Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anton R. Egorov | -- | 3805 | 2023-09-20 12:52:07 | | | |
| 2 | Rita Xu | -5 word(s) | 3800 | 2023-09-21 04:59:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Egorov, A.R.; Kirichuk, A.A.; Rubanik, V.V.; Tskhovrebov, A.G.; Kritchenkov, A.S. Antibacterial Activity of Chitosan and Its Derivatives. Encyclopedia. Available online: https://encyclopedia.pub/entry/49422 (accessed on 07 February 2026).
Egorov AR, Kirichuk AA, Rubanik VV, Tskhovrebov AG, Kritchenkov AS. Antibacterial Activity of Chitosan and Its Derivatives. Encyclopedia. Available at: https://encyclopedia.pub/entry/49422. Accessed February 07, 2026.
Egorov, Anton R., Anatoly A. Kirichuk, Vasili V. Rubanik, Alexander G. Tskhovrebov, Andreii S. Kritchenkov. "Antibacterial Activity of Chitosan and Its Derivatives" Encyclopedia, https://encyclopedia.pub/entry/49422 (accessed February 07, 2026).
Egorov, A.R., Kirichuk, A.A., Rubanik, V.V., Tskhovrebov, A.G., & Kritchenkov, A.S. (2023, September 20). Antibacterial Activity of Chitosan and Its Derivatives. In Encyclopedia. https://encyclopedia.pub/entry/49422
Egorov, Anton R., et al. "Antibacterial Activity of Chitosan and Its Derivatives." Encyclopedia. Web. 20 September, 2023.
Copy Citation
This research illuminates the various methods of chitosan extraction, its antibacterial properties, and its multifarious applications in diverse sectors. The choice of method is dictated by multiple variables, including the desired properties of chitosan, resource availability, cost, and environmental footprint.
chitosan
films
nanoparticles
1. Introduction
Chitosan, a biocompatible, biodegradable and bioactive aminopolysaccharide derived from chitin, has generated significant interest in various fields, particularly due to its remarkable antibacterial properties. This natural biopolymer finds extensive applications in diverse areas such as food preservation, biomedicine, cosmetics, water treatment, and agriculture. The contribution of chitosan to the medical field is especially great, e.g., as antibacterial gels [1], biosensors [2], antitumor systems [3], antioxidants [4], antifungal agents [5], anti-neurodegenerative systems [6] and a lot more. The review then delves into the antibacterial activity of chitosan, exploring the various factors influencing its antimicrobial effectiveness, and presents the key chitosan-based antibacterial materials currently in use, including films, nanoparticles, nonwoven materials, and hydrogels. It also investigates the role of chitosan’s inherent structural and physicochemical properties, the characteristics of targeted microorganisms, and environmental conditions in shaping its antimicrobial properties. A detailed examination of the interaction between chitosan’s cationic density, molecular weight, water solubility, and pH, and their consequent influence on its antibacterial potency is conducted.
2. Antibacterial Activity of Chitosan and Its Derivatives
2.1. Mechanism of Antibacterial Effect
The antibacterial activity of chitosan and its derivatives is contingent upon a myriad of factors that can be categorized into three main groups:
-
The inherent structural and physicochemical traits of chitosan, which include its molecular weight and distribution, cationic density, degree of deacetylation, and balance between hydrophilic and hydrophobic properties;
-
The specific type and strain of the microorganism targeted by the chitosan;
-
Various environmental conditions, such as ionic strength, pH, temperature, etc.
The scientific literature presents three primary models elucidating the antimicrobial mechanisms of chitosan. The first model places emphasis on the interaction between positively-charged macromolecules and the negatively-charged cell surface. The second model centers on the penetration of chitosan molecules into the interior of the cell. The third model highlights the chelation by chitosan of pivotal metal ions essential for bacterial survival.
2.1.1. Model Focusing on the Interaction of a Polycation with Anionic Sites on the Bacterial Cell Surface
The first model primarily focuses on the interaction between the positively-charged chitosan polycation and negatively-charged segments of the microbial cell surface. This interaction is mediated by Coulombic electrostatic forces between the protonated amine groups of chitosan (NH3+) and negatively-charged residues on the surface of the bacterial cell. Moreover, the protonated amine groups of chitosan compete effectively even with Ca2+ ions, which, in conventional conditions, electrostatically interact with the negatively-charged areas of the bacteria surface. This electrostatic interaction of chitosan with bacterial surface and the displacement of Ca2+ ions lead to at least two adverse effects on the microbial cell:
-
A significant alteration in membrane permeability properties, which induces an internal osmotic imbalance which can, finally, result in breakage of the bacterial cell wall;
-
the hydrolytic breakdown of peptidoglycans in the microorganism’s cell wall, resulting in the leakage of intracellular content into the environment.
This model was meticulously examined in various studies. The adhesion of chitosan to the bacterial cell surface can be directly visualized using scanning electron microscopy or transmission electron microscopy [7]. Amorim et al. [8] performed ultrastructural analyses of the clinical isolates S. aureus and E. coli by transmission electron microscopy before and after chitosan treatment. They observed that chitosan effectively adheres to the surface of bacterial cells and causes the bacterial cell wall to rupture. Zhenzhen Zhang et al. used scanning electron microscopy and revealed that a novel cationic chitosan derivative, 3,6-O-[N-(2-aminoethyl)-acetamide-yl]-chitosan, kills bacteria by disrupting their membranes [9]. Similar results were obtained by Jenny Kim’s group, who confirmed that ultrastructural alterations in P. acnes cells were identified under the influence of positively-charged chitosan-based nanoparticles. They detected chitosan molecules attached to bacterial cell surfaces [10].
In addition to the direct method of microscopic observation of bacterial membrane rupture, a very convenient spectrophotometric method for observing this process is described in the literature. This approach is based on the following fact. If the antibacterial system can damage the membrane of the bacterial cell, then the contents of the bacterial cell leak into the external environment. This, in turn, results in a strong increase in adsorption at 260 nm. Using this approach, it was confirmed that chitosan and many of its cationic derivatives (triazole betaine [11], betainic- and sulfur-containing betaine derivatives [12][13], trimethylaminobenzyl [14], and many others) effectively destroy the integrity of the bacterial cell wall.
2.1.2. Model Focusing on the Penetration of Chitosan Polycation into the Bacterial Cell
Another proposed mechanism involves chitosan binding to microbial DNA, which inhibits the protein synthesis cascade. It is clear that such a model requires the penetration of chitosan macromolecules into the cells of microorganisms [15][16]. It should be noted that only oligomeric molecules of chitosan can traverse the bacterial cell wall and reach the intracellular space. Studies using confocal laser scanning microscopy [17][18][19][20][21][22] have confirmed the presence of chitosan oligomers (chains with a small number of monomeric links) within E. coli cells. However, despite this model being accepted as a possible mechanism, the likelihood of its realization is considered significantly lower compared with the first model, even for chitosan oligomers [23]. The prevailing statement is that chitosan acts as a membrane disruptor rather than a penetrating substance [23][24].
2.1.3. Model Focusing on Chelating by Chitosan Metal Ions
The third model posits that chitosan acts as an agent that chelates metal ions and binds cations necessary for microbial growth [25][26]. Chitosan exhibits a high chelating capacity for various metal ions (including Ni2+, Zn2+, Co2+, Fe3+, Mg2+ and Cu2+) and is widely used for the removal or extraction of metal ions in various industries [27]. Metal ions that are linked to the molecules of a microorganism’s cell wall through coordination and ionic interactions are crucial for the stability of the cell wall. Chitosan-mediated chelation of such metal ions is often associated with a possible form of antimicrobial action [28]. It is worth noting that the coordination binding of metal cations via chelation is facilitated by free non-protonated amino groups in chitosan molecules [29].
The structure of complexes of chitosan with metal cations can be different, and this very strongly depends on the degree of deacetylation of chitosan, its molecular weight and pH, the nature and oxidation state of the metal center, as well as on the molar ratio of chitosan and the metal cation [30][31][32][33][34][35]. In Scheme 1, researchers show one of the possible structures of a coordination compound that includes chitosan as a macromolecular ligand [36].

Scheme 1. Complex of chitosan with a divalent metal ion.
Nonetheless, the antimicrobial mechanism of chitosan proposed in the third model appears to be of secondary significance. This is due to the fact that the amino groups available for coordinating interactions with metal centers are somewhat constrained at pH levels relevant to the infection process, and the formation of complexes attains saturation based on the concentration of metals.
Drawing from the extensive experience and contemplation spanning numerous years, researchers hold the view that the foremost mechanism underpinning the antibacterial efficacy of chitosan hinges on the electrostatic interaction between the cationic chitosan macromolecule and the negatively-charged sites on the bacterial cell membrane. The second and third models, while insightful and pertinent, should be regarded as supplementary and intriguing yet not supplant the primary significance of the first model.
2.2. The Effect of Cationic Density and Ways to Increase It
High cationic density in the macromolecules of chitosan and its derivatives leads to a strong electrostatic interaction with the negatively-charged segments of the bacterial surface [37][38]. The cationic density of chitosan macromolecules is determined by its degree of deacetylation [39]. The highest cationic density is characteristic for chitosans with the highest degree of deacetylation because it is the deacetylated free amino groups of chitosan that can undergo effective protonation, forming positively-charged NH3+ fragments [40]. For this reason, chitosans with a high degree of deacetylation have significantly greater antibacterial activity against both Gram-positive and Gram-negative bacteria compared with chitosans with a low degree of deacetylation [41].
Increasing the cationic density of chitosan can be achieved using two strategies: (1) increasing the degree of deacetylation; (2) introducing into the chitosan chain substituents containing cationic fragments. Using the second strategy results in derivatives of chitosan that typically possess greater antibacterial effectiveness than the original chitosan [42][43].
The main advantage of chitosan over other polysaccharides (cellulose, starch, carrageenan, alginic and hyaluronic acids, etc.) lies in the much greater ease of its chemical modification [44]. The presence of an amino group along with the primary alcohol function allows N-substituted, O-modified or N,O-substituted derivatives of chitosan to be obtained (Scheme 2). Chitosan derivatization is primarily carried out to improve its physicochemical and biological properties. For example, N-quaternized chitosan has excellent water solubility and significantly greater antibacterial activity compared with starting chitosan, N-substituted tetrazole [45], triazole [46], azide [47], selenodiazole [43], oxadiazole [48], and many other derivatives. In fact, the literature describes significantly more examples of N-substituted antibacterial chitosan derivatives than O-substituted ones. This is clearly due to the greater reactivity of the amino group compared with the hydroxyl group (for example, the nucleophilic properties of the amino group are much greater than the nucleophilic ability of the OH group).

Scheme 2. Chitosan derivatization.
Figure 1 showcases instances of substituents introduced into the chitosan backbone through N-substitution, aimed at crafting exceptionally potent antibacterial derivatives. These illustrations provide a broad overview of the extensive array of chemical structures exhibited by these substituents.

Figure 1. Examples of chemical structures of substituents of antibacterial chitosan N-derivatives.
Indeed, if chitosan is treated with an alkylating reagent, for example, diethylaminoethyl chloride, then under conventional conditions, the substitution occurs at both nucleophilic centers, with a significant predominance of N-substitution [49][50][51]. There are certain strategies for controlling this synthetic process that allow for the preparation of chitosan derivatives selectively at the desired reaction center with the desired degree of substitution. To selectively obtain N-substituted antibacterial chitosan derivatives, the pH of the reaction medium should be lowered to strongly acidic values. This approach leads to a sharp loss of the nucleophilic power of the hydroxyl group and only a slight decrease in the nucleophilicity of the amino group, that is, it has a differentiating effect [51]. If researchers aim to obtain selectively O-substituted polymers, then a synthetic strategy based on the protection of the amino group should be used [52][53][54][55]. The benzylidene protection of the amino group stands out for its high level of effectiveness and practical convenience in preparation. This remarkably straightforward synthetic process entails treating chitosan with an excess of benzaldehyde. Rapidly forming a Schiff base, this intermediate undergoes a subsequent reaction with an alkylating reagent via the hydroxyl group, yielding the intended O-derivative. Subsequently, a straightforward and swift deprotection step is executed through the familiar acid hydrolysis of the Schiff base. These well-established methodologies within chitosan chemistry are succinctly illustrated in Scheme 3.
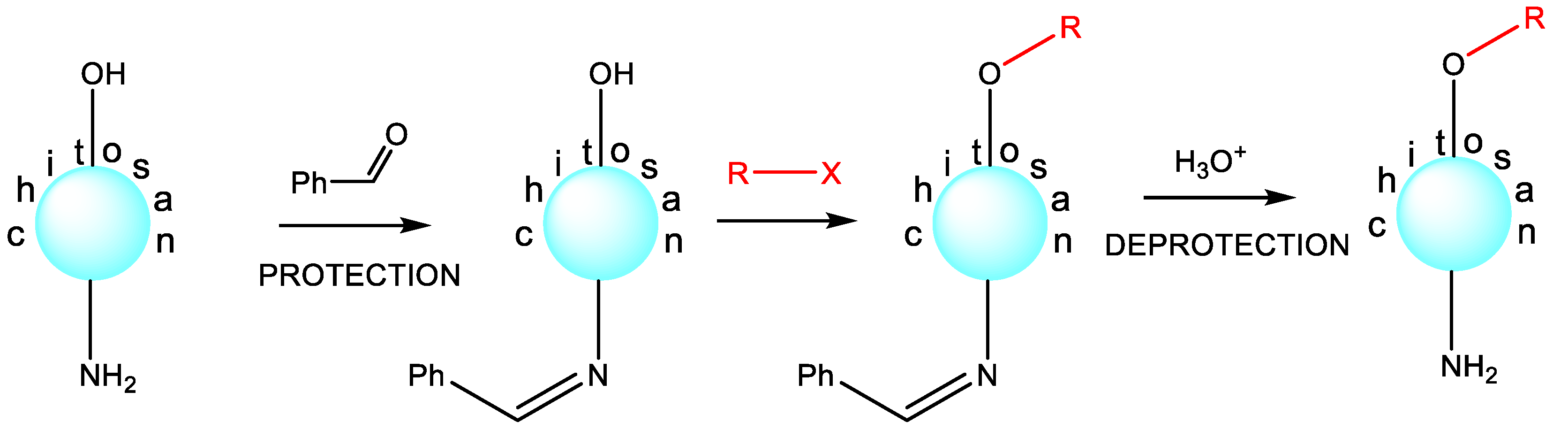
Scheme 3. Selective O-substitution.
2.3. The Influence of Molecular Weight on the Antibacterial Effect
It can be argued that there is a relationship known as “the molecular weight of chitosan—the antibacterial effect”, even though, it seemed, not so long ago, that such a statement was only a crude hypothesis. Indeed, these correlations exist, but to this day, they cannot be called unambiguous. There are examples in the literature illustrating the increased activity of low-molecular-weight chitosan against E. coli compared with high-molecular-weight chitosan. However, there are absolutely opposite examples regarding the same bacterium [56]. For example, the literature reported no effect of the molecular weight of chitosan on the antibacterial activity against the same E. coli [57]. These seemingly contradictory results obviously suggest that the question of the influence of molecular weight on activity is a complex issue and requires an individual solution in each specific case.
The molecular weight of chitosan is an extremely important factor that makes it possible for this polymer to penetrate into a bacterial cell [58]. This is important for the development of the antibacterial effect, according to the second model. The cell wall of bacteria is a very reliable barrier that prevents the penetration of undesired external substances into the bacterial cell. In general, when a bacterial cell appears, either cases of disease (ionic pumps, clathrin-mediated endocytosis, etc.) or ordinary diffusion through the pores occur [40][59][60]. Proteins with a molecular weight of 50–100 kDa readily penetrate through the pores of a bacterial cell [61]. Chitosans of low molecular weight (50–100 kDa, hydrodynamic diameter about 50 nm) have also been detected through bacterial pores [62].
In summary, the role of molecular weight in chitosan’s antibacterial properties is significant, with various factors contributing to its effectiveness:
-
Surface activity: smaller chitosan particles with a higher surface area-to-volume ratio exhibit enhanced surface activity, facilitating interactions with bacterial cells;
-
Penetration: smaller particles can more readily breach bacterial cell walls, disrupting their functionality and inducing cell demise;
-
Diffusion and dispersibility: smaller chitosan particles disperse and diffuse more effectively in solutions, leading to uniform distribution and improved contact with bacterial cells, thereby enhancing antibacterial action.
The discussed examples and rationale underline that, while the overarching trend suggests that smaller chitosan particles (lower molecular mass) possess heightened antibacterial effects, specific outcomes can hinge on diverse factors, encompassing bacteria type, pH levels, temperature, and experimental conditions. Consequently, refining the molecular weight of chitosan for optimal antibacterial applications often necessitates meticulous study and experimentation.
2.4. Evaluating the Interplay of Water Attraction and Repulsion and Solubility in Antibacterial Potency
Within the realm of antibacterial agents, especially those boasting a polymeric architecture, water takes on a pivotal role in orchestrating their antimicrobial functions. The foundation of this is the incapability of completely desiccated forms to unleash the stored energy intrinsic to their chemical structures, energy that is required for interaction with bacteria. This context converges seamlessly with the fact that bacteria, whether in in vitro or in vivo settings, invariably reside in contact with water. This dynamic sets the stage for the equilibrium between hydrophilic (water-attracting) and hydrophobic (water-repelling) traits of an antimicrobial agent to emerge as a fundamental driver of its functional mechanism and efficacy.
Chitosan, poised as a prospective contender in the realm of antibacterial weaponry, finds its solubility in water under strict governance by its inherently hydrophilic essence. Yet, lamentably, the inherent water solubility of chitosan trails on the side of inadequacy, erecting a barrier to its widespread adoption in the ambit of antibacterial applications [63].
Enhancing chitosan’s solubility through chemical alteration is a promising approach, offering the potential to widen its use as an antibacterial compound [64]. Thus, the pursuit of water-compatible chitosan and its derivative forms has become a crucial goal in antimicrobial research. Various chemical modification techniques like embedding mono- or oligosaccharide units into the chitosan structure, alkylation, acylation, quaternization, and metallization have been used to reach this objective. For example, one can obtain a quaternized version of chitosan, specifically its ammonium salt, by incorporating a quaternary ammonium group into the chitosan backbone. One notable study demonstrated that a quaternized chitosan variant, N,N,N-trimethylammonium chitosan chloride, had superior antibacterial capabilities, a wider spectrum of antimicrobial activity, and an accelerated rate of bacterial cell destruction than its unmodified counterpart [15][65][66].
Nevertheless, this is not to suggest that quaternized chitosan always outperforms in terms of antibacterial potency. It has been documented that integrating an N,N-dimethylaminobenzyl fragment or an N-pyridylmethyl group into the chitosan backbone did not boost antibacterial activity against S. aureus compared with the original chitosan [67]. Interestingly, even when these polymers showed a higher degree of quaternization than N,N,N-trimethylammonium chitosan chloride, their antibacterial effectiveness against S. aureus remained stagnant. This evidence illustrates that an increased level of N-substitution with N,N-dimethylaminobenzyl substituents can disrupt the hydrophilic–hydrophobic balance and lessen the likelihood of interaction between these chitosan variants and the bacterial cell wall. Similarly, Liu et al. elaborated highly antibacterial systems based on p-coumaric-acid-modified quaternized chitosan [68].
The antimicrobial potential of chitosan derivatives is significantly influenced by the size and characteristics of the spacer, given the consequential modifications to the polymer’s shape and charge density. Such modifications also alter its interaction mechanism with the cytoplasmic membrane [69]. In this context, the hydrophobic characteristics inherent to N-substituted chitosan confer a beneficial edge to the interaction between the polymer molecule and bacterial entities. An illustrative instance lies in N-hexadecanyl chitosan, the antimicrobial prowess of which was assessed against E. coli, P. aeruginosa, S. aureus, and B. cereus. Across the spectrum of scrutinized microorganisms, the antibacterial efficacy of these innovative alkyl derivatives surged notably beyond that of the initial chitosan. This augmentation finds its rationale in the hydrophobic nature of the introduced substituent, which engenders heightened antibacterial attributes [70].
Another study directed its focus on the potentiation of the antimicrobial influence of the low-molecular-weight derivative, namely mercaptoundecanoic-acid-grafted chitosan, alongside their corresponding nanoparticles loaded with carvacrol. This augmentation of antibacterial efficacy was ascribed to the hydrophobic aryl substituent’s role in expediting the derivative’s assimilation into bacterial cells. This, in turn, accentuated the hydrophobic interaction between the derivative and the cell, yielding an intensified antibacterial effect [71].
In a recent investigation, the hydrophobic attributes of chitosan underwent enhancement while its antibacterial efficacy was upheld through the grafting of dodecenylsuccinyl chains onto phthaloylchitosan, primarily targeting the C-6 position of the glucopyranose cyclic chain. The synthesis of dodecenylsuccinylated phthaloylchitosan was accomplished through a sequential process involving phthaloylation, dodecenylsuccinylation, and subsequent hydrazinolysis. This chemical alteration of chitosan yielded a remarkable surge in antibacterial potency specifically against Gram-positive bacteria.
A film derived from a solution of this chitosan derivative exhibited superior inhibition of bacterial growth and a more effective vapor barrier compared with a film composed of the unmodified chitosan. As a result, the authors propose the application of this refined film as a coating for perishable food products, thereby augmenting their shelf life by inhibiting bacterial proliferation [72].
These observations find further affirmation in the following insight: the impact of acyl group length on antibacterial effectiveness becomes evident when hindering the growth of E. coli with acyl chitosan derivatives. The elevation in antibacterial activity attributed to acyl groups is likely grounded in the heightened hydrophobicity conferred by the acyl-substituted polymer. For instance, N-hexanoylchitosan sulfate, characterized by a more pronounced hydrophobic character due to its longer acyl chain (six carbon atoms), exhibited a more robust inhibitory effect in comparison to the relatively less hydrophobic N-propanoylchitosan sulfate (three carbon atoms) [73].
Chitosan’s solubility in water significantly impacts its antibacterial activity:
-
Amino Groups and Solubility. Chitosan’s amino groups can be protonated, enabling them to dissolve in water, especially under acidic conditions;
-
Molecular Weight Influence. Lower-molecular-weight chitosan tends to be more soluble in water, which can lead to better dispersion and increased interaction with bacterial cells;
-
Antibacterial Activity. Higher solubility increases chitosan’s bioavailability and therefore its antibacterial activity.
However, why are water-soluble forms of chitosan characterized by a significantly greater antibacterial effect? The following explanation seems logical and reasonable. In their dissolved state, soluble chitosan and its cationic counterparts adopt a disassociated, lengthened structure. This particular shape allows for more effective engagement with the bacterial cell surface [74]. This underpins the superior antibacterial capabilities of soluble chitosan derivatives compared with those in insoluble forms.
Indeed, water solubility stands as a pivotal property of chitosan intricately linked to its antibacterial efficacy. This association underscores why chitosan derivatives or modifications are frequently engineered to enhance this characteristic, catering to distinct applications. Nevertheless, the interplay between chitosan’s solubility and its antibacterial activity is intricate and can be influenced by a myriad of other factors, including pH, temperature, and the precise bacterial strain under scrutiny.
Consequently, a continuous and evolving research endeavor is imperative to comprehensively fathom and refine this facet of chitosan’s antibacterial attributes. This pursuit is geared toward unraveling the nuanced dynamics and optimizing the interplay between solubility and antibacterial potency, enabling the harnessing of chitosan’s potential across diverse applications.
2.5. Effect of pH on Chitosan Solubility and Its Antibacterial Activity
Researchers have already emphasized that even high-molecular-weight chitosan in acidic environments becomes soluble. This solubility exposes its positively-charged amino groups (−NH3+), which interact with the negatively-charged components of bacterial cell walls. As a result, it damages the bacterial cell wall, disrupting metabolism and leading to the death of the bacteria. Therefore, chitosan’s antibacterial activity is high under acidic conditions [75].
Conversely, when confronted with higher pH levels, the solubility of chitosan diminishes, subsequently curbing its potential to engage with bacterial cells. Consequently, as the environmental pH escalates, the antibacterial efficacy of chitosan experiences a decline.
The permeability of the bacterial cell wall stands as a pivotal determinant in the efficacy of antibacterial agents. This significance stems from the composition and role of the bacterial cell wall, which serves as a protective barricade against detrimental agents. Amplifying the permeability of the bacterial cell wall holds the consequence of rendering it more penetrable to antibacterial agents, enabling them to more effectively infiltrate the bacterial cell and perturb its functions. This mechanistic principle frequently underlies the mode of action for numerous antibiotics and antimicrobial substances [76].
In the case of chitosan, an increase in the permeability of the bacterial cell wall is one of the key mechanisms by which it exerts its antibacterial activity. The positively-charged amino groups of chitosan can interact with the negatively-charged components of the bacterial cell wall, leading to increased permeability [77]. This increased permeability disrupts the balance of substances entering and exiting the bacterial cell, leading to cell damage and death [78][79]. It is important to note that different bacterial species may have varying levels of cell wall permeability, influencing their susceptibility to different antibacterial agents, including chitosan.
Chitosan can affect bacterial metabolism, a crucial aspect of its antibacterial activity. When chitosan interacts with bacterial cells, it can disrupt normal metabolic processes that are essential for the survival and proliferation of the bacteria [79]. The disruption of these processes can occur in several ways. For example, chitosan can interfere with the synthesis of proteins and nucleic acids, impede energy production processes, or disrupt the balance of ions across the cell membrane. Each of these impacts can lead to a decrease in the bacterial cell’s viability [80][81]. Moreover, chitosan’s ability to increase the permeability of the bacterial cell wall can also contribute to these metabolic disruptions. This is because an increase in cell wall permeability can lead to an imbalance in the flow of substances into and out of the cell, which can disrupt normal cellular metabolism [81].
Undoubtedly, the coagulation process assumes a role in chitosan’s antibacterial efficacy. Coagulation, characterized by the aggregation of particles, can be provoked when chitosan interacts with bacterial cells. Under acidic conditions, chitosan has the capacity to trigger the coagulation of proteins. These coagulated protein clusters wield the potential to disrupt the integrity of the bacterial cell membrane, hamper the microorganism’s metabolic functions, or even culminate in cell lysis [82]. Furthermore, the aggregation of coagulated proteins can give rise to a barricade that obstructs bacteria from accessing essential nutrients or expelling waste products. This consequential hindrance to vital processes can significantly contribute to the bacteria’s eventual demise.
References
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistarà, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293.
- Chauhan, S.; Thakur, A. Chitosan-Based Biosensors-A Comprehensive Review. Mater. Today Proc. 2023, in press.
- Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942.
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan, and their derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31.
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332.
- De Gaetano, F.; d’Avanzo, N.; Mancuso, A.; De Gaetano, A.; Paladini, G.; Caridi, F.; Venuti, V.; Paolino, D.; Ventura, C.A. Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone. Pharmaceuticals 2022, 15, 1206.
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173.
- Gaspar, M.E.d.F.A.; Peixoto, C.A.; da Silva Amorim, R.V. Ultrastructural analysis of chitosan antibacterial activity against clinical isolates of Staphylococcus aureus and Escherichia coli. Adv. Microbiol. 2019, 9, 893–903.
- Yan, F.; Dang, Q.; Liu, C.; Yan, J.; Wang, T.; Fan, B.; Cha, D.; Li, X.; Liang, S.; Zhang, Z. 3,6-O--chitosan exerts antibacterial activity by a membrane damage mechanism. Carbohydr. Polym. 2016, 149, 102–111.
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan–Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239.
- Kritchenkov, A.S.; Egorov, A.R.; Dysin, A.P.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V. Ultrasound-assisted Cu(I)-catalyzed azide-alkyne click cycloaddition as polymer-analogous transformation in chitosan chemistry. High antibacterial and transfection activity of novel triazol betaine chitosan derivatives and their nanoparticles. Int. J. Biol. Macromol. 2019, 137, 592–603.
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Kritchenkov, I.S.; Kurliuk, A.V.; Shakola, T.V.; Khrustalev, V.N. Ultrasound-assisted catalyst-free phenol-yne reaction for the synthesis of new water-soluble chitosan derivatives and their nanoparticles with enhanced antibacterial properties. Int. J. Biol. Macromol. 2019, 139, 103–113.
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; Tskhovrebov, A.G.; Yagafarov, N.Z.; et al. Ultrasound-assisted catalyst-free thiol-yne click reaction in chitosan chemistry: Antibacterial and transfection activity of novel cationic chitosan derivatives and their based nanoparticles. Int. J. Biol. Macromol. 2020, 143, 143–152.
- Kritchenkov, A.S.; Zhaliazniak, N.V.; Egorov, A.R.; Lobanov, N.N.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; et al. Chitosan derivatives and their based nanoparticles: Ultrasonic approach to the synthesis, antimicrobial and transfection properties. Carbohydr. Polym. 2020, 242, 116478.
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136.
- Jing, Y.J.; Hao, Y.J.; Qu, H.; Shan, Y.; Li, D.S.; Du, R.Q. Studies on the antibacterial activities and mechanisms of chitosan obtained from cuticles of housefly larvae. Acta Biol. Hung. 2007, 58, 75–86.
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205.
- Kaya, M.; Baran, T.; Erdogan, S.; Mentes, A.; Ozusaglam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 72–81.
- Vinsova, J.; Vavrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607.
- Kaya, M.; Asan-Ozusaglam, M.; Erdogan, S. Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J. Biosci. Bioeng. 2016, 121, 678–684.
- Arkoun, M.; Ardila, N.; Heuzey, M.C.; Ajji, A. Chitosan-Based Structures/Coatings with Antibacterial Properties; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–389.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible Antimicrobial Films Based on Chitosan Matrix. J. Food Sci. 2002, 67, 1162–1169.
- Lim, S.-H.; Hudson, S.M. Review of Chitosan and Its Derivatives as Antimicrobial Agents and Their Uses as Textile Chemicals. J. Macromol. Sci. Part C 2003, 43, 223–269.
- Yuan, L.; Yao, Q.; Liang, Y.; Dan, Y.; Wang, Y.; Wen, H.; Yang, Y.; Dan, W. Chitosan based antibacterial composite materials for leather industry: A review. J. Leather Sci. Eng. 2021, 3, 12.
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868.
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904.
- Li, B.; Shan, C.L.; Ge, M.Y.; Wang, L.; Fang, Y.; Wang, Y.L.; Xie, G.-L.; Sun, G.C. Antibacterial Mechanism of Chitosan and its Applications in Protection of Plant from Bacterial Disease. Asian J. Chem. 2013, 25, 10033–10036.
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Malcata, F.X. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134.
- Amin, A.; Goher, M.; El-Shamy, A.; Abdel-Wahed, A.-W. Synthesis and Characterization of Chitosan-Amidoxime Chelating Resin (CACR) and Application for lead Removal from Aqueous Medium. Egypt. J. Aquat. Biol. Fish. 2020, 24, 623–637.
- McIlwee, H.A.; Schauer, C.L.; Praig, V.G.; Boukherroub, R.; Szunerits, S. Thin chitosan films as a platform for SPR sensing of ferric ions. Analyst 2008, 133, 673–677.
- Nikiforova, T.E.; Kozlov, V.A.; Islyaikin, M.K. Regularities and mechanism of heavy metal cations sorption and (or) proton desorption by chitosan from aqueous solutions. Can. J. Chem. 2019, 97, 621–628.
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085.
- Maksimović, Z.; Petrović, S.; Pavlović, M.; Kovačević, N.; Kukić, J. Antioxidant activity of Filipendula hexapetala flowers. Fitoterapia 2007, 78, 265–267.
- Gomes, J.R.B.; Jorge, M.; Gomes, P. Interaction of chitosan and chitin with Ni, Cu and Zn ions: A computational study. J. Chem. Thermodyn. 2014, 73, 121–129.
- Hernández, R.B.; Yola, O.R.; Mercê, A.L.R. Chemical equilibrium in the complexation of first transition series divalent cations Cu2+, Mn2+ and Zn2+ with chitosan. J. Braz. Chem. Soc. 2007, 18, 1388–1396.
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Recent research progress on preparation and application of N, N, N-trimethyl chitosan. Carbohydr. Res. 2016, 434, 27–32.
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984.
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265.
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194.
- Lan, W.; Du, J.; Sun, Y.; Xie, J. Insight into the antibacterial activity and mechanism of chitosan caffeic acid graft against Pseudomonas fluorescens. Int. J. Food Sci. Technol. 2023, 58, 1317–1325.
- Egorov, A.R.; Khubiev, O.; Rubanik, V.V.; Rubanik, V.V., Jr.; Lobanov, N.N.; Savilov, S.V.; Kirichuk, A.A.; Kritchenkov, I.S.; Tskhovrebov, A.G.; Kritchenkov, A.S. The first selenium containing chitin and chitosan derivatives: Combined synthetic, catalytic and biological studies. Int. J. Biol. Macromol. 2022, 209, 2175–2187.
- Egorov, A.R.; Artemjev, A.A.; Kozyrev, V.A.; Sikaona, D.N.; Rubanik, V.V.; Rubanik, V.V., Jr.; Kritchenkov, I.S.; Yagafarov, N.Z.; Khubiev, O.M.; Tereshina, T.A.; et al. Synthesis of Selenium-Containing Chitosan Derivatives and Their Antibacterial Activity. Appl. Biochem. Microbiol. 2022, 58, 132–135.
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084.
- Kritchenkov, A.S.; Egorov, A.R.; Krytchankou, I.S.; Dubashynskaya, N.V.; Volkova, O.V.; Shakola, T.V.; Kurliuk, A.V.; Skorik, Y.A. Synthesis of novel 1H-tetrazole derivatives of chitosan via metal-catalyzed 1,3-dipolar cycloaddition. Catalytic and antibacterial properties of chitosan and its nanoparticles. Int. J. Biol. Macromol. 2019, 132, 340–350.
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Kurasova, M.N.; Dysin, A.P.; Kurliuk, A.V.; Shakola, T.V.; et al. Active antibacterial food coatings based on blends of succinyl chitosan and triazole betaine chitosan derivatives. Food Packag. Shelf Life 2020, 25, 100534.
- Kritchenkov, A.S.; Egorov, A.R.; Skorik, Y.A. Azide pre-click modification of chitosan: N-(2-azidoethyl)chitosan. Russ. Chem. Bull. 2018, 67, 1915–1919.
- Kritchenkov, A.S.; Egorov, A.R.; Artemjev, A.A.; Kritchenkov, I.S.; Volkova, O.V.; Kiprushkina, E.I.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Tskhovrebov, A.G.; et al. Novel heterocyclic chitosan derivatives and their derived nanoparticles: Catalytic and antibacterial properties. Int. J. Biol. Macromol. 2020, 149, 682–692.
- Caro-León, F.J.; López-Donaire, M.L.; Vázquez, R.; Huerta-Madroñal, M.; Lizardi-Mendoza, J.; Argüelles-Monal, W.M.; Fernández-Quiroz, D.; García-Fernández, L.; San Roman, J.; Vázquez-Lasa, B.; et al. DEAE/Catechol–Chitosan Conjugates as Bioactive Polymers: Synthesis, Characterization, and Potential Applications. Biomacromolecules 2023, 24, 613–627.
- Vázquez, R.; Caro-León, F.J.; Nakal, A.; Ruiz, S.; Doñoro, C.; García-Fernández, L.; Vázquez-Lasa, B.; San Román, J.; Sanz, J.; García, P.; et al. DEAE-chitosan nanoparticles as a pneumococcus-biomimetic material for the development of antipneumococcal therapeutics. Carbohydr. Polym. 2021, 273, 118605.
- Raik, S.V.; Andranovitš, S.; Petrova, V.A.; Xu, Y.; Lam, J.K.-W.; Morris, G.A.; Brodskaia, A.V.; Casettari, L.; Kritchenkov, A.S.; Skorik, Y.A. Comparative Study of Diethylaminoethyl-Chitosan and Methylglycol-Chitosan as Potential Non-Viral Vectors for Gene Therapy. Polymers 2018, 10, 442.
- Zhang, A.D.; Ding, D.R.; Ren, J.C.; Zhu, X.L.; Yao, Y.H. Synthesis, characterization, and drug delivery property of 2-N-carboxymethyl-6-O-diethylaminoethyl-chitosan. e-Polymers 2013, 13, 3.
- Kim, J.H.; Lee, Y.M. Synthesis and properties of diethylaminoethyl chitosan. Polymer 1993, 34, 1952–1957.
- Spagna, G.; Barbagallo, R.N.; Casarini, D.; Pifferi, P.G. A novel chitosan derivative to immobilize alpha-L-rhamnopyranosidase from Aspergillus niger for application in beverage technologies. Enzym. Microb. Technol. 2001, 28, 427–438.
- Zhang, A.D.; Ding, D.R.; Ren, J.C.; Zhu, X.L.; Yao, Y.H. Synthesis, Characterization, and Drug-Release Behavior of Amphiphilic Quaternary Ammonium Chitosan Derivatives. J. Appl. Polym. Sci. 2014, 131, 39890.
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochemistry 2020, 85, 154–176.
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.-B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72.
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Tan, W.N.; Hasanolbasori, M.A.; Abdullah, S.Z. Potential Antimicrobial Applications of Chitosan Nanoparticles (ChNP). J. Microbiol. Biotechnol. 2019, 29, 1009–1013.
- Gademann, K. Controlling protein transport by small molecules. Curr. Drug Targets 2011, 12, 1574–1580.
- Bajaj, H.; Acosta Gutierrez, S.; Bodrenko, I.; Malloci, G.; Scorciapino, M.A.; Winterhalter, M.; Ceccarelli, M. Bacterial Outer Membrane Porins as Electrostatic Nanosieves: Exploring Transport Rules of Small Polar Molecules. ACS Nano 2017, 11, 5465–5473.
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32.
- Raafat, D.; Bargen, K.v.; Haas, A.; Sahl, H.-G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773.
- Yusharani, M.S.; Ulfin, I.; Ni’mah, Y.L. Synthesis of water-soluble chitosan from squid pens waste as raw material for capsule shell: Temperature deacetylation and reaction time. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012070.
- Xuan Du, D.; Xuan Vuong, B. Study on Preparation of Water-Soluble Chitosan with Varying Molecular Weights and Its Antioxidant Activity. Adv. Mater. Sci. Eng. 2019, 2019, 8781013.
- Bajrami, D.; Fischer, S.; Barth, H.; Hossain, S.I.; Cioffi, N.; Mizaikoff, B. Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms. Molecules 2022, 27, 8647.
- Sahariah, P.; Cibor, D.; Zielińska, D.; Hjálmarsdóttir, M.Á.; Stawski, D.; Másson, M. The Effect of Molecular Weight on the Antibacterial Activity of N,N,N-Trimethyl Chitosan (TMC). Int. J. Mol. Sci. 2019, 20, 1743.
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Daly, W.H. Synthesis of methylated chitosan containing aromatic moieties: Chemoselectivity and effect on molecular weight. Carbohydr. Polym. 2008, 72, 740–750.
- Li, B.; Chang, G.; Dang, Q.; Liu, C.; Song, H.; Chen, A.; Yang, M.; Shi, L.; Zhang, B.; Cha, D. Preparation and characterization of antibacterial, antioxidant, and biocompatible p-coumaric acid modified quaternized chitosan nanoparticles. Int. J. Biol. Macromol. 2023, 242, 125087.
- Luna, M.; Beltran, O.; Encinas-Basurto, D.A.; Ballesteros-Monrreal, M.G.; Topete, A.; Hassan, N.; López-Mata, M.A.; Reyes-Márquez, V.; Valdez, M.A.; Juarez, J. High antibacterial performance of hydrophobic chitosan-based nanoparticles loaded with Carvacrol. Colloids Surf. B Biointerfaces 2022, 209, 112191.
- Mansour, H.; El-Sigeny, S.; Shoman, S.; Abu-Serie, M.M.; Tamer, T.M. Preparation, Characterization, and Bio Evaluation of Fatty N- Hexadecanyl Chitosan Derivatives for Biomedical Applications. Polymers 2022, 14, 4011.
- Bernal-Mercado, A.T.; Juarez, J.; Valdez, M.A.; Ayala-Zavala, J.F.; Del-Toro-Sánchez, C.L.; Encinas-Basurto, D. Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms. Molecules 2022, 27, 699.
- Inta, O.; Yoksan, R.; Limtrakul, J. Hydrophobically modified chitosan: A bio-based material for antimicrobial active film. Mater. Sci. Eng. C 2014, 42, 569–577.
- Huang, R.H.; Du, Y.M.; Zheng, L.S.; Liu, H.; Fan, L.H. A new approach to chemically modified chitosan sulfates and study of their influences on the inhibition of Escherichia coli and Staphylococcus aureus growth. React. Funct. Polym. 2004, 59, 41–51.
- Phaechamud, T.; Charoenteeraboon, J. Antibacterial Activity and Drug Release of Chitosan Sponge Containing Doxycycline Hyclate. AAPS PharmSciTech 2008, 9, 829–835.
- Tang, W.; Wang, J.; Hou, H.; Li, Y.; Wang, J.; Fu, J.; Lu, L.; Gao, D.; Liu, Z.; Zhao, F.; et al. Review: Application of chitosan and its derivatives in medical materials. Int. J. Biol. Macromol. 2023, 240, 124398.
- Jones, S. Permeability rules for antibiotic design. Nat. Biotechnol. 2017, 35, 639.
- Young, D.H.; Köhle, H.; Kauss, H. Effect of Chitosan on Membrane Permeability of Suspension-Cultured Glycine max and Phaseolus vulgaris Cells 1. Plant Physiol. 1982, 70, 1449–1454.
- Leksono, T.; Mus, S.; Annisa, Z. Antibacterial Potential of Chitosan Extracted from Rama Shrimp (Thalassina Anomala) Carapace. J. Phys. Conf. Ser. 2019, 1351, 012088.
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449.
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335.
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960.
- Xia, Y.; Yang, R.; Wang, H.; Li, Y.; Fu, C. Application of chitosan-based materials in surgical or postoperative hemostasis. Front. Mater. 2022, 9, 994265.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
21 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




