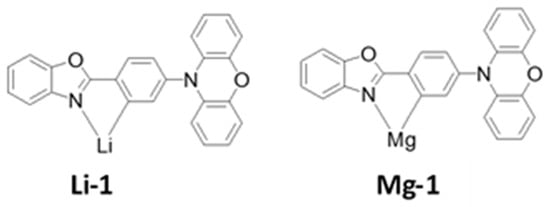
Figure 29. Molecular structures of Lithium (Li) and Magnesium (Mg)-based blue emitters.
References
- Hamada, Y.; Adachi, C.; Tsutsui, T.; Saito, S. Organic Electroluminescent Devices with Bright Blue Emission. Optoelectron. Tokyo 1992, 7, 83–93.
- Hamada, Y.; Adachi, C.; Tsutsui, T.; Saito, S. Blue-Light-Emitting Organic Electroluminescent Devices with Oxadiazole Dimer Dyes as an Emitter. Jpn. J. Appl. Phys. 1992, 31, 1812–1816.
- Grem, G.; Leditzky, G.; Ullrich, B.; Leising, G. Realization of a Blue-Light-Emitting Device Using Poly(p-Phenylene). Adv. Mater. 1992, 4, 36–37.
- Grem, G.; Leditzky, G.; Ullrich, B.; Leising, G. Blue Electroluminescent Device Based on a Conjugated Polymer. Synth. Met. 1992, 51, 383–389.
- Hosokawa, C.; Kawasaki, N.; Sakamoto, S.; Kusumoto, T. Bright Blue Electroluminescence from Hole Transporting Polycarbonate. Appl. Phys. Lett. 1998, 61, 2503.
- Hosokawa, C.; Tokailin, H.; Higashi, H.; Kusumoto, T. Transient Behavior of Organic Thin Film Electroluminescence. Appl. Phys. Lett. 1998, 60, 1220.
- Hosokawa, C.; Tokailin, H.; Higashi, H.; Kusumoto, T. Transient Electroluminescence from Hole Transporting Emitting Layer in Nanosecond Region. Appl. Phys. Lett. 1998, 63, 1322.
- Vestweber, H.; Sander, R.; Greiner, A.; Heitz, W.; Mahrt, R.F.; Bässler, H. Electroluminescence from Polymer Blends and Molecularly Doped Polymers. Synth. Met. 1994, 64, 141–145.
- Hosokawa, C.; Tokailin, H.; Higashi, H.; Kusumoto, T. Efficient Electroluminescence of Distyrylarylene with Hole Transporting Ability. J. Appl. Phys. 1998, 78, 5831.
- Tokailin, T.H.; Matsuura, M.; Higashi, H.; Hosokawa, C.; Kusumoto, T.; Tokailin, H.; Matsuura, M.; Kusumoto, T. Characteristics of Blue Organic Electroluminescent Devices with Distyryl Arylene Derivatives. Electron. Imaging 1993, 1910, 38–47.
- Wen, S.; Lee, M.; Chen, C.H. Recent Development of Blue Fluorescent OLED Materials and Devices. J. Disp. Technol. 2005, 1, 90–99.
- Hosokawa, C.; Matsuura, M.; Eida, M.; Fukuoka, K.; Tokailin, H.; Kusumoto, T. 4.1: Invited Paper: Full-Color Organic EL Display. SID Symp. Dig. Tech. Pap. 1998, 29, 7–10.
- Ho, M.H.; Chang, C.M.; Chu, T.Y.; Chen, T.M.; Chen, C.H. Iminodibenzyl-Substituted Distyrylarylenes as Dopants for Blue and White Organic Light-Emitting Devices. Org. Electron. 2008, 9, 101–110.
- Wind, M.; Wiesler, U.M.; Saalwächter, K.; Müllen, K.; Spiess, H.W. Shape-Persistent Polyphenylene Dendrimers—Restricted Molecular Dynamics from Advanced Solid-State Nuclear Magnetic Resonance Techniques. Adv. Mater. 2001, 13, 752–756.
- Rosenfeldt, S.; Dingenouts, N.; Pötschke, D.; Ballauff, M.; Berresheim, A.J.; Müllen, K.; Lindner, P. Analysis of the Spatial Dimensions of Fully Aromatic Dendrimers. Angew. Chem. Int. Ed. 2004, 43, 109–112.
- Specification, E.P. EP000875947B1. 2002. Available online: https://data.epo.org/publication-server/document?iDocId=2079949&iFormat=0 (accessed on 15 August 2023).
- Novel Green Light-Emitting Carbazole Derivatives: Potential Electroluminescent Materials-Thomas-2000-Advanced Materials-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/1521-4095%28200012%2912%3A24%3C1949%3A%3AAID-ADMA1949%3E3.0.CO%3B2-X (accessed on 16 May 2022).
- Tonzola, C.J.; Alam, M.M.; Kaminsky, W.; Jenekhe, S.A. New N-Type Organic Semiconductors: Synthesis, Single Crystal Structures, Cyclic Voltammetry, Photophysics, Electron Transport, and Electroluminescence of a Series of Diphenylanthrazolines. J. Am. Chem. Soc. 2003, 125, 13548–13558.
- Figueira-Duarte, T.M.; Del Rosso, P.G.; Trattnig, R.; Sax, S.; List, E.J.W.; Mullen, K. Designed Suppression of Aggregation in Polypyrene: Toward High-Performance Blue-Light-Emitting Diodes. Adv. Mater. 2010, 22, 990–993.
- Xing, Y.; Xu, X.; Zhang, P.; Tian, W.; Yu, G.; Lu, P.; Liu, Y.; Zhu, D. Carbazole–Pyrene-Based Organic Emitters for Electroluminescent Device. Chem. Phys. Lett. 2005, 408, 169–173.
- Sotoyama, W.; Sato, H.; Kinoshita, M.; Takahashi, T.; Matsuura, A.; Kodama, J.; Sawatari, N.; Inoue, H. 45.3: Tetra-Substituted Pyrenes: New Class of Blue Emitter for Organic Light-Emitting Diodes. SID Symp. Dig. Tech. Pap. 2003, 34, 1294–1297.
- Miura, Y.; Yamano, E.; Tanaka, A.; Yamauchi, J. Generation, Isolation, and Characterization of N-(Arylthio)-7-Tert-Butyl- and N-(Arylthio)-2,7-Di-Tert-Butyl-1-Pyrenylaminyl Radicals. J. Org. Chem. 1994, 59, 3294–3300.
- Grill, E.; Hauge, B.; Schiefelbein, J.; Martinez-Zapater, J.; Gil, P.; Iba, K.; Bevilacqua, P.C.; Kierzek, R.; Johnson, K.A.; Turner, D.H. Dynamics of Ribozyme Binding of Substrate Revealed by Fluorescence-Detected Stopped-Flow Methods. Science 1992, 258, 1355–1358.
- Kim, J.; Beardslee, R.; Phillips, D.T.; Offen, H.W. Fluorescence Lifetimes of Pyrene Monomer and Excimer at High Pressures. J. Chem. Phys. 1969, 51, 1638.
- Kropp, J.L.; Dawson, W.R.; Windsor, M.W. Radiative and Radiationless Processes in Aromatic Molecules. Pyrene. J. Phys. Chem. 1969, 73, 1747–1752.
- Zhao, Z.; Li, J.H.; Chen, X.; Wang, X.; Lu, P.; Yang, Y. Solution-Processable Stiff Dendrimers: Synthesis, Photophysics, Film Morphology, and Electroluminescence. J. Org. Chem. 2009, 74, 383–395.
- Jia, W.-L.; McCormick, T.; Liu, Q.-D.; Fukutani, H.; Motala, M.; Wang, R.-Y.; Tao, Y.; Wang, S. Diarylamino Functionalized Pyrene Derivatives for Use in Blue OLEDs and Complex Formation. J. Mater. Chem. 2004, 14, 3344–3350.
- Lampkins, A.J.; O’Neil, E.J.; Smith, B.D. Bio-Orthogonal Phosphatidylserine Conjugates for Delivery and Imaging Applications. J. Org. Chem. 2008, 73, 6053–6058.
- Vollmer, A.; Lorbach, D.; List, E.J.W.; Müllen, K.; Loi, M.A.; Manca, M.; Baumgarten, M.; Koch, N.; Trattnig, R.; Sax, S.; et al. Deep Blue Polymer Light Emitting Diodes Based on Easy to Synthesize, Non-Aggregating Polypyrene. Opt. Express 2011, 19, A1281–A1293.
- Wu, K.C.; Ku, P.J.; Lin, C.S.; Shih, H.T.; Wu, F.I.; Huang, M.J.; Lin, J.J.; Chen, I.C.; Cheng, C.H. The Photophysical Properties of Dipyrenylbenzenes and Their Application as Exceedingly Efficient Blue Emitters for Electroluminescent Devices. Adv. Funct. Mater. 2008, 18, 67–75.
- Zhao, Z.; Xu, X.; Wang, H.; Lu, P.; Yu, G.; Liu, Y. Zigzag Molecules from Pyrene-Modified Carbazole Oligomers: Synthesis, Characterization, and Application in OLEDs. J. Org. Chem. 2008, 73, 594–602.
- Zhao, Z.; Xu, X.; Jiang, Z.; Lu, P.; Yu, G.; Liu, Y. Oligo(2,7-Fluorene Ethynylene)s with Pyrene Moieties: Synthesis, Characterization, Photoluminescence, and Electroluminescence. J. Org. Chem. 2007, 72, 8345–8353.
- Chan, K.; Lim, J.; Yang, X.; Dodabalapur, A.; Jabbour, G.E.; Sellinger, A. High-Efficiency Pyrene-Based Blue Light Emitting Diodes: Aggregation Suppression Using a Calixarene 3D-Scaffold. Chem. Commun. 2012, 48, 5106–5108.
- Feng, X.; Hu, J.-Y.; Yi, L.; Seto, N.; Tao, Z.; Redshaw, C.; Elsegood, M.R.J.; Yamato, T. Pyrene-Based Y-shaped Solid-State Blue Emitters: Synthesis, Characterization, and Photoluminescence. Chem. Asian J. 2012, 7, 2854–2863.
- Wang, Z.; Xu, C.; Wang, W.; Dong, X.; Zhao, B.; Pigments, B.J.-D. Novel Pyrene Derivatives: Synthesis, Properties and Highly Efficient Non-Doped Deep-Blue Electroluminescent Device. Dye. Pigment. 2012, 92, 732–736.
- Sonar, P.; Soh, M.S.; Cheng, Y.H.; Henssler, J.T.; Sellinger, A. 1,3,6,8-Tetrasubstituted Pyrenes: Solution-Processable Materials for Application in Organic Electronics. Org. Lett. 2010, 12, 3292–3295.
- Moorthy, J.N.; Natarajan, P.; Venkatakrishnan, P.; Huang, D.F.; Chow, T.J. Steric Inhibition of π-Stacking: 1,3,6,8-Tetraarylpyrenes as Efficient Blue Emitters in Organic Light Emitting Diodes (OLEDs). Org. Lett. 2007, 9, 5215–5218.
- Otsubo, T.; Aso, Y.; Takimiya, K. Functional Oligothiophenes as Advanced Molecular Electronic Materials. J. Mater. Chem. 2002, 12, 2565–2575.
- Kumchoo, T.; Promarak, V.; Sudyoadsuk, T.; Sukwattanasinitt, M.; Rashatasakhon, P. Dipyrenylcarbazole Derivatives for Blue Organic Light-Emitting Diodes. Chem. Asian J. 2010, 5, 2162–2167.
- Zhao, Z.; Xu, X.; Wang, F.; Yu, G.; Lu, P.; Liu, Y.; Zhu, D. Synthesis and Characterization of Light-Emitting Materials Composed of Carbazole, Pyrene and Fluorene. Synth. Met. 2006, 156, 209–214.
- Mikroyannidis, J.A.; Fenenko, L.; Adachi, C. Synthesis and Photophysical Characteristics of 2,7-Fluorenevinylene-Based Trimers and Their Electroluminescence. J. Phys. Chem. B 2006, 110, 20317–20326.
- Tang, C.; Liu, F.; Xia, Y.; Xie, L.; Wei, A.; Li, S.-B.; Fan, Q.-L.; Huang, W. Efficient 9-Alkylphenyl-9-Pyrenylfluorene Substituted Pyrene Derivatives with Improved Hole Injection for Blue Light-Emitting Diodes. J. Mater. Chem. 2006, 16, 4074–4080.
- Tang, C.; Liu, F.; Xia, Y.; Lin, J.; Xie, L.; Zhong, G.-Y.; Fan, Q.-L.; Huang, W. Fluorene-Substituted Pyrenes—Novel Pyrene Derivatives as Emitters in Nondoped Blue OLEDs. Org. Electron. 2006, 7, 155–162.
- Peng, Z.; Tao, S.; Zhang, X.; Tang, J.; Lee, C.S.; Lee, S.T. New Fluorene Derivatives for Blue Electroluminescent Devices: Influence of Substituents on Thermal Properties, Photoluminescence, and Electroluminescence. J. Phys. Chem. C 2008, 112, 2165–2169.
- Liu, F.; Xie, L.H.; Tang, C.; Liang, J.; Chen, Q.Q.; Peng, B.; Wei, W.; Cao, Y.; Huang, W. Facile Synthesis of Spirocyclic Aromatic Hydrocarbon Derivatives Based on O-Halobiaryl Route and Domino Reaction for Deep-Blue Organic Semiconductors. Org. Lett. 2009, 11, 3850–3853.
- Tao, S.; Zhou, Y.; Lee, C.S.; Zhang, X.; Lee, S.T. High-Efficiency Nondoped Deep-Blue-Emitting Organic Electroluminescent Device. Chem. Mater. 2010, 22, 2138–2141.
- Thangthong, A.; Prachumrak, N.; Tarsang, R.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuka, T.; Promarak, V. Blue Light-Emitting and Hole-Transporting Materials Based on 9, 9-Bis (4-Diphenylaminophenyl) Fluorenes for Efficient Electroluminescent Devices. Mater. Chem. 2012, 22, 6869–6877.
- Jou, J.; Chen, Y.; Tseng, J.; Wu, R.Z.; Shyue, J.J.; Thomas, K.R.J.; Kapoor, N.; Chen, C.-T.; Lin, Y.-P.; Wang, P.-H.; et al. The Use of a Polarity Matching and High-Energy Exciton Generating Host in Fabricating Efficient Purplish-Blue OLEDs from a Sky-Blue Emitter. J. Mater. Chem. 2012, 22, 15500–15506.
- Tao, S.; Peng, Z.; Zhang, X.; Wang, P.; Lee, C.-S.; Lee, S.-T. Highly Efficient Non-doped Blue Organic Light-emitting Diodes Based on Fluorene Derivatives with High Thermal Stability. Adv. Funct. Mater. 2005, 15, 1716–1721.
- Lai, S.; Tong, Q.; Chan, M.; Ng, T.; Lo, M.-F.; Lee, S.-T.; Lee, C.-S. Distinct Electroluminescent Properties of Triphenylamine Derivatives in Blue Organic Light-Emitting Devices. J. Mater. Chem. 2011, 21, 1206–1211.
- Thomas, K.; Velusamy, M.; Lin, J.T.; Chuen, C.H.; Tao, Y.-T. Hexaphenylphenylene Dendronised Pyrenylamines for Efficient Organic Light-Emitting Diodes. J. Mater. Chem. 2005, 15, 4453–4459.
- Tao, S.; Zhou, Y.; Lee, C.S.; Lee, S.T.; Huang, D.; Zhang, X. Highly Efficient Nondoped Blue Organic Light-Emitting Diodes Based on Anthracene-Triphenylamine Derivatives. J. Phys. Chem. C 2008, 112, 14603–14606.
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314.
- Kumar, D.; Thomas, K.R.J.; Lin, C.C.; Jou, J.H. Pyrenoimidazole-Based Deep-Blue-Emitting Materials: Optical, Electrochemical, and Electroluminescent Characteristics. Chem. Asian J. 2013, 8, 2111–2124.
- Figueira-Duarte, T.M.; Simon, S.C.; Wagner, M.; Druzhinin, S.I.; Zachariasse, K.A.; Müllen, K. Polypyrene Dendrimers. Angew. Chem. Int. Ed. 2008, 47, 10175–10178.
- Kotchapradist, P.; Prachumrak, N.; Tarsang, R.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Pyrene-Functionalized Carbazole Derivatives as Non-Doped Blue Emitters for Highly Efficient Blue Organic Light-Emitting Diodes. J. Mater. Chem. C 2013, 1, 4916–4924.
- Adachi, C.; Tsutsui, T.; Saito, S. Blue Light-emitting Organic Electroluminescent Devices. Appl. Phys. Lett. 1998, 56, 799.
- Shi, J.; Tang, C.W. Anthracene Derivatives for Stable Blue-Emitting Organic Electroluminescence Devices. Appl. Phys. Lett. 2002, 80, 3201.
- Shih, B.P.; Chuang, C.; Chien, C.; Diau, E.W.; Shu, C. Highly Efficient Non-Doped Blue-Light-Emitting Diodes Based on an Anthrancene Derivative End-Capped with Tetraphenylethylene Groups. Adv. Funct. Mater. 2007, 17, 3141–3146.
- Kim, S.; Yang, B.; Ma, Y.; Lee, J.; Park, J. Exceedingly Efficient Deep-Blue Electroluminescence from New Anthracenes Obtained Using Rational Molecular Design. J. Mater. Chem. 2008, 18, 3376–3384.
- Zheng, C.; Zhao, W.; Wang, Z.; Huang, D.; Ye, J.; Ou, X.; Zhang, X.; Lee, C.; Lee, S. Highly Efficient Non-Doped Deep-Blue Organic Light-Emitting Diodes Based on Anthracene Derivatives. J. Mater. Chem. 2010, 20, 1560–1566.
- Chem, J.M.; Kim, S.H.; Cho, I.; Sim, M.K.; Park, S.; Park, S.Y. Highly Efficient Deep-Blue Emitting Organic Light Emitting Diode Based on the Multifunctional Fluorescent Molecule Comprising Covalently Bonded Carbazole and Anthracene Moieties. J. Mater. Chem. 2011, 21, 9139–9148.
- Zhuang, S.; Shangguan, R.; Jin, J.; Tu, G.; Wang, L.; Chen, J.; Ma, D.; Zhu, X. Efficient Nondoped Blue Organic Light-Emitting Diodes Based on Phenanthroimidazole-Substituted Anthracene Derivatives. Org. Electron. 2012, 13, 3050–3059.
- Park, J. Highly Efficient Dual-Core Derivatives with EQEs as High as 8.38% at High Brightness for OLED Blue Emitters. J. Mater. Chem. 2019, 7, 14709–14716.
- Lai, W.Y.; Zhu, R.; Fan, Q.L.; Hou, L.T.; Cao, Y.; Huang, W. Monodisperse Six-Armed Triazatruxenes: Microwave-Enhanced Synthesis and Highly Efficient Pure-Deep-Blue Electroluminescence. Macromolecules 2006, 39, 3707–3709.
- Lai, W.Y.; He, Q.Y.; Zhu, R.; Chen, Q.Q.; Huang, W. Kinked Star-Shaped Fluorene/Triazatruxene Co-Oligomer Hybrids with Enhanced Functional Properties for High-Performance, Solution-Processed, Blue Organic Light-Emitting Diodes. Adv. Funct. Mater. 2008, 18, 265–276.
- You, A.; Be, M.A.Y.; In, I. High Efficiency Deep-Blue Organic Light-Emitting Diode with a Blue Dye in Low-Polarity Host. Appl. Phys. Lett. 2008, 92, 193314.
- Wang, L.; Jiang, Y.; Luo, J.; Zhou, Y.; Zhou, J.; Wang, J.; Pei, J.; Cao, Y. Highly Efficient and Color-Stable Deep-Blue Organic Light-Emitting Diodes Based on a Solution-Processible Dendrimer. Adv. Mater. 2009, 21, 4854–4858.
- Zhu, M.; Ye, T.; Li, C.G.; Cao, X.; Zhong, C.; Ma, D.; Qin, J.; Yang, C. Efficient Solution-Processed Nondoped Deep-Blue Organic Light-Emitting Diodes Based on Fluorene-Bridged Anthracene Derivatives Appended with Charge Transport Moieties. J. Phys. Chem. C 2011, 115, 17965–17972.
- Zou, Y.; Zou, J.; Ye, T.; Li, H.; Yang, C.; Wu, H.; Ma, D.; Qin, J.; Cao, Y. Unexpected Propeller-Like Hexakis(Fluoren-2-Yl)Benzene Cores for Six-Arm Star-Shaped Oligofluorenes: Highly Efficient Deep-Blue Fluorescent Emitters and Good Hole-Transporting Materials. Adv. Funct. Mater. 2013, 23, 1781–1788.
- Zhen, C.G.; Dai, Y.F.; Zeng, W.J.; Ma, Z.; Chen, Z.K.; Kieffer, J. Achieving Highly Efficient Fluorescent Blue Organic Light-Emitting Diodes through Optimizing Molecular Structures and Device Configuration. Adv. Funct. Mater. 2011, 21, 699–707.
- Huang, H.; Fu, Q.; Zhuang, S.; Liu, Y.; Wang, L.; Chen, J.; Ma, D.; Yang, C. Novel Deep Blue OLED Emitters with 1,3,5-Tri(Anthracen-10-Yl)Benzene-Centered Starburst Oligofluorenes. J. Phys. Chem. C 2011, 115, 4872–4878.
- Liu, C.; Li, Y.; Zhang, Y.; Yang, C.; Wu, H.; Qin, J.; Cao, Y. Solution-Processed, Undoped, Deep-Blue Organic Light-Emitting Diodes Based on Starburst Oligofluorenes with a Planar Triphenylamine Core. Chemistry 2012, 18, 6928–6934.
- Zhi, B.; Gao, Q.; Li, Z.H.; Xia, P.F.; Wong, M.S.; Cheah, K.W.; Chen, C.H. Efficient Deep-Blue Organic Light-Emitting Diodes: Arylamine-Substituted Oligofluorenes. Adv. Funct. Mater. 2007, 17, 3194–3199.
- Zhang, T.; Wang, R.; Ren, H.; Chen, Z.; Li, J. Deep Blue Light-Emitting Polymers with Fluorinated Backbone for Enhanced Color Purity and Efficiency. Polymer 2012, 7, 1529–1534.
- Meunmart, D.; Prachumrak, N.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promark, V. Bis(4-Diphenylaminophenyl)Carbazole End-Capped Fluorene as Solution-Processed Deep-Blue Light-Emitting and Hole-Transporting Materials for Electroluminescent Devices. Tetrahedron Lett. 2012, 28, 3615–3618.
- Liu, C.; Gu, Y.; Fu, Q.; Sun, N.; Zhong, C.; Ma, D.; Qin, J.; Yang, C. Nondoped Deep-Blue Organic Light-Emitting Diodes with Color Stability and Very Low Efficiency Roll-off: Solution-Processable Small-Molecule Fluorophores by Phosphine Oxide Linkage. Chem.-A Eur. J. 2012, 18, 13828–13835.
- Liu, C.; Li, Y.; Li, Y.; Yang, C.; Wu, H.; Qin, J.; Cao, Y. Efficient Solution-Processed Deep-Blue Organic Light-Emitting Diodes Based on Multibranched Oligofluorenes with a Phosphine Oxide Center. Chem. Mater. 2013, 25, 3320–3327.
- Giovanella, U.; Botta, C.; Galeotti, F.; Vercelli, B.; Battiato, S.; Pasini, M. Perfluorinated Polymer with Unexpectedly Efficient Deep Blue Electroluminescence for Full-Colour OLED Displays and Light Therapy Applications. J. Mater. Chem. C Mater. 2013, 1, 5322–5329.
- McDowell, J.J.; Maier-Flaig, F.; Wolf, T.J.A.; Unterreiner, A.N.; Lemmer, U.; Ozin, G. Synthesis and Application of Photolithographically Patternable Deep Blue Emitting Poly(3,6-Dimethoxy-9,9-Dialkylsilafluorene)s. ACS Appl. Mater. Interfaces 2014, 6, 83.
- Hu, L.; Liu, S.; Xie, G.; Yang, W.; Zhang, B. Bis(Benzothiophene-S,S-Dioxide) Fused Small Molecules Realize Solution-Processible, High-Performance and Non-Doped Blue Organic Light-Emitting Diodes. J. Mater. Chem. C Mater. 2020, 8, 1002–1009.
- Liu, X.; Liu, W.; Dongyu, W.; Wei, X.; Wang, L.; Wang, H.; Miao, Y.; Xu, H.; Yu, J.; Xu, B. Deep-Blue Fluorescent Emitter Based on a 9,9-Dioctylfluorene Bridge with a Hybridized Local and Charge-Transfer Excited State for Organic Light-Emitting Devices with EQE Exceeding 8%. J. Mater. Chem. C. 2020, 8, 14117–14124.
- Fischer, A.; Forget, S.; Chénais, S.; Castex, M.; Adès, D.; Siove, A.; Denis, C.; Maisse, P.; Geffroy, B. Highly Efficient Multilayer Organic Pure-Blue-Light Emitting Diodes with Substituted Carbazoles Compounds in the Emitting Layer. J. Phys. D Appl. Phys. 2006, 39, 917.
- Tseng, R.J.; Chiechi, R.C.; Wudl, F.; Yang, Y. Highly Efficient 7,8,10-Triphenylfluoranthene-Doped Blue Organic Light-Emitting Diodes for Display Application. Appl. Phys. Lett. 2006, 88, 093512.
- Jou, J.H.; Chiang, P.H.; Lin, Y.P.; Chang, C.Y.; Lai, C.L. Hole-Transporting-Layer-Free High-Efficiency Fluorescent Blue Organic Light-Emitting Diodes. Appl. Phys. Lett. 2007, 91, 043504.
- Jeon, Y.; Lee, J.; Kim, J.; Lee, C.; Gong, M. Deep-Blue OLEDs Using Novel Efficient Spiro-Type Dopant Materials. Org. Electron. 2010, 11, 1844–1852.
- Li, Z.; Jiao, B.; Wu, Z.; Liu, P.; Ma, L.; Lei, X.; Wang, D.; Zhou, G.; Hu, H.; Hou, X. Fluorinated 9,9′-Spirobifluorene Derivatives as Host Materials for Highly Efficient Blue Organic Light-Emitting Devices. J. Mater. Chem. C Mater. 2013, 1, 2183–2192.
- Yu, J.; Chen, Z.; Sakuratani, Y.; Suzuki, H.; Tokita, M.; Miyata, S. A Novel Blue Light Emitting Diode Using Tris(2,3-Methyl-8-Hydroxyquinoline) Aluminum(III) as Emitter. Jpn. J. Appl. Phys. 1999, 38, 6762–6763.
- Chan, L.; Lee, R.; Hsieh, C.; Yeh, H.; Chen, C. Optimization of High-Performance Blue Organic Light-Emitting Diodes Containing Tetraphenylsilane Molecular Glass Materials. J. Am. Chem. Soc. 2002, 78, 6469–6479.
- Liu, D.; Du, M.; Chen, D.; Ye, K.; Zhang, Z.; Liu, Y.; Wang, Y. A Novel Tetraphenylsilane–Phenanthroimidazole Hybrid Host Material for Highly Efficient Blue Fluorescent, Green and Red Phosphorescent. J. Mater. Chem. C 2015, 3, 4394–4401.
- Jou, J.H.; Li, J.L.; Sahoo, S.; Dubey, D.K.; Kumar Yadav, R.A.; Joseph, V.; Thomas, K.R.J.; Wang, C.W.; Jayakumar, J.; Cheng, C.H. Enabling a 6.5% External Quantum Efficiency Deep-Blue Organic Light-Emitting Diode with a Solution-Processable Carbazole-Based Emitter. J. Phys. Chem. C 2018, 122, 24295–24303.
- Yang, J.; Liu, X.; Liu, Z.; Wang, L.; Sun, J.; Guo, Z.; Xu, H.; Wang, H.; Zhao, B.; Xie, G. Protonation-Induced Dual Fluorescence of a Blue Fluorescent Material with Twisted A–π–D–π–A Configuration. J. Mater. Chem. C Mater. 2020, 8, 2442–2450.
- Wu, Q.; Braveenth, R.; Zhang, H.Q.; Bae, I.J.; Kim, M.; Chai, K.Y. Oxadiazole-Based Highly Efficient Bipolar Fluorescent Emitters for Organic Light-Emitting Diodes. Molecules 2018, 23, 843.
- Wang, Y.; Huang, H.; Wang, Y.; Zhu, Q.; Qin, J. Simple Oxadiazole Derivatives as Deep Blue Fluorescent Emitter and Bipolar Host for Organic Light-Emitting Diodes. OptMa 2018, 84, 278–283.
- Tang, C.; VanSlyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915.
- Wu, C.-C.; Lin, Y.-T.; Wong, K.-T.; Chen, R.-T.; Chien, Y.-Y. Efficient Organic Blue-Light-Emitting Devices with Double Confinement on Terfluorenes with Ambipolar Carrier Transport Properties. Adv. Mater. 2004, 16, 61–65.
- Lee, M.-T.; Liao, C.-H.; Tsai, C.-H.; Chen, H. Highly Efficient, Deep-blue Doped Organic Light-emitting Devices. Adv. Mater. 2002, 80, 2493–2497.
- Baldo, M.; Thompson, M.; Forrest, S. High-Efficiency Fluorescent Organic Light-Emitting Devices Using a Phosphorescent Sensitizer. Nature 2000, 403, 750–753.
- Kido, J.; Kimura, M.; Science, K.N. Multilayer White Light-Emitting Organic Electroluminescent Device. Science 1995, 267, 1332–1334.
- Benmansour, B.H.; Shioya, T.; Sato, Y.; Bazan, G.C. Anthracene-Containing Binaphthol Chromophores for Light-Emitting Diode (LED) Fabrication. Adv. Funct. Mater. 2003, 13, 883–886.
- Holmes, R.J.; Forrest, S.R.; Sajoto, T.; Tamayo, A.; Djurovich, P.I.; Thompson, M.E.; Brooks, J.; Tung, Y.J.; D’Andrade, B.W.; Weaver, M.S.; et al. Saturated Deep Blue Organic Electrophosphorescence Using a Fluorine-Free Emitter. Appl. Phys. Lett. 2005, 87, 243507.
- Xiaohui Yang, B.; Wang, Z.; Madakuni, S.; Li, J.; Jabbour, G.E.; Jabbour, G.E.; Yang, X.; Li, J.; Wang, Z.; Madakuni, S. Efficient Blue- and White-Emitting Electrophosphorescent Devices Based on Platinum(II) Chloride. Adv. Mater. 2008, 20, 2405–2409.
- Sasabe, H.; Takamatsu, J.I.; Motoyama, T.; Watanabe, S.; Wagenblast, G.; Langer, N.; Molt, O.; Fuchs, E.; Lennartz, C.; Kido, J. High-Efficiency Blue and White Organic Light-Emitting Devices Incorporating a Blue Iridium Carbene Complex. Adv. Mater. 2010, 22, 5003–5007.
- Hang, X.-C.; Fleetham, T.; Turner, E.; Brooks, J.; Li, J.; Hang, X.; Fleetham, T.; Turner, E.; Li, J.; Brooks, J. Highly Efficient Blue-Emitting Cyclometalated Platinum(II) Complexes by Judicious Molecular Design. Angew. Chem. Int. Ed. 2013, 52, 6753–6756.
- Ertl, C.D.; Cerdá, J.; Junquera-Hernández, J.M.; Pertegás, A.; Bolink, H.J.; Constable, E.C.; Neuburger, M.; Ortí, E.; Housecroft, C.E. Colour Tuning by the Ring Roundabout: + Emitters with Sulfonyl-Substituted Cyclometallating Ligands. RSC Adv. 2015, 5, 42815–42827.
- He, L.; Wang, Z.; Duan, L.; Yang, C.; Tang, R.; Song, X.; Pan, C. Toward Fluorine-Free Blue-Emitting Cationic Iridium Complexes: To Generate Emission from the Cyclometalating Ligands with Enhanced Triplet Energy. Dalton Trans. 2016, 45, 5604–5613.
- Shavaleev, N.M.; Scopelliti, R.; Grätzel, M.; Nazeeruddin, M.K. Phosphorescent cationic iridium(III) complexes with cyclometalating 1H-indazole and 2H--triazole ligands. Inorganica Chim. Acta 2012, 388, 84–87.
- Li, J.; Djurovich, P.I.; Alleyne, B.D.; Yousufuddin, M.; Ho, N.N.; Thomas, J.C.; Peters, J.C.; Bau, R.; Thompson, M.E. Synthetic Control of Excited-State Properties in Cyclometalated Ir(III) Complexes Using Ancillary Ligands. Inorg. Chem. 2005, 44, 1713–1727.
- Adachi, C.; Kwong, R.C.; Djurovich, P.; Adamovich, V.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Endothermic Energy Transfer: A Mechanism for Generating Very Efficient High-Energy Phosphorescent Emission in Organic Materials. Appl. Phys. Lett. 2001, 79, 2082.
- Holmes, R.J.; D’Andrade, B.W.; Forrest, S.R.; Ren, X.; Li, J.; Thompson, M.E. Efficient, Deep-Blue Organic Electrophosphorescence by Guest Charge Trapping. Appl. Phys. Lett. 2003, 83, 3818.
- Lee, S.J.; Park, K.M.; Yang, K.; Kang, Y. Blue Phosphorescent Ir(III) Complex with High Color Purity: Fac-Tris(2′,6′-Difluoro-2,3′-Bipyridinato-N,C 4′)Iridium(III). Inorg. Chem. 2009, 48, 1030–1037.
- Kang, Y.; Chang, Y.L.; Lu, J.S.; Ko, S.B.; Rao, Y.; Varlan, M.; Lu, Z.H.; Wang, S. Highly Efficient Blue Phosphorescent and Electroluminescent Ir(III) Compounds. J. Mater. Chem. C Mater. 2012, 1, 441–450.
- Hsieh, C.H.; Wu, F.I.; Fan, C.H.; Huang, M.J.; Lu, K.Y.; Chou, P.Y.; Yang, Y.H.O.; Wu, S.H.; Chen, I.C.; Chou, S.H.; et al. Design and Synthesis of Iridium Bis(Carbene) Complexes for Efficient Blue Electrophosphorescence. Chem.-A Eur. J. 2011, 17, 9180–9187.
- Fan, C.; Li, Y.; Yang, C.; Wu, H.; Qin, J.; Cao, Y. Phosphoryl/Sulfonyl-Substituted Iridium Complexes as Blue Phosphorescent Emitters for Single-Layer Blue and White Organic Light-Emitting Diodes by Solution Process. Chem. Mater. 2012, 24, 4581–4587.
- Fan, C.; Zhu, L.; Jiang, B.; Zhong, C.; Ma, D.; Qin, J.; Yang, C. Efficient Blue and Bluish-Green Iridium Phosphors: Fine-Tuning Emissions of FIrpic by Halogen Substitution on Pyridine-Containing Ligands. Org. Electron. 2013, 14, 3163–3171.
- Kessler, F.; Watanabe, Y.; Sasabe, H.; Katagiri, H.; Nazeeruddin, M.K.; Grätzel, M.; Kido, J. High-Performance Pure Blue Phosphorescent OLED Using a Novel Bis-Heteroleptic Iridium(III) Complex with Fluorinated Bipyridyl Ligands. J. Mater. Chem. C Mater. 2013, 1, 1070–1075.
- Park, H.J.; Kim, J.N.; Yoo, H.J.; Wee, K.R.; Kang, S.O.; Cho, D.W.; Yoon, U.C. Rational Design, Synthesis, and Characterization of Deep Blue Phosphorescent Ir(III) Complexes Containing (4′-Substituted-2′-Pyridyl)-1,2,4-Triazole Ancillary Ligands. J. Org. Chem. 2013, 78, 8054–8064.
- Lee, S.; Kim, S.O.; Shin, H.; Yun, H.J.; Yang, K.; Kwon, S.K.; Kim, J.J.; Kim, Y.H. Deep-Blue Phosphorescence from Perfluoro Carbonyl-Substituted Iridium Complexes. J. Am. Chem. Soc. 2013, 135, 14321–14328.
- Liu, L.; Liu, X.; Wu, K.; Ding, J.; Zhang, B.; Xie, Z.; Wang, L. Efficient Solution-Processed Blue Phosphorescent Organic Light-Emitting Diodes with Halogen-Free Solvent to Optimize the Emissive Layer Morphology. Org. Electron. 2014, 15, 1401–1406.
- Sun, D.; Zhou, X.; Li, H.; Sun, X.; Ren, Z.; Yan, S. Multi-3,3 ′ -Bicarbazole-Substituted Arylsilane Host Materials with Balanced Charge Transport for Highly E Ffi Cient Solution-Processed Blue Phosphorescent Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 32, 17802–17810.
- Byeon, S.Y.; Choi, J.M.; Lee, J.Y. Dyes and Pigments Pyridoindole Based Intramolecular Charge Transfer Type Host Material for Blue Phosphorescent Organic Light-Emitting Diodes. Dye. Pigment. 2016, 134, 285–290.
- Park, S.R.; Kim, S.M.; Kang, J.H.; Lee, J.H.; Suh, M.C. Bipolar Host Materials with Carbazole and Dipyridylamine Groups Showing High Triplet Energy for Blue Phosphorescent Organic Light Emitting Diodes. Dye. Pigment. 2017, 141, 217–224.
- Kim, M.; Lee, I.H.; Lee, J.; Hyun, S.; Park, K.; Kang, Y. Chemistry Phenanthridine Based Ir(III) Complex for Blue Phosphorescent Organic Light-Emitting Diodes with Long-Term Operational Stability. J. Ind. Eng. Chem. 2018, 58, 386–390.
- Yuan, J.; Jiang, H.; Yang, Q.; Xiang, Y.; Zhang, Y.; Dai, Y.; Li, P.; Zheng, C.; Xie, G.; Chen, R. A solution-processable wholly-aromatic bipolar host material for highly efficient blue electroluminescent devices. J. Mater. Chem. C 2021, 9, 687–692.
- Kang, J.; Zaen, R.; Park, K.M.; Lee, K.H.; Lee, J.Y.; Kang, Y. Cyclometalated Platinum(II) β-Diketonate Complexes as Single Dopants for High-Efficiency White OLEDs: The Relationship between Intermolecular Interactions in the Solid State and Electroluminescent Efficiency. Cryst. Growth Des. 2020, 20, 6129–6138.
- Liao, K.Y.; Hsu, C.W.; Chi, Y.; Hsu, M.K.; Wu, S.W.; Chang, C.H.; Liu, S.H.; Lee, G.H.; Chou, P.T.; Hu, Y.; et al. Pt(II) Metal Complexes Tailored with a Newly Designed Spiro-Arranged Tetradentate Ligand; Harnessing of Charge-Transfer Phosphorescence and Fabrication of Sky Blue and White OLEDs. Inorg. Chem. 2015, 54, 4029–4038.
- Klimes, K.; Zhu, Z.Q.; Li, J. Efficient Blue Phosphorescent OLEDs with Improved Stability and Color Purity through Judicious Triplet Exciton Management. Adv. Funct. Mater. 2019, 29, 1903068.
- Liu, Q.D.; Wang, R.; Wang, S. Blue Phosphorescent Zn(II) and Orange Phosphorescent Pt(II) Complexes of 4,4′-Diphenyl-6,6′-Dimethyl-2,2′-Bipyrimidine. Dalton Trans. 2004, 2073–2079.
- Xu, H.; Xu, Z.F.; Yue, Z.Y.; Yan, P.F.; Wang, B.; Jia, L.W.; Li, G.M.; Sun, W.B.; Zhang, J.W. A Novel Deep Blue-Emitting ZnII Complex Based on Carbazole-Modified 2-(2-Hydroxyphenyl)Benzimidazole: Synthesis, Bright Electroluminescence, and Substitution Effect on Photoluminescent, Thermal, and Electrochemical Properties. J. Phys. Chem. C 2008, 112, 15517–15525.
- Oh, C.S.; Lee, J.Y. High Triplet Energy Zn Complexes as Host Materials for Green and Blue Phosphorescent Organic Light-Emitting Diodes. Dye. Pigment. 2013, 99, 374–377.
- Chaskar, A.; Chen, H.-F.; Wong, K.-T.; Chaskar, A.; Chen, H.-F.K.; Wong, K.-T. Bipolar Host Materials: A Chemical Approach for Highly Efficient Electrophosphorescent Devices. Adv. Mater. 2011, 23, 3876–3895.
- Soo Yook, K.; Yeob Lee, J.; Yook, K.S.; Lee, J.Y. Organic Materials for Deep Blue Phosphorescent Organic Light-emitting Diodes. Adv. Mater. 2012, 24, 3169–3190.
- Zhu, M.; Yang, C. Blue Fluorescent Emitters: Design Tactics and Applications in Organic Light-Emitting Diodes. Chem. Soc. Rev. 2013, 42, 4963–4976.
- Endo, A.; Ogasawara, M.; Takahashi, A.; Yokoyama, D.; Kato, Y.; Adachi, C.; Adachi, C.; Endo, A.; Yokoyama, D.; Ogasawara, M.; et al. Thermally Activated Delayed Fluorescence from Sn4+–Porphyrin Complexes and Their Application to Organic Light Emitting Diodes—A Novel Mechanism for Electroluminescence. Adv. Mater. 2009, 21, 4802–4806.
- Cui, L.-S.; Nomura, H.; Kim, J.U.; Nakanotani, H.; Adachi, C.; Cui, L.; Geng, Y.; Kim, J.U.; Nakanotani, H.; Adachi, C.; et al. Controlling Singlet–Triplet Energy Splitting for Deep-Blue Thermally Activated Delayed Fluorescence Emitters. Angew. Chem. Int. Ed. 2017, 56, 1571–1575.
- Cai, X.; Su, S.-J.; Cai, X.Y.; Su, S.-J. Marching Toward Highly Efficient, Pure-Blue, and Stable Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes. Adv. Funct. Mater. 2018, 28, 1802558.
- Su, S.J.; Chiba, T.; Takeda, T.; Kido, J. Pyridine-Containing Triphenylbenzene Derivatives with High Electron Mobility for Highly Efficient Phosphorescent OLEDs. Adv. Mater. 2008, 20, 2125–2130.
- Cui, L.S.; Deng, Y.L.; Tsang, D.P.K.; Jiang, Z.Q.; Zhang, Q.; Liao, L.S.; Adachi, C. Controlling Synergistic Oxidation Processes for Efficient and Stable Blue Thermally Activated Delayed Fluorescence Devices. Adv. Mater. 2016, 28, 7620–7625.
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally Activated Delayed Fluorescence Materials towards the Breakthrough of Organoelectronics. Adv. Mater. 2014, 26, 7931–7958.
- Shin, H.; Lee, S.; Kim, K.H.; Moon, C.K.; Yoo, S.J.; Lee, J.H.; Kim, J.J. Blue Phosphorescent Organic Light-Emitting Diodes Using an Exciplex Forming Co-Host with the External Quantum Efficiency of Theoretical Limit. Adv. Mater. 2014, 26, 4730–4734.
- Hirata, S.; Sakai, Y.; Masui, K.; Tanaka, H.; Lee, S.Y.; Nomura, H.; Nakamura, N.; Yasumatsu, M.; Nakanotani, H.; Zhang, Q.; et al. Highly Efficient Blue Electroluminescence Based on Thermally Activated Delayed Fluorescence. Nat. Mater. 2014, 14, 330–336.
- Lee, J.; Shizu, K.; Tanaka, H.; Nomura, H.; Yasuda, T.; Adachi, C. Oxadiazole- and Triazole-Based Highly-Efficient Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes. J. Mater. Chem. C Mater. 2013, 1, 4599–4604.
- Li, W.; Pan, Y.; Xiao, R.; Peng, Q.; Zhang, S.; Ma, D.; Li, F.; Shen, F.; Wang, Y.; Yang, B.; et al. Employing ~100% Excitons in OLEDs by Utilizing a Fluorescent Molecule with Hybridized Local and Charge-Transfer Excited State. Adv. Funct. Mater. 2014, 24, 1609–1614.
- Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient Blue Organic Light-Emitting Diodes Employing Thermally Activated Delayed Fluorescence. Nat. Photonics 2014, 8, 326–332.
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Youn Lee, S.; Yasuda, T.; Adachi, C.; Zhang, Q.; et al. Nearly 100% Internal Quantum Efficiency in Undoped Electroluminescent Devices Employing Pure Organic Emitters. Adv. Mater. 2015, 27, 2096–2100.
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m -Terphenyl-Modifed Sulfone Derivative as a Host Material for High-Efficiency Blue and Green Phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406.
- Jeon, S.O.; Earmme, T.; Jenekhe, S.A. New Sulfone-Based Electron-Transport Materials with High Triplet Energy for Highly Efficient Blue Phosphorescent Organic Light-Emitting Diodes. J. Mater. Chem. C Mater. 2014, 2, 10129–10137.
- Duan, L.; Qiao, J.; Sun, Y.; Qiu, Y.; Duan, L.; Qiao, J.; Sun, Y.D.; Qiu, Y. Strategies to Design Bipolar Small Molecules for OLEDs: Donor-Acceptor Structure and Non-Donor-Acceptor Structure. Adv. Mater. 2011, 23, 1137–1144.
- Hsu, F.M.; Chien, C.H.; Hsieh, Y.J.; Wu, C.H.; Shu, C.F.; Liu, S.W.; Chen, C.T. Highly Efficient Red Electrophosphorescent Device Incorporating a Bipolar Triphenylamine/Bisphenylsulfonyl-Substituted Fluorene Hybrid as the Host. J. Mater. Chem. 2009, 19, 8002–8008.
- Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of Efficient Thermally Activated Delayed Fluorescence Materials for Pure Blue Organic Light Emitting Diodes. J. Am. Chem. Soc. 2012, 134, 14706–14709.
- Romain, M.; Tondelier, D.; Geffroy, B.; Shirinskaya, A.; Jeannin, O.; Rault-Berthelot, J.; Poriel, C. Spiro-Configured Phenyl Acridine Thioxanthene Dioxide as a Host for Efficient PhOLEDs. Chem. Commun. 2014, 51, 1313–1315.
- Lee, C.W.; Lee, J.Y. A Hole Transport Material with Ortho-Linked Terphenyl Core Structure for High Power Efficiency in Blue Phosphorescent Organic Light-Emitting Diodes. Org. Electron. 2014, 15, 399–404.
- Seo, J.A.; Gong, M.S.; Lee, J.Y. Thermally Stable Indoloacridine Type Host Material for High Efficiency Blue Phosphorescent Organic Light-Emitting Diodes. Org. Electron. 2014, 15, 3773–3779.
- Park, M.S.; Lee, J.Y. High Efficiency Green and Blue Phosphorescent Organic Light-Emitting Diodes Using Pyrroloacridine Type Hole Transport Material. Mol. Cryst. Liq. Cryst. 2013, 584, 145–152.
- Kim, M.; Lee, J.Y. Improved Power Efficiency in Deep Blue Phosphorescent Organic Light-Emitting Diodes Using an Acridine Core Based Hole Transport Material. Org. Electron. 2012, 13, 1245–1249.
- Kim, M.; Lee, J.Y. Pyridine-Modified Acridine-Based Bipolar Host Material for Green Phosphorescent Organic Light-Emitting Diodes. Chem. Asian J. 2012, 7, 899–902.
- Park, M.S.; Lee, J.Y. Indolo Acridine-Based Hole-Transport Materials for Phosphorescent OLEDs with over 20% External Quantum Efficiency in Deep Blue and Green. Chem. Mater. 2011, 23, 4338–4343.
- Seo, J.A.; Jeon, S.K.; Gong, M.S.; Lee, J.Y.; Noh, C.H.; Kim, S.H. Long Lifetime Blue Phosphorescent Organic Light-Emitting Diodes with an Exciton Blocking Layer. J. Mater. Chem. C Mater. 2015, 3, 4640–4645.
- Nakanotani, H.; Higuchi, T.; Furukawa, T.; Masui, K.; Morimoto, K.; Numata, M.; Tanaka, H.; Sagara, Y.; Yasuda, T.; Adachi, C. High-Efficiency Organic Light-Emitting Diodes with Fluorescent Emitters. Nat. Commun. 2014, 5, 4016.
- Nakao, K.; Sasabe, H.; Komatsu, R.; Hayasaka, Y.; Ohsawa, T.; Kido, J.; Nakao, K.; Sasabe, H.; Komatsu, R.; Hayasaka, Y.; et al. Significant Enhancement of Blue OLED Performances through Molecular Engineering of Pyrimidine-Based Emitter. Adv. Opt. Mater. 2017, 5, 1600843.
- Grimsdale, A.C.; Jacob, J. Polymer-Based LEDs and Solar Cells. Polym. Sci. A Compr. Ref. 2012, 8, 261–282.
- Liou, G.S.; Yen, H.J. Polyimides. Polym. Sci. A Compr. Ref. 2012, 5, 497–535.
- Wu, S.; Aonuma, M.; Zhang, Q.; Huang, S.; Nakagawa, T.; Kuwabara, K.; Adachi, C. High-Efficiency Deep-Blue Organic Light-Emitting Diodes Based on a Thermally Activated Delayed Fluorescence Emitter. J. Mater. Chem. C Mater. 2013, 2, 421–424.
- High-Efficiency Diphenylsulfon Derivative-Based Organic Light-Emitting Diode Exhibiting Thermally-Activated Delayed Fluorescence|Request PDF. Available online: https://www.researchgate.net/publication/303448146_High-efficiency_diphenylsulfon_derivative-based_organic_light-emitting_diode_exhibiting_thermally-activated_delayed_fluorescence (accessed on 15 May 2022).
- Serevičius, T.; Nakagawa, T.; Kuo, M.C.; Cheng, S.H.; Wong, K.T.; Chang, C.H.; Kwong, R.C.; Xia, S.; Adachi, C. Enhanced Electroluminescence Based on Thermally Activated Delayed Fluorescence from a Carbazole–Triazine Derivative. Phys. Chem. Chem. Phys. 2013, 15, 15850–15855.
- Kim, M.; Kyu Jeon, S.; Hwang, S.-H.; Yeob Lee, J.; Kim, M.; Hwang, S.; Jeon, S.K.; Lee, J.Y. Stable Blue Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes with Three Times Longer Lifetime than Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2015, 27, 2515–2520.
- Su, L.; Cao, F.; Cheng, C.; Tsuboi, T.; Zhu, Y.; Deng, C.; Zheng, X.; Wang, D.; Liu, Z.; Zhang, Q. High Fluorescence Rate of Thermally Activated Delayed Fluorescence Emitters for Efficient and Stable Blue OLEDs. ACS Appl. Mater. Interfaces 2020, 12, 31706–31715.
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly Efficient Organic Light-Emitting Diodes from Delayed Fluorescence. Nature 2012, 492, 234–238.
- Cho, Y.J.; Yook, K.S.; Lee, J.Y. A Universal Host Material for High External Quantum Efficiency Close to 25% and Long Lifetime in Green Fluorescent and Phosphorescent OLEDs. Adv. Mater. 2014, 26, 4050–4055.
- Masui, K.; Nakanotani, H.; Adachi, C. Analysis of Exciton Annihilation in High-Efficiency Sky-Blue Organic Light-Emitting Diodes with Thermally Activated Delayed Fluorescence. Org. Electron. 2013, 14, 2721–2726.
- Cho, Y.J.; Yook, K.S.; Lee, J.Y. Cool and Warm Hybrid White Organic Light-Emitting Diode with Blue Delayed Fluorescent Emitter Both as Blue Emitter and Triplet Host. Sci. Rep. 2015, 5, 7859.
- Lee, D.R.; Hwang, S.H.; Jeon, S.K.; Lee, C.W.; Lee, J.Y. Benzofurocarbazole and Benzothienocarbazole as Donors for Improved Quantum Efficiency in Blue Thermally Activated Delayed Fluorescent Devices. Chem. Commun. 2015, 51, 8105–8107.
- Yi, C.L.; Ko, C.L.; Yeh, T.C.; Chen, C.Y.; Chen, Y.S.; Chen, D.G.; Chou, P.T.; Hung, W.Y.; Wong, K.T. Harnessing a New Co-Host System and Low Concentration of New TADF Emitters Equipped with Trifluoromethyl- and Cyano-Substituted Benzene as Core for High-Efficiency Blue OLEDs. ACS Appl. Mater. Interfaces 2020, 12, 2724–2732.
- Numata, M.; Yasuda, T.; Adachi, C. High Efficiency Pure Blue Thermally Activated Delayed Fluorescence Molecules Having 10 H -Phenoxaborin and Acridan Units. Chem. Commun. 2015, 51, 9443–9446.
- Tsai, W.L.; Huang, M.H.; Lee, W.K.; Hsu, Y.J.; Pan, K.C.; Huang, Y.H.; Ting, H.C.; Sarma, M.; Ho, Y.Y.; Hu, H.C.; et al. A Versatile Thermally Activated Delayed Fluorescence Emitter for Both Highly Efficient Doped and Non-Doped Organic Light Emitting Devices. Chem. Commun. 2015, 51, 13662–13665.
- Li, W.; Li, W.; Gan, L.; Li, M.; Zheng, N.; Ning, C.; Chen, D.; Wu, Y.C.; Su, S.J. J-Aggregation Enhances the Electroluminescence Performance of a Sky-Blue Thermally Activated Delayed-Fluorescence Emitter in Nondoped Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2020, 12, 2717–2723.
- Yu, Y.J.; Wang, X.Q.; Liu, J.F.; Jiang, Z.Q.; Liao, L.S. Harvesting Triplet Excitons for Near-Infrared Electroluminescence via Thermally Activated Delayed Fluorescence Channel. iScience 2021, 24, 102123.
- Evaristo De Sousa, L.; Dos, L.; Born, S.; Henrique De Oliveira Neto, P.; De Silva, P. Triplet-to-Singlet Exciton Transfer in Hyperfluorescent OLED Materials. J. Mater. Chem. C Mater. 2022, 10, 4914–4922.
- Dias, F.B.; Bourdakos, K.N.; Jankus, V.; Moss, K.C.; Kamtekar, K.T.; Bhalla, V.; Santos, J.; Bryce, M.R.; Monkman, A.P. Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters. Adv. Mater. 2013, 25, 3707–3714.
- Li, Z.W.; Peng, L.Y.; Song, X.F.; Chen, W.K.; Gao, Y.J.; Fang, W.H.; Cui, G. Room-Temperature Phosphorescence and Thermally Activated Delayed Fluorescence in the Pd Complex: Mechanism and Dual Upconversion Channels. J. Phys. Chem. Lett. 2021, 12, 5944–5950.
- Mamada, M.; Inada, K.; Komino, T.; Potscavage, W.J.; Nakanotani, H.; Adachi, C. Highly Efficient Thermally Activated Delayed Fluorescence from an Excited-State Intramolecular Proton Transfer System. ACS Cent. Sci. 2017, 3, 769–777.
- Madayanad Suresh, S.; Hall, D.; Beljonne, D.; Olivier, Y.; Zysman-Colman, E.; Madayanad Suresh, S.; Hall, D.; Zysman-Colman, E.; Beljonne, D.; Olivier, Y. Multiresonant Thermally Activated Delayed Fluorescence Emitters Based on Heteroatom-Doped Nanographenes: Recent Advances and Prospects for Organic Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 1908677.
- Weissenseel, S.; Gottscholl, A.; Bönnighausen, R.; Dyakonov, V.; Sperlich, A.; Maximilian, J. Long-Lived Spin-Polarized Intermolecular Exciplex States in Thermally Activated Delayed Fluorescence-Based Organic Light-Emitting Diodes. Sci. Adv. 2021, 7, eabj9961.
- Sakai, Y.; Sagara, Y.; Nomura, H.; Nakamura, N.; Suzuki, Y.; Miyazaki, H.; Adachi, C. Zinc Complexes Exhibiting Highly Efficient Thermally Activated Delayed Fluorescence and Their Application to Organic Light-Emitting Diodes. Chem. Commun. 2015, 51, 3181–3184.
- Kanno, H.; Ishikawa, K.; Nishio, Y.; Endo, A.; Adachi, C.; Shibata, K. Highly Efficient and Stable Red Phosphorescent Organic Light-Emitting Device Using BisZinc(II) as Host Material. Appl. Phys. Lett. 2007, 90, 123509.
- Hamada, Y.; Sano, T.; Fujii, H.; Nishio, Y.; Takahashi, H.; Shibata, K. White-Light-Emitting Material for Organic Electroluminescent Devices. Jpn. J. Appl. Phys. 1996, 35, L1339.
- Norikazu, N.; Shinichi, W.; Keiichi, M.; Tsuneo, F. A Novel Blue Light Emitting Material Prepared from 2-(o-Hydroxyphenyl)Benzoxazole. Chem. Lett. 2006, 23, 1741–1742.
- Osawa, M. Highly Efficient Blue-Green Delayed Fluorescence from Copper(i) Thiolate Complexes: Luminescence Color Alteration by Orientation Change of the Aryl Ring. Chem. Commun. 2014, 50, 1801–1803.
- Kang, L.; Chen, J.; Teng, T.; Chen, X.L.; Yu, R.; Lu, C.Z. Experimental and Theoretical Studies of Highly Emissive Dinuclear Cu(i) Halide Complexes with Delayed Fluorescence. Dalton Trans. 2015, 44, 11649–11659.
- Zhang, Q.; Chen, J.; Wu, X.Y.; Chen, X.L.; Yu, R.; Lu, C.Z. Outstanding Blue Delayed Fluorescence and Significant Processing Stability of Cuprous Complexes with Functional Pyridine–Pyrazolate Diimine Ligands. Dalton Trans. 2015, 44, 6706–6710.
- Zink, D.M.; Bächle, M.; Baumann, T.; Nieger, M.; Kühn, M.; Wang, C.; Klopper, W.; Monkowius, U.; Hofbeck, T.; Yersin, H.; et al. Synthesis, Structure, and Characterization of Dinuclear Copper(I) Halide Complexes with P^N Ligands Featuring Exciting Photoluminescence Properties. Inorg. Chem. 2013, 52, 2292–2305.
- Deaton, J.C.; Switalski, S.C.; Kondakov, D.Y.; Young, R.H.; Pawlik, T.D.; Giesen, D.J.; Harkins, S.B.; Miller, A.J.M.; Mickenberg, S.F.; Peters, J.C. E-Type Delayed Fluorescence of a Phosphine-Supported Cu 2(μ-Nar 2) 2 Diamond Core: Harvesting Singlet and Triplet Excitons in OLEDs. J. Am. Chem. Soc. 2010, 132, 9499–9508.
- Igawa, S.; Hashimoto, M.; Kawata, I.; Yashima, M.; Hoshino, M.; Osawa, M. Highly Efficient Green Organic Light-Emitting Diodes Containing Luminescent Tetrahedral Copper(i) Complexes. J. Mater. Chem. C Mater. 2012, 1, 542–551.
- Osawa, M.; Kawata, I.; Ishii, R.; Igawa, S.; Hashimoto, M.; Hoshino, M. Application of Neutral d 10 Coinage Metal Complexes with an Anionic Bidentate Ligand in Delayed Fluorescence-Type Organic Light-Emitting Diodes. J. Mater. Chem. C Mater. 2013, 1, 4375–4383.
- Volz, D.; Zink, D.M.; Bocksrocker, T.; Friedrichs, J.; Nieger, M.; Baumann, T.; Lemmer, U.; Bräse, S. Molecular Construction Kit for Tuning Solubility, Stability and Luminescence Properties: Heteroleptic MePyrPHOS-Copper Iodide-Complexes and Their Application in Organic Light-Emitting Diodes. Chem. Mater. 2013, 25, 3414–3426.
- Ma, Y.; Che, C.M.; Chao, H.Y.; Zhou, X.; Chan, W.H.; Shen, J. High Luminescence Gold(I) and Copper(I) Complexes with a Triplet Excited State for Use in Light-Emitting Diodes. Adv. Mater. 1999, 11, 852–857.
- Ma, Y.G.; Chan, W.H.; Zhou, X.M.; Che, C.M. Light-Emitting Diode Device from a Luminescent Organocopper(I) Compound. New J. Chem. 1999, 23, 263–265.
- Cu(I) Complexes–Thermally Activated Delayed Fluorescence. Photophysical Approach and Material Design|Request PDF. Available online: https://www.researchgate.net/publication/304779001_CuI_complexes_-_Thermally_activated_delayed_fluorescence_Photophysical_approach_and_material_design (accessed on 17 May 2022).
- Tomé, L.I.N.; Domínguez-Pérez, M.; Cláudio, A.F.M.; Freire, M.G.; Marrucho, I.M.; Oscar Cabeza, O.C.; Coutinho, J.A.P. On the Interactions between Amino Acids and Ionic Liquids in Aqueous Media. J. Phys. Chem. B 2009, 113, 13971–13979.
- Housecroft, C.E.; Constable, E.C. TADF: Enabling Luminescent Copper(I) Coordination Compounds for Light-Emitting Electrochemical Cells. J. Mater. Chem. C Mater. 2022, 10, 4456–4482.
- Teng, T.; Xiong, J.; Cheng, G.; Zhou, C.; Lv, X.; Li, K. Solution-Processed Oleds Based on Thermally Activated Delayed Fluorescence Copper(I) Complexes with Intraligand Charge-Transfer Excited State. Molecules 2021, 26, 1125.
- Lv, L.L.; Yuan, K.; Wang, Y.C. Theoretical Studying of Basic Photophysical Processes in a Thermally Activated Delayed Fluorescence Copper(I) Complex: Determination of Reverse Intersystem Crossing and Radiative Rate Constants. Org. Electron. 2017, 51, 207–219.
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) Complexes–Thermally Activated Delayed Fluorescence. Photophysical Approach and Material Design. Coord. Chem. Rev. 2016, 325, 2–28.
- Czerwieniec, R.; Yersin, H. Diversity of Copper(I) Complexes Showing Thermally Activated Delayed Fluorescence: Basic Photophysical Analysis. Inorg. Chem. 2015, 54, 4322–4327.
- Linfoot, C.L.; Leitl, M.J.; Richardson, P.; Rausch, A.F.; Chepelin, O.; White, F.J.; Yersin, H.; Robertson, N. Thermally Activated Delayed Fluorescence (TADF) and Enhancing Photoluminescence Quantum Yields of + Complexes-Photophysical, Structural, and Computational Studies. Inorg. Chem. 2014, 53, 10854–10861.
- Xu, H.; Yang, T.; Wang, F.; Zhang, J.; Zhang, X.; Wang, H.; Xu, B. Thermally Activated Delayed Fluorescence of Copper(I) Complexes Using N, N′-Heteroaromatic of 2-(5-Phenyl-1,2,3-Triazole)Pyridine as Ligand. J. Lumin. 2019, 205, 82–86.
- Stoïanov, A.; Gourlaouen, C.; Vela, S.; Daniel, C. Luminescent Dinuclear Copper(I) Complexes as Potential Thermally Activated Delayed Fluorescence (TADF) Emitters: A Theoretical Study. J. Phys. Chem. A 2018, 122, 1413–1421.
- Leitl, M.J.; Zink, D.M.; Schinabeck, A.; Baumann, T.; Volz, D.; Yersin, H. Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties. Top. Curr. Chem. 2016, 374, 25.
- Leitl, M.J.; Krylova, V.A.; Djurovich, P.I.; Thompson, M.E.; Yersin, H. Phosphorescence versus Thermally Activated Delayed Fluorescence. Controlling Singlet-Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds. J. Am. Chem. Soc. 2014, 136, 16032–16038.
- Chen, X.L.; Yu, R.; Zhang, Q.K.; Zhou, L.J.; Wu, X.Y.; Zhang, Q.; Lu, C.Z. Rational Design of Strongly Blue-Emitting Cuprous Complexes with Thermally Activated Delayed Fluorescence and Application in Solution-Processed OLEDS. Chem. Mater. 2013, 25, 3910–3920.
- Czerwieniec, R.; Yu, J.; Yersin, H. Blue-Light Emission of Cu(I) Complexes and Singlet Harvesting. Inorg. Chem. 2011, 50, 8293–8301.
- Lee, C.F.; Chin, K.F.; Peng, S.M.; Che, C.M. A Luminescent Tetrameric Zinc(II) Complex Containing the 7-Azaindolate Ligand. Photophysical Properties and Crystal Structure. J. Chem. Soc. Dalton Trans. 1993, 3, 467–470.
- Yu, G.; Yin, S.; Liu, Y.; Shuai, Z.; Zhu, D. Structures, Electronic States, and Electroluminescent Properties of a Zinc(II) 2-(2-Hydroxyphenyl)Benzothiazolate Complex. J. Am. Chem. Soc. 2003, 125, 14816–14824.
- Xu, X.; Liao, Y.; Yu, G.; You, H.; Di, C.; Su, Z.; Ma, D.; Wang, Q.; Li, S.; Wang, S.; et al. Charge Carrier Transporting, Photoluminescent, and Electroluminescent Properties of Zinc(II)-2-(2-Hydroxyphenyl)Benzothiazolate Complex. Chem. Mater. 2007, 19, 1740–1748.
- Singh, S.P.; Mohapatra, Y.N.; Qureshi, M.; Manoharan, S.S. White Organic Light-Emitting Diodes Based on Spectral Broadening in Electroluminescence Due to Formation of Interfacial Exciplexes. Appl. Phys. Lett. 2005, 86, 113505.
- Hao, Y.; Meng, W.; Xu, H.; Wang, H.; Liu, X.; Xu, B. White Organic Light-Emitting Diodes Based on a Novel Zn Complex with High CRI Combining Emission from Excitons and Interface-Formed Electroplex. Org. Electron. 2011, 12, 136–142.
- Roh, S.G.; Kim, Y.H.; Seo, K.D.; Lee, D.H.; Kim, H.K.; Park, Y.I.; Park, J.W.; Lee, J.H. Synthesis, Photophysical, and Electroluminescent Device Properties of Zn(II)-Chelated Complexes Based on Functionalized Benzothiazole Derivatives. Adv. Funct. Mater. 2009, 19, 1663–1671.
- Li, Z.; Dellali, A.; Malik, J.; Motevalli, M.; Nix, R.M.; Olukoya, T.; Peng, Y.; Ye, H.; Gillin, W.P.; Hernández, I.; et al. Luminescent Zinc(II) Complexes of Fluorinated Benzothiazol-2-Yl Substituted Phenoxide and Enolate Ligands. Inorg. Chem. 2013, 52, 1379–1387.
- Wang, R.; Deng, L.; Fu, M.; Cheng, J.; Li, J. Novel Zn II Complexes of 2-(2-Hydroxyphenyl)Benzothiazoles Ligands: Electroluminescence and Application as Host Materials for Phosphorescent Organic Light-Emitting Diodes. J. Mater. Chem. 2012, 22, 23454–23460.
- Son, H.J.; Han, W.S.; Chun, J.Y.; Kang, B.K.; Kwon, S.N.; Ko, J.; Su, J.H.; Lee, C.; Sung, J.K.; Sang, O.K. Generation of Blue Light-Emitting Zinc Complexes by Band-Gap Control of the Oxazolylphenolate Ligand System: Syntheses, Characterizations, and Organic Light Emitting Device Applications of 4-Coordinated Bis(2-Oxazolylphenolate) Zinc(II) Complexes. Inorg. Chem. 2008, 47, 5666–5676.
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet Exciton Confinement in Green Organic Light-Emitting Diodes Containing Luminescent Charge-Transfer Cu(I) Complexes. Adv. Funct. Mater. 2012, 22, 2327–2336.
- Hashimoto, M.; Igawa, S.; Yashima, M.; Kawata, I.; Hoshino, M.; Osawa, M. Highly Efficient Green Organic Light-Emitting Diodes Containing Luminescent Three-Coordinate Copper(I) Complexes. J. Am. Chem. Soc. 2011, 133, 10348–10351.
- Cheng, G.; So, G.K.M.; To, W.P.; Chen, Y.; Kwok, C.C.; Ma, C.; Guan, X.; Chang, X.; Kwok, W.M.; Che, C.M. Luminescent Zinc(II) and Copper(I) Complexes for High-Performance Solution-Processed Monochromic and White Organic Light-Emitting Devices. Chem. Sci. 2015, 6, 4623–4635.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
19 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




