Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abdelrahman Zaky | -- | 3662 | 2023-09-19 19:25:28 | | | |
| 2 | Rita Xu | -4 word(s) | 3658 | 2023-09-20 03:39:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Seaweed Biorefineries for Carbon Capture and Storage. Encyclopedia. Available online: https://encyclopedia.pub/entry/49393 (accessed on 08 February 2026).
Johnston KG, Abomohra A, French CE, Zaky AS. Seaweed Biorefineries for Carbon Capture and Storage. Encyclopedia. Available at: https://encyclopedia.pub/entry/49393. Accessed February 08, 2026.
Johnston, Katherine G., Abdelfatah Abomohra, Christopher E. French, Abdelrahman S. Zaky. "Seaweed Biorefineries for Carbon Capture and Storage" Encyclopedia, https://encyclopedia.pub/entry/49393 (accessed February 08, 2026).
Johnston, K.G., Abomohra, A., French, C.E., & Zaky, A.S. (2023, September 19). Seaweed Biorefineries for Carbon Capture and Storage. In Encyclopedia. https://encyclopedia.pub/entry/49393
Johnston, Katherine G., et al. "Seaweed Biorefineries for Carbon Capture and Storage." Encyclopedia. Web. 19 September, 2023.
Copy Citation
Seaweeds are among the most important biomass feedstocks for the production of third-generation biofuels. They are also efficient in carbon sequestration during growth and produce a variety of high-value chemicals. Given these characteristics together with the relatively high carbohydrate content, seaweeds have been discussed as an ideal means for CO2 capture and biofuel production.
macroalgae
seaweeds
yeast
biomass conversion
1. Introduction
Since the industrial revolution, extensive utilisation of fossil fuels has led to increasing greenhouse gas (GHG) emissions, mainly CO2. This has resulted in an increase in global temperatures, leading to more and more extreme weather phenomena. In response, countries around the world signed the 2016 Paris Agreement as a commitment to combat the ongoing crisis [1]. However, according to the Intergovernmental Panel on Climate Change (IPCC) 2018 special report, today’s actions are not sufficient to stop the rise in temperature, and the report states that global warming will go beyond 1.5 °C by 2030 if the present pollution rates continue [2]. Exceeding the 1.5–2.0 °C temperature limit would lead to irreversible damage to the biosphere. The report also outlines two targets for the coming century: achieving carbon neutrality by 2050 and removal of 100 gigatons of atmospheric CO2 by 2100 [3]. These goals highlight the need to replace fossil fuels with more sustainable energy sources and reduce atmospheric CO2 levels to maintain the global temperature within the safe range.
Much research is being done to find sustainable alternatives to fossil fuels, with groundbreaking development in renewable electric power. However, certain sectors, like transportation, are still reliant on liquid fuels. Biofuels have been discussed as a sustainable replacement for these liquid fuels. The term biofuel refers to the extraction of energy from organic biomass in the form of liquid fuel. Biofuels are classified into three generations, depending on their sources. First-generation biofuels are derived from food crop material. Though their production is efficient, the use of crop-based substrates poses a threat to food security. This led to the emergence of second-generation biofuels, derived from lignocellulosic biomass residues and other agricultural wastes. However, the production of second-generation biofuels is unsustainable on economic and environmental grounds due to the required pre-treatment step to degrade lignin [4], which increases the production cost and processing time. Third-generation biofuels can be produced from aquatic feedstocks including micro- and macroalgae as a solution to the aforementioned problems. In recent years, macroalgae (seaweeds) have been studied as a potential substrate for bioethanol [5][6] and biogas [7]. Seaweeds are also more efficient at carbon sequestration than land plants, making them an effective means of carbon capture and sequestration [8]. Furthermore, many seaweed-derived chemicals are of high commercial value [9][10].
Reducing the resource input and increasing the co-product potential of the production process are critical aspects for spurring the development of seaweed biorefineries. Thus, coastal-based integrated marine biorefinery (CIMB) systems were suggested for the efficient production of third-generation biofuels [11][12]. These systems utilise marine resources (seawater, marine yeast, and marine algae) to produce biofuels and high-value chemicals (HVC) through integrated biological conversion technologies (i.e., fermentation and anaerobic digestion). The integration of these marine resources and conversion technologies could significantly enhance production efficiency and totally eliminate the use of freshwater and arable land in the biofuel industry. This could also enhance the CO2 sequestration potential of the process, making biofuels a more sustainable and eco-friendly energy source [13]. CIMB systems have not yet been thoroughly researched and, therefore, they need intensive investigation [14].
2. Coastal Marine Biorefinery Systems
The concept of biorefineries for the production of liquid fuels is not new, as the processing of different biomass feedstocks into bioethanol is a common practice in many countries such as Brazil and the USA. However, such industries consume huge amounts of freshwater. It was estimated that the production of 1 L of bioethanol requires 5–10 L of water during the fermentation process alone, with the total water footprint ranging between 1388 to 9812 L when taking into account conventional routes of biomass production [15][16]. Given the major concerns regarding freshwater shortages, this production method is unsustainable, and a coastal marine biorefinery could provide a solution. This is a conceptual refinery system that relies on the use of marine components (seawater, yeast, and seaweeds) to produce biofuels and other valuable products. In the case of bioethanol, seaweeds are used as a feedstock, seawater as a growth medium and marine yeast for marine fermentation (Figure 1). Though research has been done on each individual element, “coastal”, “marine”, and “biorefinery”, a single system combining all three elements has not yet been considered [14].
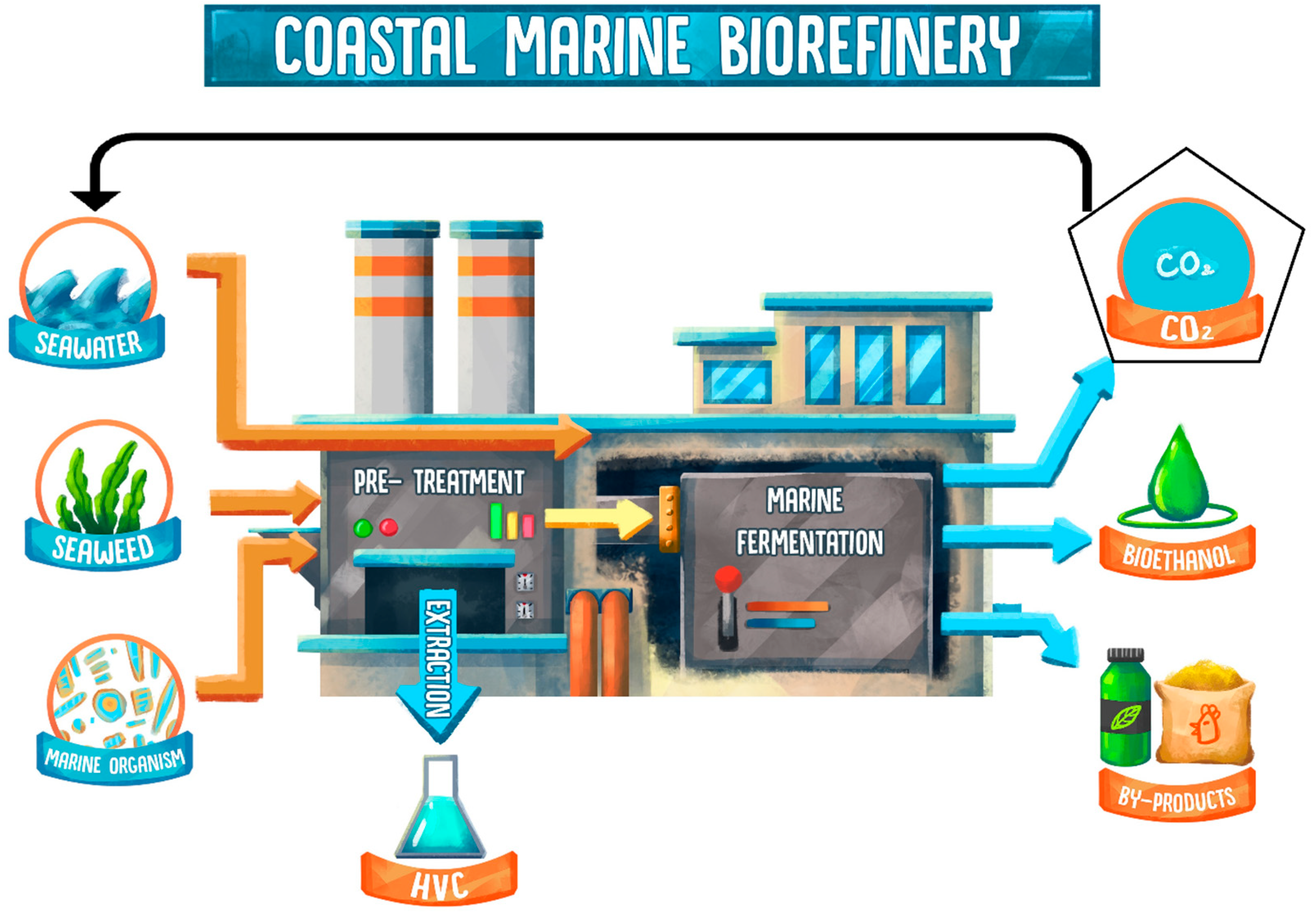
Figure 1. Visual diagram of a coastal seaweed marine biorefinery. Marine components include seawater, seaweed, and marine yeast (orange arrow). After processing, a number of outputs (blue arrows) are obtained. High-value chemicals (HVC) are extracted during the pre-treatment step, while bioethanol and by-products (e.g., plant fertilizer and salted animal feed) are produced during or after the fermentation. Produced CO2 is captured and stored in seawater or utilised to promote the growth of seaweed.
All major components of the coastal marine biorefineries offer environmental and economic advantages compared to their conventional counterparts. As will be detailed later in this research, macroalgal biomass is an ideal substrate for bioethanol production. As macroalgae generally grow in marine environments, they do not require freshwater or arable land and therefore do not compete with food production. The successful use of seaweed as a substrate in a biorefinery has been investigated in several studies [17][18][19][20][21]. Though often titled “marine biorefineries”, these papers focused solely on the marine nature of the feedstock where, in reality, the majority of the processes still rely on the use of freshwater and conventional terrestrial yeast strains. The potential use of seawater and marine microorganisms for marine fermentation has nonetheless been demonstrated. Isolated marine yeast strains, such as Saccharomyces cerevisiae AZ65, have shown a high capability of producing bioethanol from glucose and molasses in seawater media [15][22]. These strains also produce more bioethanol from glucose compared to terrestrial yeasts and are more tolerant to fermentation inhibitors [22][23]. Seawater’s mineral content further eliminates the need for mineral nutrients and enables the production of sea salts and salted animal feed as co-products. The process of marine fermentation can also yield high-quality distilled water [24]. Furthermore, the high-salt environment may reduce the chances of microbial contamination within a bioreactor [14].
The importance of coastal locations must also be highlighted. As both the substrate and media are marine sourced, the establishment of marine biorefineries along coastal regions would decrease transportation costs. Furthermore, coastal sites are easy water access points for arid and semi-arid areas. From an economic perspective, coastal locations enable rural regeneration, providing jobs to former coastal industrial sites that have historically been hard to maintain [25].
Though coastal marine seaweed biorefineries can operate on a stand-alone basis, there is the potential to pair such a system with other biorefineries and energy outlets to maximise the valuable outputs and minimise the cost. Such emerging combined systems are called coastal integrated marine biorefineries (CIMB). As can be seen in Figure 2, integrated biorefineries may combine both seaweed and microalgal refineries, with certain by-products of each serving as inputs for the other. CO2 and spent seaweed hydrolysate may be used as organic and inorganic carbon substrates for the growth of microalgae [26][27]. Each fraction of microalgae has a certain potential industrial application. Lipids can be extracted and processed into biodiesel, proteins sold as livestock feed, and carbohydrates fermented into bioethanol. Aside from the primary metabolites, microalgae contain a host of HVCs, many of which have commercial uses. These include unsaturated long-chain fatty acids, shown to have a number of health benefits, as well as pigments, such as chlorophylls, phycocyanin, and carotenoids [28][29]. As both biorefineries require electricity, this can be provided from sustainable sources, such as wind, solar, or wave energy. Conversely, coastal integrated marine biorefineries may also serve as a means of energy storage for renewable electricity. For example, peaks in solar electricity production generally surpass simultaneous energy demands. Excess electricity is often lost as there are currently no means of storage. Coastal integrated marine biorefineries enable the storage of excess renewable electricity by using it to produce biofuels. This integrated biorefinery system would lead to the production of less waste from each individual system and an increase in HVC and co-product yields [14].
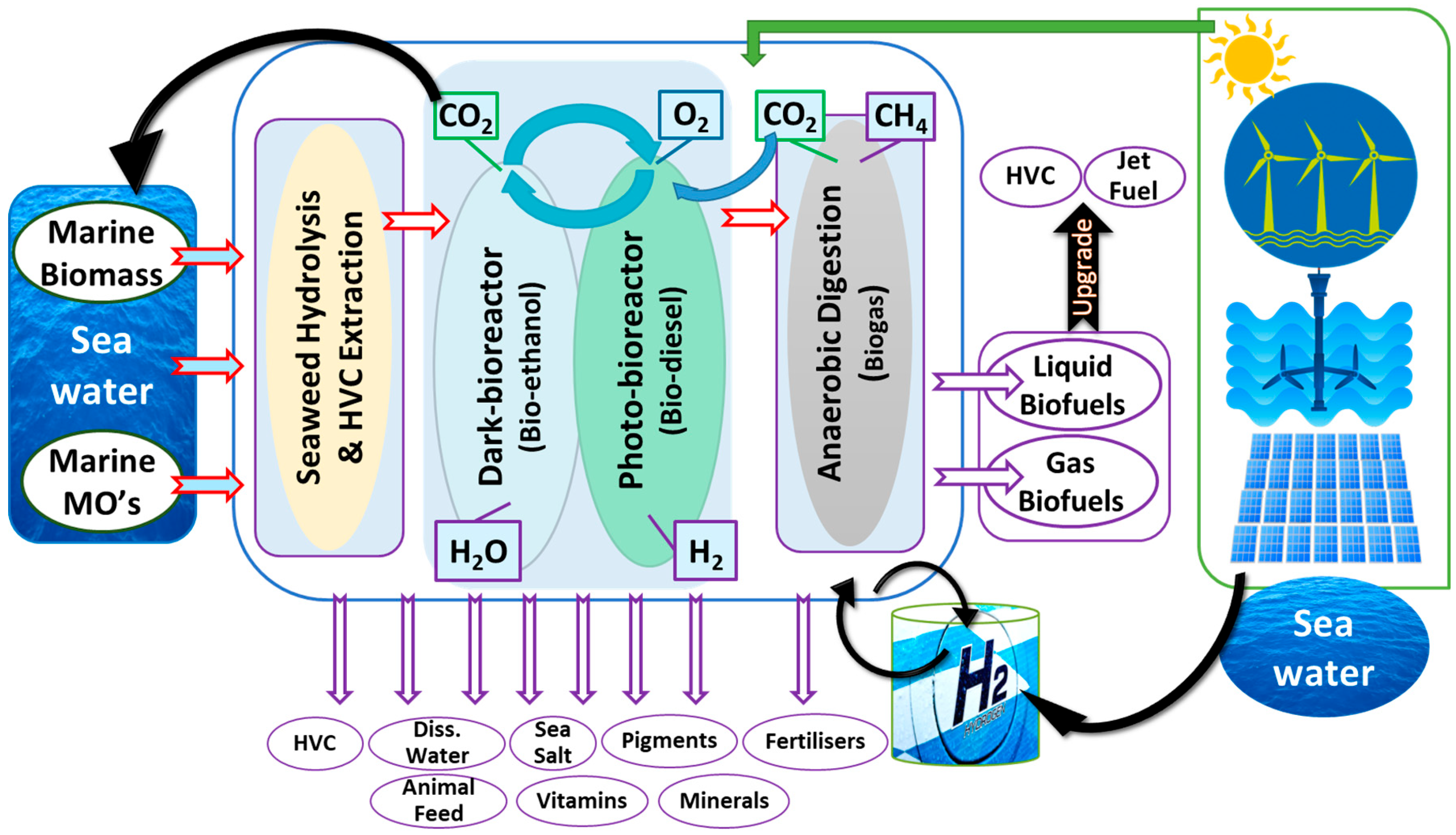
Figure 2. Coastal Integrated Marine Biorefinery (CIMB) system. Marine components (seawater, marine biomass, and marine microorganisms) serve as inputs for the biorefineries. Different biological and biochemical conversion processes are performed (blue box). Biofuels and high-value chemicals are obtained (purple arrows and ovals). Renewable energy sources (green box/arrow) are integrated with the biological system to improve efficiency.
3. Seaweed
Unlike terrestrial plants, algae do not require freshwater or agricultural land, two rapidly depleting world resources [30]. Furthermore, they can serve as a means for bioremediation as they have been shown to eliminate heavy metals and other contaminants in wastewater such as microplastic [31][32]. Macroalgae are also capable of removing high concentrations of nitrogen and phosphorous from coastal waters [33][34]. Therefore, seaweeds have been discussed as a potential feedstock for bioethanol and biogas production coupled with heavy metal removal [35]. The main groups of marine macroalgae include species of the phyla Rhodophyta (red), Chlorophyta (green), and Phaeophyta (brown), which are differentiated mainly based on their pigmentation. Seaweeds have a wide variation in biochemical composition and, therefore, have many applications in HVC production [14].
4. Evaluation of CO2 Removal and CCS by Seaweeds
Over the course of the 21st century, an estimated 100–1000 gigatons (Gt) of CO2 need to be removed from the atmosphere to mitigate the impacts of global warming expected by the end of this century [36]. In recent years, planting trees has gained traction, with certain campaigns amassing millions of dollars in funds [37]. Such efforts highlight the widespread interest in finding tangible solutions to the current crisis. However, afforestation is mostly suited to tropical regions, where fast plant growth is possible [38]. Furthermore, tree monoculture and planting in arid regions have been shown to have a negative environmental impact, increasing water scarcity and the creation of “Green Deserts” [39][40]. Other carbon capture and sequestration (CCS) technologies are therefore necessary to combat climate change.
Seaweeds have been discussed as an alternative carbon sink to terrestrial biomass. Seaweeds do not use freshwater or arable land. Furthermore, as the ocean covers more than 70% of the Earth’s surface, the available area for seaweed growth is much larger than what can be grown on land. Most importantly, the biomass productivity of seaweeds is much higher than that of terrestrial plants. Whereas the carbon productivity of second-generation lignocellulosic crops is less than 1 kg Carbon (C) m−2 year−1, seaweed productivity ranges between 1 and 3.4 kg C m−2 year−1, depending on the species [41].
Seaweed carbon sequestration is part of blue carbon sequestration, referring to the removal of atmospheric CO2 by marine ecosystems through the accumulation and sequestration of carbon by marine organisms. Blue carbon sequestration accounts for around 55 to 71% of all biological carbon sequestration on the planet [42][43]. Wild seaweeds (naturally grown seaweed) have already been shown to be an effective means of carbon removal, permanently sequestering on average 0.634 Gt CO2 per year, mainly through deep sea biomass exportation or coastal sediment burial [44]. A study by Johnston et al. determined how much time is required to sequester 100 Gt of CO2 by growing seaweed biomass in large seaweed farms based on the available marine area. To achieve that, three scenarios (A, B, and C) have been explored based on the total cultivation area. Scenario A accounts for 5.7 M km2 which is the inshore coastal area that is suitable for seaweed cultivation. Scenario B accounts for 100 M km2 which is the total ocean area that could be used for seaweed farming. Scenario C accounts for 47 M km2, which is the ecologically available ocean area for seaweed farming. In order to determine the total seaweed biomass production from each scenario, the average productivity of wild (naturally grown) seaweed and the productivity of the highly productive cultivated seaweed species, M. pyrifera and Ulva sp., were used in the calculation. The biomass production in each scenario determines the number of years required to sequester 100 Gt of CO2 [14].
Scenario A represents the total time and biomass needed to remove 100 Gt of CO2 when growing seaweeds on inshore coastal surface areas. Using an area of 5.7 million km2, a total removal of excess CO2 can be achieved in less than 11.28 years based on average wild seaweed NPP. When selecting highly productive species, the time goes down to 5.65 years using Ulva sp. and 3.64 years using M. pyrifera [14].
In Scenario B, the surface area for seaweed farming can be expanded to include the total inshore and offshore ocean surface area that can be theoretically used for seaweed farming. Based on Ulva sp., a theoretical growth area of around 100 million km2 can be farmed with 0.838 kg C m−2 yr−1 NPP to remove 100 Gt of CO2 in just 116.8 days. Total yearly CO2 removal using Ulva sp. farms in this scenario is 17 times higher than that of growth limited to inshore coastal sites [14].
Though Ulva sp. can be theoretically grown over 100 M km2 of the ocean when only considering certain factors such as temperature, light, depth, and pH, the model does not consider all ecological limits on seaweed growth. A better model taking into account these constraints estimates that the ocean surface area ecologically available for seaweed farms is in fact 48 million km2. Therefore, in Scenario C, a total target CO2 removal, considering NPP of wild seaweed, M. pyrifera, and Ulva sp. would take 1.34, 0.43, and 0.67 years, respectively. Across all seaweed classes, the yearly rate of CO2 removal was 8.42 times greater for seaweed farms compared to inshore coastal areas [14].
5. Future Perspectives
According to the Intergovernmental Panel on Climate Change (IPCC) and the European Commission, a number of targets must be met in order for the planet to not reach a stage of irreversible climate change. Firstly, human activity must be carbon neutral by 2050 [45]. Second, a minimum of 100 GtCO2 must be removed from the atmosphere using carbon dioxide removal (CDR) strategies by 2100 [3]. Both objectives aim to maintain global temperature increase to below 2 °C. The results show total sequestration of 100 GtCO2 could take less than 12 years, based on wild seaweed cultivated in inshore coastal sites alone. This is already considerably shorter than the time scale left until the 2100 deadline. However, this period can be significantly reduced if highly productive seaweed species are selected for farming. Therefore, seaweed cultivation is an efficient means of atmospheric carbon dioxide removal [14].
Scenario B of seaweed farms was limited to Ulva sp. as the used model was specifically designed for that genus of Chlorophyta. Though other types of macroalgae can generally grow within the same niche as Ulva, growth over 100 million km2, around 10% of the total ocean area, is restricted to Ulva sp. [46]. However, Both the scale at which seaweed may be grown and, consequently, the efficient carbon capture and sequestration power of seaweed. Indeed, seaweed farms have the potential to offset total carbon emissions from entire industrial sectors. Seaweed farming on 3.8% of the West Coast Exclusive Economic Zones could offset carbon emissions for the entire Californian land farming sector. Moreover, only an estimated 474 km2 of seaweed farms are required to completely offset the entire global seafood aquaculture industry [47].
However, the Ulva sp. growth model does not take into account the ecological constraints of all seaweed species. A broader more accurate model estimates that 48 million km2 of ocean surface could be used for seaweed farming [47]. Under these conditions, 100 GtCO2 removal could be achieved in under a year when farming M. pyrifera and Ulva sp. Furthermore, as the yearly productivity of both species exceeds the minimum CDR target, there is the potential to go beyond the IPCC’s requirements. Further CO2 removal would contribute to “negative carbon” emissions. This could not only completely limit global warming but also reverse the 1.3 °C temperature rise that has already occurred [48]. Indeed, the removal of the IPCC’s upper limit, 1000 GtCO2, would undo 20 years of global GHG emissions [36][49].
Only the available surface area for seaweed growth was explored in this analysis. However, improvements in seaweed farming conditions could enhance seaweed productivity and would thus shorten the time needed for CO2 removal and/or a decrease in necessary surface area. There is, however, a lack of research on seaweed farming conditions and their direct impact on seaweed NPP. Certain studies have focussed on the various factors that influence biomass production but not NPP [50][51]. Aside from temperature, the most limiting parameter is the rate of photosynthesis, itself limited by multiple physiological processes [52]. A study by Golberg and Liberzon showed that the use of an external mixing system, one that would cycle seaweed culture plots, enabling optimised light exposure, could increase total energy gain by two orders of magnitude [53]. However, practical technologies based on this principle have yet to be developed.
Though seaweed farming could be a means of carbon capture, a number of studies have highlighted the economic and environmental costs of such a strategy [47][54]. There can be some debate on the feasibility of three scenarios. For example, despite Scenario C being ecologically possible, biomass transportation from distant offshore seaweed farms to marine biorefineries becomes a major challenge, leading to increased production costs. Indeed, until more advances are made in transportation technologies, seaweed farming will be restricted to areas close to the coast [55]. Another major issue is the environmental consequences of seaweed farming. This includes concerns regarding the release of artificial and organic materials into the environment, as well as the noise disturbance to marine wildlife [56]. However, seaweed farming has also been shown to have a number of ecological benefits. Macroalgae mitigate ocean acidification whilst replenishing oxygen supplies by removing CO2 and producing O2 through photosynthesis [54]. This is particularly important in hypoxic environments, resulting from the eutrophication of water bodies. Seaweed can further help to bioremediate nutrients and metals from agricultural and urban runoffs [57]. Aside from biochemical impacts, seaweed farms can also serve as a means of wave attenuation, providing protection from extreme weather phenomena [54].
To further explore the economic potential of seaweed, the mass and value of the bioethanol and HVC that could be produced from the biomass were calculated. In 2018, worldwide oil consumption was estimated at around 4622 million tonnes (Mtoe) [58][59]. According to the results, bioethanol production from coastal sites could generate around 6310 Mtoe of bioethanol. This value more than exceeds planetary oil requirements. In fact, total seafarm bioethanol production, estimated at 53,200 Mtoe, greatly exceeds the 2018 global energy demand of 13,864.9 Mtoe [58]. It is worth noting that, in the study by Johnston et al., bioethanol estimates were based on production using fresh water and genetically modified E. coli [14][60]. Further research is needed for bioethanol production on such a scale within a coastal marine biorefinery, using seawater and marine yeast. Nonetheless, the volumes of bioethanol that could be produced using carbon capture seaweed could meet worldwide energy demands and replace the petrol industry, a main driver of CO2 emissions. Climate protection policies could also lead to the expansion of the bioethanol market. The Renewable Fuels Standard (RFS) mandates the blending of 36 billion gallons of renewable fuels by 2022, of which only 42% can be corn-based ethanol [61]. The remaining gap can be filled by seaweed bioethanol, a market only set to grow in the coming years. Global bioethanol production is projected to rise by 14%, with the biofuels market set to reach USD 246.52 billion by 2024, at a compound growth rate of 4.92% [62][63].
Turning to HVC, like all seaweed species, the chemical composition of M. pyrifera varies greatly depending on environmental conditions [64][65][66]. Specific values and parameters chosen for the calculation came down to the quality of the study conducted by Johnston et al. [14][67]. Considerable amounts of phlorotannin can be extracted. Many phlorotannins have commercial value as they have been shown to have anti-oxidant, anti-diabetic, radioprotective, hepatoprotective, and anti-inflammatory activity [68]. Indeed, phlorotannin is the most valuable compound (USD ~70/kg) that can be extracted from M. pyrifera [69]. However, given the low production yields, they tend to generate the least revenue. Optimisation of extraction procedures could increase the total volume and revenue of the product. However, given the generally low phlorotannin content of seaweed, there is a ceiling limit [70].
The most interesting seaweed HVC are alginate and mannitol as both sugars have multi-billion-dollar valuations. Alginate is the most abundant of the extractable HVC and is also the most lucrative while mannitol is the second most profitable. This is in contrast to bioethanol, which, despite having the highest production volumes, is the lowest-grossing product. Given their abundance, polysaccharides are the most cost-effective HVC for future investments. Furthermore, the market for algal sugars is set to expand in the coming years. Alginate is finding increasing pharmaceutical and biomedical applications while mannitol, a low-calorie sweetener, is facing increasing demand in a health-concerned population [71][72].
Though large quantities of proteins can be extracted from M. pyrifera, as most have not been characterised and, therefore, they currently have no commercial applications; however, they may have tremendous potential, especially as animal feed. Brown seaweeds also contain fucoidan, a sugar with interesting properties and commercial value. However, due to a lack of efficient extraction procedures, no values could be estimated for fucoidan from M. pyrifera [73][74].
The compound estimations were based on the individual extraction values of each chemical. This means that the extraction process was optimised for a single compound. For the simultaneous extraction of all HVC and the production of bioethanol, the design of a downstream process is necessary. However, in such downstream production processes, product yields and valuations may decrease as the extraction procedures are not tailored to the individual chemicals. Furthermore, the product valuations in the study conducted by Johnston et al. are based on current market prices. With an influx of HVC on the market, the increased supply may exceed the demand, leading to an overall drop in price. However, a supply increase and price fall make a product more accessible, thus opening its use to further markets. The average price of each compound is also based on its bulk sale value. Laboratory-grade chemicals sell for a higher price but, in turn, entail higher purification costs. Nonetheless, seaweed biorefineries represent a potential multi-billion-dollar business that could potentially aid in the removal of excess CO2 and help combat climate change [14].
References
- Obergassel, W.; Arens, C.; Hermwille, L.; Kreibich, N.; Mersmann, F.; Ott, H.E.; Wang-Helmreich, H. Phoenix from the ashes: An analysis of the Paris Agreement to the United Nations Framework Convention on Climate Change: Part 1. Geography 2015, 27, 243–262.
- United Nations Paris Climate Agreement Moves Closer to Entry into Force in 2016—United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/blog/2016/09/paris-climate-agreement-moves-closer-to-entry-into-force-in-2016/ (accessed on 1 October 2021).
- Hood, R. Global Warming. In A Companion to Applied Ethics; Blackwell Publishing Ltd.: Oxford, UK, 2018; pp. 674–684. ISBN 9780470996621.
- Elsayed, M.; Abomohra, A.; Ai, P.; Jin, K.; Fan, Q.; Zhang, Y. Acetogenesis and methanogenesis liquid digestates for pretreatment of rice straw: A holistic approach for efficient biomethane production and nutrient recycling. Energy Convers. Manag. 2019, 195, 447–456.
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Abomohra, A. Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ. Sci. Pollut. Res. 2020, 27, 32481–32493.
- Zaky, A.; Abomohra, A. Marine-Based Biorefinery: A Path Forward to a Sustainable Future. Ferment 2023, 9, 554.
- Faisal, S.; Zaky, A.; Wang, Q.; Huang, J.; Abomohra, A. Integrated Marine Biogas: A Promising Approach towards Sustainability. Ferment 2022, 8, 520.
- Hu, C.; Wang, M.; Lapointe, B.E.; Brewton, R.A.; Hernandez, F.J. On the Atlantic pelagic Sargassum’s role in carbon fixation and sequestration. Sci. Total Environ. 2021, 781, 146801.
- Khan, F.; Jeong, G.J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384.
- Spagnuolo, D.; Di Martino, A.; Zammuto, V.; Armeli Minicante, S.; Spanò, A.; Manghisi, A.; Gugliandolo, C.; Morabito, M.; Genovese, G. Conventional vs. Innovative Protocols for the Extraction of Polysaccharides from Macroalgae. Sustainability 2022, 14, 5750.
- Zaky, A.S.; Kumar, S.; Welfle, A.J. Integrated Approaches and Future Perspectives. In Waste-to-Energy; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 613–651.
- Zaky, A.S. Introducing a Marine Biorefinery System for the integrated production of biofuels, high-value-chemicals and co-products: A path forward to a sustainable future. Processes 2021, 9, 1841.
- Zaky, A.S.; Greetham, D.; Louis, E.J.; Tucker, G.A.; Du, C. A new isolation and evaluation method for marine-derived yeast spp. with potential applications in industrial biotechnology. J. Microbiol. Biotechnol. 2016, 26, 1891–1907.
- Katherine G. Johnston; Abdelfatah Abomohra; Christopher E. French; Abdelrahman S. Zaky; Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustain. 2023, 15, 13193.
- Zaky, A.S.; Greetham, D.; Tucker, G.A.; Du, C. The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Sci. Rep. 2018, 8, 12127.
- Gerbens-Leenes, W.; Hoekstra, A.Y.; Van Der Meer, T.H. The water footprint of bioenergy. Proc. Natl. Acad. Sci. USA 2009, 106, 10219–10223.
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359.
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620.
- Zollmann, M.; Robin, A.; Prabhu, M.; Polikovsky, M.; Gillis, A.; Greiserman, S.; Golberg, A. Green technology in green macroalgal biorefineries. Phycologia 2019, 58, 516–534.
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511.
- Zaky, A.S.; Carter, C.E.; Meng, F.; French, C.E. A Preliminary Life Cycle Analysis of Bioethanol Production Using Seawater in a Coastal Biorefinery Setting. Process 2021, 9, 1399.
- Zaky, A.S.; French, C.E.; Tucker, G.A.; Du, C. Improving the productivity of bioethanol production using marine yeast and seawater-based media. Biomass Bioenergy 2020, 139, 105615.
- Greetham, D.; Zaky, A.S.; Du, C. Exploring the tolerance of marine yeast to inhibitory compounds for improving bioethanol production. Sustain. Energy Fuels 2019, 3, 1545–1553.
- Zaky, A.S. Marine Fermentation, the Sustainable Approach for Bioethanol Production. EC Microbiol. 2017, 25–27. Available online: https://api.semanticscholar.org/CorpusID:212470469 (accessed on 25 July 2023).
- Organisation for Economic Co-operation and Development. Meeting Policy Challenges for a Sustainable Bioeconomy; OECD iLibrary: Paris, France, 2018; ISBN 9789264292338.
- Abomohra, A.; Almutairi, A.W. A close-loop integrated approach for microalgae cultivation and efficient utilization of agar-free seaweed residues for enhanced biofuel recovery. Bioresour. Technol. 2020, 317, 124027.
- El-Hefnawy, M.E.; Alhayyani, S.; Ismail, A.; El-Sherbiny, M.; Al-Harbi, M.; Abomohra, A.; Sakran, M.; Zidan, N. Integrated approach for enhanced crude bio-oil yield from microalgae cultivated on the aqueous phase of hydrothermal co-liquefaction with agar-free seaweed residues. J. Clean. Prod. 2023, 392, 136286.
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553.
- Beyer, A.S.; Meier, J.; Jiménez-Muñoz, M.; Meixner, R.; Ende, S.S.W.; Abomohra, A.; Henjes, J. New microalgae media formulated with completely recycled phosphorus originating from agricultural sidestreams. J. Appl. Phycol. 2023, 1, 1–16.
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234.
- Michalak, I. The application of seaweeds in environmental biotechnology. Adv. Bot. Res. 2020, 95, 85–111.
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. A macroalgae-based biotechnology for water remediation: Simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J. Environ. Manag. 2017, 191, 275–289.
- Jiang, Z.; Liu, J.; Li, S.; Chen, Y.; Du, P.; Zhu, Y.; Liao, Y.; Chen, Q.; Shou, L.; Yan, X.; et al. Kelp cultivation effectively improves water quality and regulates phytoplankton community in a turbid, highly eutrophic bay. Sci. Total Environ. 2020, 707, 135561.
- Xiao, X.; Agusti, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep. 2017, 7, 46613.
- Abomohra, A.; El-Hefnawy, M.E.; Wang, Q.; Huang, J.; Li, L.; Tang, J.; Mohammed, S. Sequential bioethanol and biogas production coupled with heavy metal removal using dry seaweeds: Towards enhanced economic feasibility. J. Clean. Prod. 2021, 316, 128341.
- IPCC. Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2019.
- Niu, S.; Mai, C.; McKim, K.G.; McCrickard, S. Investigating How YouTubers Participate in a Social Media Campaign. Proc. ACM Human-Comput. Interact. 2021, 5, 1–26.
- Bala, G.; Caldeira, K.; Wickett, M.; Phillips, T.J.; Lobell, D.B.; Delire, C.; Mirin, A. Combined climate and carbon-cycle effects of large-scale deforestation. Proc. Natl. Acad. Sci. USA 2007, 104, 6550–6555.
- Zastrow, M. China’s tree-planting drive could falter in a warming world. Nature 2019, 573, 474–475.
- Hulvey, K.B.; Hobbs, R.J.; Standish, R.J.; Lindenmayer, D.B.; Lach, L.; Perring, M.P. Benefits of tree mixes in carbon plantings. Nat. Clim. Chang. 2013, 3, 869–874.
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46.
- Mitra, A.; Zaman, S. Blue Carbon Reservoir of the Blue Planet; Springer: New Delhi, India, 2015; ISBN 978-81-322-2106-7.
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon: A Rapid Response Assessment; UNEP/Earthprint: Nairobi, Kenya, 2009; ISBN 9788277010601.
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742.
- Net-Zero Emissions Must Be Met by 2050 or COVID-19 Impact on Global Economies Will Pale Beside Climate Crisis, Secretary-General Tells Finance Summit. Available online: https://press.un.org/en/2020/sgsm20411.doc.htm (accessed on 16 September 2022).
- Van den Hoek, C.; Breeman, A.M.; Stam, W.T. The Geographic Distribution of Seaweed Species in Relation to Temperature: Present and Past. In Expected Effects of Climatic Change on Marine Coastal Ecosystems; Springer: Dordrecht, The Netherlands, 1990; pp. 55–67.
- Froehlich, H.E.; Afflerbach, J.C.; Frazier, M.; Halpern, B.S. Blue Growth Potential to Mitigate Climate Change through Seaweed Offsetting. Curr. Biol. 2019, 29, 3087–3093.e3.
- NOAA National Centers for Environmental Information. Monthly Global Climate Report for July 2020. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202007 (accessed on 22 July 2020).
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E.; et al. Mitigation Pathways Compatible with 1.5 °C in the Context of Sustainable Development. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 93–174.
- Smale, D.A.; Pessarrodona, A.; King, N.; Burrows, M.T.; Yunnie, A.; Vance, T.; Moore, P. Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci. Rep. 2020, 10, 12161.
- Tait, L.W.; Schiel, D.R. Ecophysiology of Layered Macroalgal Assemblages: Importance of Subcanopy Species Biodiversity in Buffering Primary Production. Front. Mar. Sci. 2018, 5, 444.
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406.
- Golberg, A.; Liberzon, A. Modeling of smart mixing regimes to improve marine biorefinery productivity and energy efficiency. Algal Res. 2015, 11, 28–32.
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 100.
- Lehahn, Y.; Ingle, K.N.; Golberg, A. Global potential of offshore and shallow waters macroalgal biorefineries to provide for food, chemicals and energy: Feasibility and sustainability. Algal Res. 2016, 17, 150–160.
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M. The environmental risks associated with the development of seaweed farming in Europe—Prioritizing key knowledge gaps. Front. Mar. Sci. 2019, 6, 107.
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The Bioremediation Potential of Seaweeds: Recycling Nitrogen, Phosphorus, and Other Waste Products. In Blue Biotechnology; Wiley-VCH Verlag GmbH & Co., KgaA: Weinheim, Germany, 2018; pp. 217–239.
- BP. BP Statistical Review of World Energy 2019; BP: London, UK, 2019.
- FuelsEurope. Refining Products for Our Everyday Life; FuelsEurope: Brussels, Belgium, 2020.
- Camus, C.; Ballerino, P.; Delgado, R.; Olivera-Nappa, Á.; Leyton, C.; Buschmann, A.H. Scaling up bioethanol production from the farmed brown macroalga Macrocystis pyrifera in Chile. Biofuels Bioprod. Biorefining 2016, 10, 673–685.
- DOE (U.S. Department of Energy). National Algal Biofuels Technology Review; U.S. Department of Energy: Washington, DC, USA, 2016.
- OECD. Biofuels. In OECD-FAO Agricultural Outlook 2017–2026; OECD Publishing: Paris, France, 2017.
- U.S. Department of Energy. Energy Efficiency and Renewable Energy Powering the Blue Economy: Exploring Opportunities for Marine Renewable Energy in Maritime Markets; U.S. Department of Energy: Washington, DC, USA, 2019.
- Ravanal, M.C.; Sharma, S.; Gimpel, J.; Reveco-Urzua, F.E.; Øverland, M.; Horn, S.J.; Lienqueo, M.E. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 2017, 26, 287–293.
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010.
- Ravanal, M.C.; Pezoa-Conte, R.; von Schoultz, S.; Hemming, J.; Salazar, O.; Anugwom, I.; Jogunola, O.; Mäki-Arvela, P.; Willför, S.; Mikkola, J.P.; et al. Comparison of different types of pretreatment and enzymatic saccharification of Macrocystis pyrifera for the production of biofuel. Algal Res. 2016, 13, 141–147.
- Leyton, A.; Pezoa-Conte, R.; Mäki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Improvement in carbohydrate and phlorotannin extraction from Macrocystis pyrifera using carbohydrate active enzyme from marine Alternaria sp. as pretreatment. J. Appl. Phycol. 2017, 29, 2039–2048.
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361.
- Hernández-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 475–516. ISBN 9780857095121.
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599.
- Marketresearchfuture Alginates Market Research Report Information—By Source (Laminaria, Macrocystis, Ascophyllum, and Others), by Function (Thickener, Emulsifier, Stabilizer, Acidity Regulator, Others), by Region—Forecast Till 2027. Available online: https://www.marketresearchfuture.com/reports/alginates-market-1581 (accessed on 25 July 2023).
- Grandviewresearch Mannitol Market Size, Share & Trends Analysis Report by Application (Food Additive, Pharmaceuticals, Industrial, Surfactants), and Segment Forecasts, 2015–2024. Available online: https://www.grandviewresearch.com/industry-analysis/mannitol-market (accessed on 25 July 2023).
- Lorbeer, A.J.; Charoensiddhi, S.; Lahnstein, J.; Lars, C.; Franco, C.M.M.; Bulone, V.; Zhang, W. Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 2017, 29, 1515–1526.
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
868
Revisions:
2 times
(View History)
Update Date:
20 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




