Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Pérez de la Lastra | -- | 5719 | 2023-09-19 09:03:09 | | | |
| 2 | Camila Xu | Meta information modification | 5719 | 2023-09-19 09:39:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Andrés, C.M.C.; Pérez De La Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors. Encyclopedia. Available online: https://encyclopedia.pub/entry/49364 (accessed on 06 March 2026).
Andrés CMC, Pérez De La Lastra JM, Juan CA, Plou FJ, Pérez-Lebeña E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors. Encyclopedia. Available at: https://encyclopedia.pub/entry/49364. Accessed March 06, 2026.
Andrés, Celia María Curieses, José Manuel Pérez De La Lastra, Celia Andrés Juan, Francisco J. Plou, Eduardo Pérez-Lebeña. "Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors" Encyclopedia, https://encyclopedia.pub/entry/49364 (accessed March 06, 2026).
Andrés, C.M.C., Pérez De La Lastra, J.M., Juan, C.A., Plou, F.J., & Pérez-Lebeña, E. (2023, September 19). Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors. In Encyclopedia. https://encyclopedia.pub/entry/49364
Andrés, Celia María Curieses, et al. "Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors." Encyclopedia. Web. 19 September, 2023.
Copy Citation
Polyphenolic compounds are characterized by having at least one benzene ring with two or more hydroxyl (OH) groups. There are more than 8000 polyphenolic compounds in nature, classified into flavonoids, phenolic acids, lignans, and stilbenes.
polyphenols
antioxidant/pro-oxidant features
NRF2/ARE axis
1. Introduction
In biology, the production of reactive oxygen species (ROS) is a physiological and regulated process, but an uncontrolled increase can become pathological, lead to oxidative stress (OS), and trigger various diseases, as a consequence of an imbalance between radical production and endogenous antioxidant defense systems, which may be unable to mitigate their production. Over the last few years, there has been an abundance of research on the generation and effects of ROS on biological processes, mainly on proteins, lipids, and cellular DNA [1].
Polyphenolic compounds are characterized by having at least one benzene ring with two or more hydroxyl (OH) groups [2]. There are more than 8000 polyphenolic compounds in nature, classified into flavonoids, phenolic acids, lignans, and stilbenes [3]. The elemental structure of flavonoids, called aglycone, has two benzene rings linked by a three-carbon bridge, with a C6-C3-C6 skeleton [3].
Numerous studies have demonstrated the health benefits of dietary polyphenols, but some confusion remains as to their mechanistic action in vivo [4]. Currently, phenolic compounds are incorporated into foods because of their multiple functions, including their antioxidant and anti-inflammatory capacity, and they possess antimicrobial properties [5]. Stilbenes, phenolic acids, anthocyanins, flavanols, and isoflavones are all examples of polyphenols with anti-infective potential, as they can induce and/or modulate the synthesis of host defense peptides (HDPs) in various animal species. However, further research is needed to fully understand the complex interactions between polyphenols and HDPs [6]. Some of the properties of polyphenols are summarized in Figure 1.
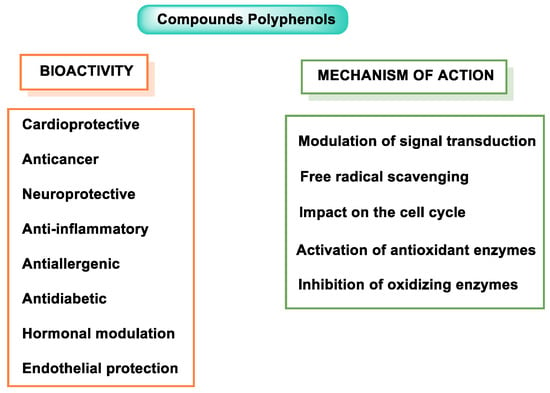
Figure 1. Polyphenol properties and mode of action.
In recent decades, polyphenols have received enormous attention from scientists and nutritionists for their role in human health, as they can help prevent or even treat diseases such as cancer, cardiovascular disease (CVD), and neurological disorders [7]. Their therapeutic effects are based on the antioxidant capacity as free radical scavengers and their involvement in the regulation of cell signaling pathways and gene regulation and expression [8].
Its well-known antioxidant effect is based on its ability to donate a proton H+ to free radicals, such as hydroxyl, peroxyl, etc., which in turn lose their reactivity, forming a relatively stable flavonoid radical, called a phenoxyl radical [9]. This proton is derived from the OH group present in the aromatic rings of polyphenols. This mechanism is explained in some detail for three polyphenols have been selected: resveratrol, hydroxytyrosol, and luteolin. Figure 2 characterizes the main mechanisms of antioxidant action.
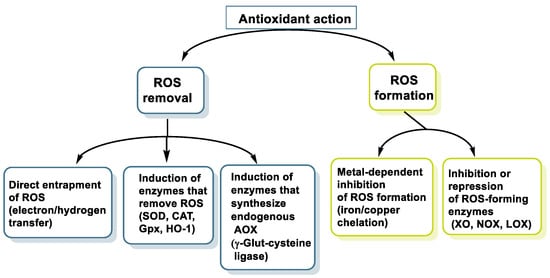
Figure 2. Main mechanisms of antioxidant action.
Polyphenols also act as chelators of metals such as Fe, reducing the kinetics in the Fenton reaction [10]. They are also involved in the inhibition of oxidant enzymes, such as lipoxygenase (LO) [11], cyclooxygenase (COX) [12], myeloperoxidase (MPO) [13], NADPH oxidase (NOx) [14], and xanthine oxidase (XO) [15], thus preventing the generation of reactive oxygen species (ROS) [16], and stimulate others with antioxidant properties, such as catalase (CAT) [17] and superoxide dismutase (SOD) [18].
It is worth mentioning the importance of the efforts being made in the development of new synthetic antioxidants. One such effort is the work of Rabecca Jenifer et al. on the rational design of heterocyclic molecules incorporated into sugar-triazole derivatives for antioxidant studies [19].
2. Chemical Structure of Polyphenols and Antioxidant Properties Based on Their Aromatic Resonant Structure
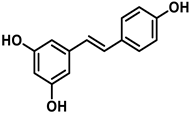
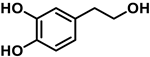
To carry out this study, three polyphenols have been selected which are usually present in the human diet and which are widely known, that is, resveratrol, hydroxytyrosol, and luteolin. Their molecular structure is expressed in Table 1:
Table 1. Chemical structure of resveratrol, hydroxytyrosol, and luteolin.
| Resveratrol | Hydroxytyrosol | Luteolin |
|---|---|---|
 |
 |
 |
Resveratrol is a stilbenoid that occurs naturally in various plants in response to injury or attack by pathogens such as fungi or bacteria [20]. Some studies have indicated that resveratrol activates sirtuin SIRT1 and improves mitochondria function [21]. In cells treated with resveratrol, it is observed that the action of superoxide dismutase depends on manganese MnSOD (SOD2), which increases up to 14 times [22]. There are also numerous studies associating beneficial effects of resveratrol in cancer and in cardio- and neuro-protective effects [23].
Hydroxytyrosol is a derivative of catechol found in a variety of natural sources, particularly olive oil and wine. Olives, leaves, and olive pulp contain large amounts of oleuropein, the most common hydroxytyrosol derivative [24]. HT is a new safe food for human consumption, with a level of unobserved adverse effects of 50 mg/kg of body weight per day, as evaluated by the European Food Safety Authority (EFSA) [25]. The Mediterranean diet is characterized by the regular intake of olive oil, which positively affects human health, including a reduction in cardiovascular disease rates [26][27]. Consuming olive oil, hydroxytyrosol, and oleuropein may inhibit the oxidation of LDL cholesterol, a risk factor for atherosclerosis, heart attack, or stroke [28].
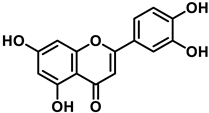
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a flavone present, in its glycosylated form, in different fruits and vegetables, and numerous studies have indicated that it has antioxidant, anticarcinogenic, anti-inflammatory, and neuroprotective effects [29]. Luteolin inhibited lipopolysaccharide LPS-induced release of TNF-α, IL-6, and NO by macrophages and significantly decreased the secretion of INF-γ, IL-6, COX-2, and iNOS in alveolar macrophage and peripheral macrophage cell lines [30]. This anti-inflammatory action was accompanied by suppression of the transcription factor NF-kβ (nuclear factor enhancer of kappa light chains of activated β cells; its defective regulation is associated with cancer, inflammatory and autoimmune diseases, septic shock, viral infections or inadequate immune development). Luteolin also suppresses inflammation in brain tissues and regulates different cell signaling pathways in neurodegenerative diseases and neuronal cell death [31].
The characteristic antioxidant potential of these three compounds is based on the phenolic OH group, which can donate H+ and remove the unpaired electron through the resonant electron system [32]. The OH groups donate a proton to various radicals •X such as hydroxyl, peroxyl, superoxide, or peroxynitrous acid, which lose their reactivity and in turn form a relatively stable flavonoid radical, called the phenoxyl radical [33]. Figure 3, Figure 4 and Figure 5 show the preferred positions for the formation of phenoxyl radicals for resveratrol, hydroxytyrosol, and luteolin, respectively. Figure 6, Figure 7 and Figure 8 show the resonant structures after formation of the phenoxyl radical in the different positions that are advantageous.
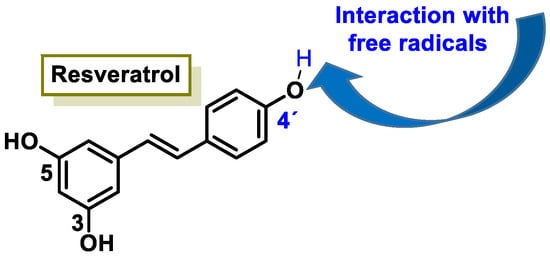
Figure 3. For resveratrol, phenoxyl radical positions at C-4′.
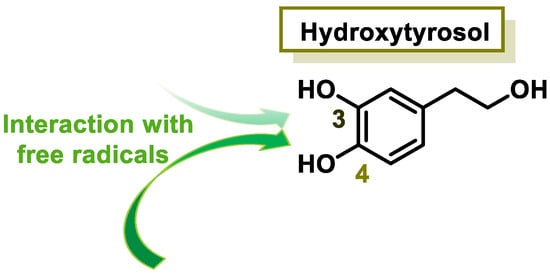
Figure 4. For hydroxytyrosol, phenoxyl radical positions at C-3 and C-4.
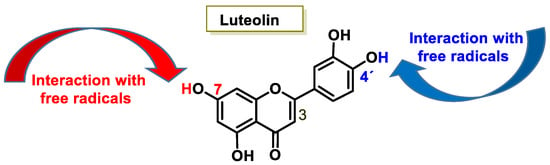
Figure 5. For luteolin, phenoxyl radical positions at C-7 and C-4′.
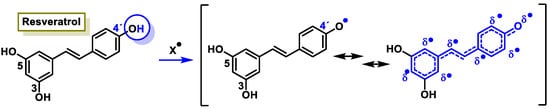
Figure 6. For resveratrol, electron delocalization at C-4′ positions.
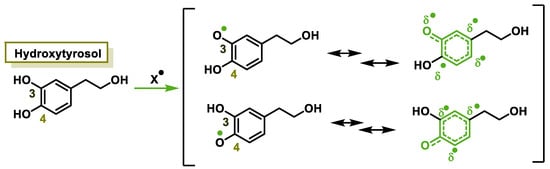
Figure 7. For hydroxytyrosol, electron delocalization at C-3 and C-4.
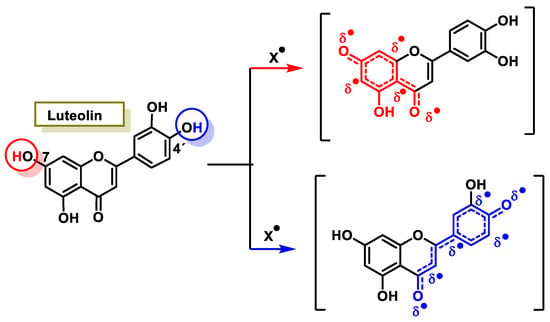
Figure 8. For luteolin, electron delocalization from C-7 and C-4′ positions.
For hydroxytyrosol, the odd electron is delocalized by the two benzene nuclei through the C=C double bond.
In hydroxytyrosol, the odd electron is only delocalized by the benzene nucleus, whether the radical is generated at the OH of C-3 or C-4.
The interaction of a FR (•X) at the OH of the C-7 position resulted in the electron delocalization throughout the A-ring (Figure 7). When •X is generated at C-7 and C-4′ OH positions of luteolin, there is a greater delocalization of the unpaired electron, yielding a greater number of resonant forms (in blue and red). OH located at 7 and 4′ positions were predicted to have greater antioxidant activity.
In luteolin, the radical formed at both C-7 and C-4′ is resonance-stabilized due to conjugation with the ketone or with the double bond and the ketone, respectively.
3. Oxidation of Polyphenolic Compounds to Quinones
For the selected compounds, in the aromatic groups, the presence of a phenolic group (at the B-ring of resveratrol), catechol (at the aromatic ring of hydroxytyrosol and at the B-ring of luteolin), and resorcinol (at the A-rings of resveratrol and luteolin) have been observed (Figure 9).
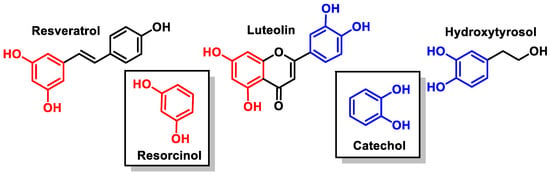
Figure 9. Catechol and resorcinol groups of resveratrol, luteolin, and hydroxytyrosol.
Resorcinol shares many characteristics with phenol and deprotonates [34]. Catechols (less acidic than resorcinol) are part of many essential chemicals and are valuable, typically nucleophilic intermediates used in synthesis [35].
Phenols and polyphenols are nucleophiles susceptible to electrophilic substitution reactions due to their high electron density [36]. The phenolic OH group facilitates the effective delocalization of the charge in the aromatic ring through resonance. In electrophilic aromatic substitution reactions, an electrophile substitutes one or more hydrogen atoms attached to the aromatic ring [37][38] (Figure 10).
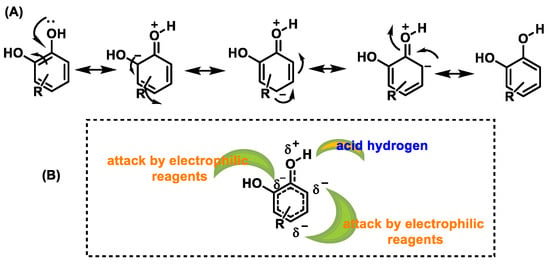
Figure 10. (A) Resonant forms of polyphenols. (B) Positions in polyphenols attacked by electrophiles.
In contrast, due to the aromatic ring system, quinones are resonance-stabilized electrophiles [39] (Figure 11).
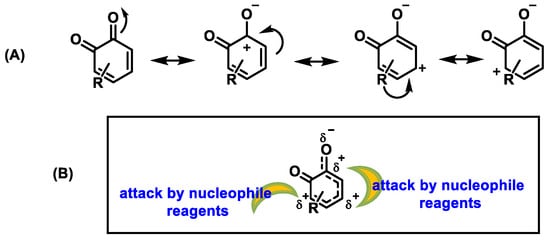
Figure 11. (A) Resonant forms of o-quinones. (B) Positions in o-quinones attacked by nucleophiles.
Polyphenols are oxidized by several oxidative enzymes, including cytochrome P450, COX-2, peroxidase, tyrosinase, XO, monoamine oxidase (MAO), and polyphenol oxidases (PPO)into highly reactive quinones [40]. PPO (widely distributed in the animal and plant world) is a group of copper-containing enzymes that catalyze the o-hydroxylation of monophenols to catechols and the oxidation of catechols to quinones in the presence of oxygen [41]. The hydroxylation reaction is kinetically slower than the oxidation reaction. As a result, quinones are obtained as products [42] (Figure 12).
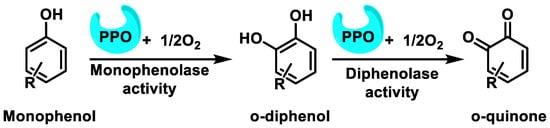
Figure 12. The oxidation reaction of phenols to o-quinones catalyzed by PPO and related enzymes.
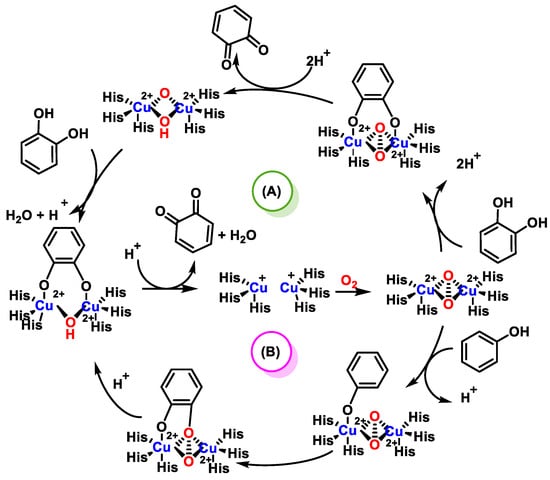
The most important structural feature of PPO is the presence in its active center of two copper atoms, bound to histidines; around the coppers, hydrophobic amino acids are located. The active sites have a trigonal pyramidal structure coordinated by the spheres formed by the three histidine ligands and the solvent molecule as a bridge. The reaction mechanism of PPO, shown in Figure 13, is a two-step catalysis.First is the oxidation of a monophenol to o-diphenol and the subsequent oxidation of this to o-quinone, i.e., a consecutive cresolase and catecholase activity. The enzyme first binds oxygen and then monophenol. A valence change of the copper ions from Cu1+ to Cu2+ occurs, forming a complex with an O-O bond where the diphenol is generated. The o-diphenol is then oxidized to o-quinone, which completes the cycle [43].
In polyphenols, the oxidation of a catechol configuration leads to the formation of an o-quinone structure, which can react with the sulfhydryl group of cysteine in glutathione or with the cysteine residues ofproteins [46]. This modification may be important for understanding the redox regulation of cellular functions that can be induced during polyphenol oxidation. Quinones formed by these reactions are active and covalently bond to nucleophilic side chains [47].
In the oxidation process of polyphenols to quinones, ROS are generated as part of the O2-reducing process, so this route is considered genotoxic [48]. Additionally, quinones are able to create DNA adducts, which cause genetic mutations [49]. ROS produce DNA strand breaks and oxidatively damaged nucleobases [50]. The genotoxicity of o-quinones is aggravated by the nuclear translocation of estrogen o-quinones by the estrogen receptor and by the nuclear translocation of polycyclic aromatic o-quinones by the aryl hydrocarbon receptor [51].
PPO is involved in the oxidation of phenolic compounds in fruits and vegetables, and this action is significant, as it reduces food quality, because oxidation induces the production of dark compounds (browning), which leads to consumer rejection and decreases the antioxidant capacity of foods [52]. The browning is generally assumed to be a direct consequence of phenolic compound oxidation by PPO action. Quinone formation can undergo polymerization processes and cause yellow and brown coloration [53].
In summary, polyphenolic compounds oxidize quite easily, despite the fact that in their FR-scavenging process, they donate a proton from the OH phenolic group and that the phenoxyl radical formed is relatively stable. The redox equilibrium between catechol and dihydroquinone and their oxidation to quinones readily drive those compounds to the oxidized state. During this process, the phenoxyl radical progressively donates reducing species, that is, H+ and e−,which are used to reduce the O2 or •O2− to H2O2 [54]. Thus, they behave analogously to the complexes present in the mitochondrial electron transport chain (ETC), but do not obtain H2O as an end product, as occurs in the ETC, but rather H2O2 and, ultimately, the hydroxyl radical (•OH).
The oxidation reactions of the three selected compounds, resveratrol, hydroxytyrosol, and luteolin will provide the corresponding o-quinones [48] (Figure 14):
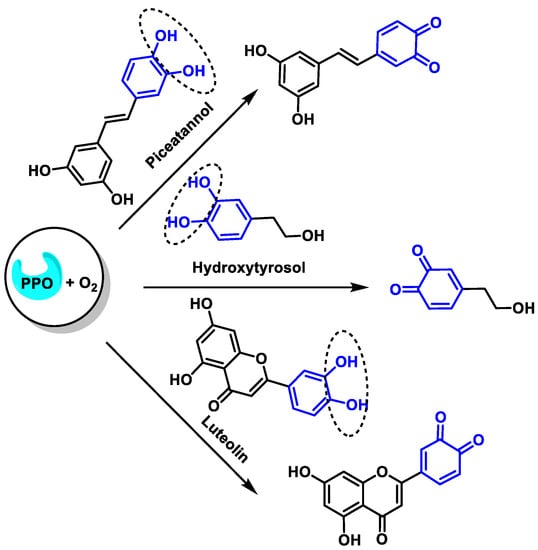
Figure 14. Oxidation of resveratrol, hydroxytyrosol, and luteolin to the corresponding o-quinones.
Enzymatic oxidation of the B-ring of resveratrol proceeds according to a two-step process, in the first of which cytochrome P450 oxidizes phenol to catechol and subsequently PPO oxidizes catechol to o-quinone [55] (Figure 15).
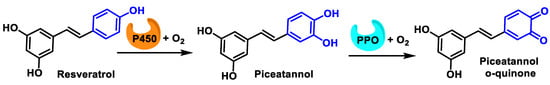
Figure 15. Oxidation of phenol to o-quinone in a two-step process.
Quinones are potent redox compounds that are readily reduced by electron-catalyzed P450/NADPH oxidoreductase 1 (NQO1), generating semiquinone radical anions [56]. The intermediate semiquinone radicals are very unstable and are easily oxidized to quinones by molecularO2. The reduction of molecular O2 generates •O2−, which is dismutated by superoxide dismutase(SOD)to H2O2, which in turn is reduced by the Fenton reaction to •OH and OH−, the highly reactive hydroxyl radical [57]. This redox cycling is responsible for OS within cells and is a major contributor to overall quinone [58]. Most quinones can also be reduced back (by 2e−) to the hydroquinone or catechol, a reduction process catalyzed by NQO1, which represents a major detoxification mechanism [57]. The redox balance between the p-quinone/hydroquinone and o-quinone/catechol pairshas an important effect on its pro-oxidant/antioxidant effects and on its cytotoxic/cytoprotective biological properties [59].
Quinones (as electrophilic compounds), are susceptible to reacting with glutathione (GSH) and usually this reaction is so simple that enzymatic catalysis by glutathione S-transferase (GST) is not necessary [60]. GSH can also be oxidized to GSSG by hydroxyl radicals generated during the quinone redox cycle [61].
Quinones are formed in the 2H+/2e− process of oxidation of catechol (1,2-dihydroxybenzene) and hydroquinone (1,4-dihydroxybenzene) [62]. However, the oxidation of resorcinol is significantly different from that of catechol and hydroquinone, including the reversibility of the process and the characteristics of the physicochemical responses [63]. The oxidation of resorcinol is a complicated process, and although theoretically its oxidation is an irreversible process forming 4-cyclohexene-1,3-dione and one would expect a similar reaction to the oxidation of catechol and hydroquinone, in practice, quinone formation is not the only possible route for the oxidation of resorcinol [64]. The oxidation pathway for resorcinol involves the formation of radical intermediates, which form dimer/polymer products [65].
4. Are Polyphenols Antioxidants, Pro-Oxidants, Or Both?
An antioxidant is a substance that inhibits or prevents oxidation, a chemical reaction of electron transfer from a substance to an oxidizing agent [66]. A pro-oxidant is any compound that accelerates oxidation or induces ROS, which can result in damage to cellular components and contribute to the development of various diseases [67]. Pro-oxidants interfere with the action of the body’s antioxidant enzymes and pathways, reducing their effectiveness in scavenging ROS and protection against oxidative damage [68]. There is a body of scientific literature that supports the idea that polyphenols are generally antioxidants that can act as pro-oxidants under certain conditions [69]. This concept is not correct and can lead to misunderstandings.
4.1. Polyphenols and Their Antioxidant Activity
Some of the mechanisms of the antioxidant activity of polyphenolic compounds are listed below (Figure 16).
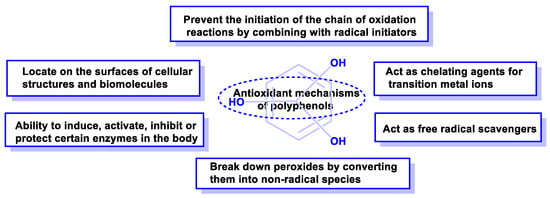
Figure 16. Mechanisms of action by which phenolic compounds present antioxidant activity.
Antioxidant capacity can be summarized in the following items:
-
They can prevent the initiation of the chain of oxidation reactions by combining with initiator radicals, such as hydroxyl radicals, e.g., caffeic acid or hydroxytyrosol [70].
-
They can decompose peroxides by converting them to non-radical species [71].
-
They can act as free radical scavengers [72].
-
They can act as chelating agents for transition metal ions and reduce the possibility of generating free radicals (as in the Fenton reaction) [73].
-
They can bind to cell membranes or combine with other biomolecules, thus reducing the consumption of metabolic antioxidants [74].
-
Polyphenolic compounds have the ability to induce, activate, inhibit, or protect enzymatic metabolism [75].
-
The antioxidant activity of polyphenols is well-known and derives from a combination of their Fe-chelating and FR-scavenging properties, together with their inhibitory activity on oxidase enzymes, lipoxygenase (LO), cyclooxygenase (COX), myeloperoxidase (MPO), NADPH oxidase (NOX), and xanthine oxidase (XO), thus preventing the endogenous generation of ROS in vivo [76].
The best-known antioxidant role of polyphenolic compounds is the scavenging of free radicals in lipid oxidation protection processes by reacting with the peroxyl radical [77]. The nature of the chemical structure of the polyphenolic compound has a major influence on its antioxidant activity. Polyphenolic antioxidants are excellent hydrogen and electron donors to hydroxyl, peroxyl, and peroxynitrite radicals and their radical intermediates are stable, due to the resonance delocalization of the unpaired electron within the aromatic ring and the absence of reactive positions towards O2 [78].
In summary, the structure of polyphenols, the number of OH groups, and substitution patterns on the benzene ring affect the antioxidant activity of polyphenols.
4.2. Polyphenols and Their Pro-Oxidant Activity
Pro-oxidant effects of polyphenols include transient reduction of Cu2+ to Cu+, formation of ROS, and potential disruption of cellular antioxidant defense, such as glutathione and glutathione-S-transferase [79].
The mechanisms underlying this effect are the formation of a labile aroxyl or a labile flavonoid-redox complex [80]. These mechanisms can produce H2O2, which can lead to Hb oxidation [81], and release Fe+2 ions to undergo auto-oxidation or even engage in a redox process, thus acting as pro-oxidants, which explains the mutagenic and genotoxic properties [82].
The pro-oxidant activity of flavonoids includes their oxidation to o- or p-quinones [83], which are highly reactive towards nucleophilic thiols and amino groups of proteins and glutathione. The B-ring structure of flavonoids (usually a catechol or a simple phenol) is readily oxidized and leads to the formation of electrophilic o-quinones, whereas resorcinol (more common in the A-ring of flavonoids) is not oxidized [84].
The definition of a pro-oxidant substance also includes substances that cause ROS or oxidative damage [85], as is the case here, which includes all reactive molecules containing FR in cells or tissues.
4.3. Polyphenols Are Both Antioxidants and Pro-Oxidants Due to the Way They Are Metabolized
There has always been a great scientific curiosity about the mechanism of action of polyphenols, their antioxidant capacity, and the relationship with the endogenous antioxidant defense [16]. At this time, although many mechanisms remain to be discovered (especially those related to its anti-inflammatory capacity), enough is already known about its antioxidant action and its influence on the prevention of different diseases. For example, in Spain, the study PREDIMED (Prevention with Mediterranean Diet) was conducted between 2003 and 2011 and included 7447 participants aged 55–80 years (58% women) at high cardiovascular risk, but initially free of the disease. It was shown that the groups treated with the Mediterranean diet achieved a statistically significant reduction in the rate of the main cardiovascular risk (myocardial infarction, stroke, or cardiovascular death) [86].
Polyphenols undergo extensive metabolism in the gastrointestinal tract (GIT) lumen, enterocyte (GIT epithelial cells responsible for the absorption of various essential nutrients), and liver [87]. Their absorption is reduced and slow [88], which contradicts the notion that their antioxidant activity may play an important role, as attributed to them.
Joseph Kanner, 2020, maintains that polyphenols generate H2O2 in the blood system, in the exogenous zone of the endothelial cell membrane. H2O2 enters the cells through aquaporin, the protein channels generally associated with water transport. The H2O2 concentration is around 0.1–2 µM and penetrates the cells between 0.01 and 0.1 µM to improve the adaptation and survival of the organism [89].
4.4. Methods for the Determination of the Antioxidant Activity
To determine antioxidant activity, methods are based on testing how an oxidizing agent induces oxidative damage to an oxidable substrate, which is inhibited or reduced in the presence of an antioxidant [90]. This inhibition is proportional to the antioxidant activity of the compound. Other assays are based on the quantification of the products formed after the oxidative process [91].
Antioxidant capacity is studied by several widely known methodologies, such as the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), the 2,2-diphenyl-1-picrylhydrazyl (DPPH), the N,N-dimethyl-ρ-phenylenediamine (DMPD), the Oxygen Radical Absorbance Capacity (ORAC), and the Ferric-Reducing Antioxidant Power (FRAP) assay. In the presence of antioxidants, the ferric form of the compound iron-tripyridyl-triazine (Fe3+-TPTZ) is reduced to the ferrous form (Fe2+-TPTZ). The Fe2+-TPTZ compound produces an intense blue coloring with an absorption maximum of 593 nm [92]. The FRAP method does not evaluate the free radical-scavenging capacity of the sample under study, but its reducing capacity by electron transfer and the ABTS and DPPH methods evaluate the capacity of the sample to neutralize free radicals [93]. The DPPH method studies the reduction of the 2,2-diphenyl-1-picrylhydrazyl radical upon reaction with H+ donor substances [94]. The generation of the DPPH radical is fast and without prior preparations, while that of the ABTS radical requires the use of chemical (manganese dioxide, potassium persulphate), enzymatic (peroxidase, myoglobulin), or electrochemical agents [92].
There are other techniques that measure antioxidant capacity, such as CUPRAC (cupric ion-reducing antioxidant capacity), ABAP (2′-azobis(2-amidopropane) depletion), DMPO (N-5,5-dimethyl-1-pyrroline oxide depletion), ORAC (oxygen radical absorbance capacity), and TRAP (total antioxidant capacity), among others [95].
5. Relationship of Polyphenols and Quinones with the Antioxidant Metabolism Pathway
Quinones are toxicological intermediates with a variety of hazardous effects in vivo, such as cytotoxicity, immunotoxicity, and carcinogenesis, but they can also be cytoprotective through the induction of antioxidant, detoxifying, and anti-inflammatory genes by modifying the cellular redox state [58].
Keap1 is the Kelch-like ECH-associated protein 1, NRF2 is the Nuclear factor erythroid 2-related factor 2, and ARE is the Antioxidant Response Elements [96]. In biology, the NRF2-ARE axis is a complex that regulates cellular antioxidant responses against oxidative stress, as well as cell proliferation [97]. Under normal conditions, the NRF2 factor is targeted by Keap1, which promotes its proteasomal degradation. Keap1 functions as a sensor of stress signals, through induced oxidation at key cysteine residues [98]. These conformational changes promote the release of NRF2 by the NRF2/Keap1 complex and once NRF2 release occurs, it translocates to the nucleus. Here, NRF2 induces phase II cytoprotective genes [99].
Phase II metabolic enzymes are a battery of critical proteins that detoxify xenobiotics (xenobiotics are chemicals extrinsic to physiological metabolism and to which an organism is exposed by ingestion, inhalation, or through the skin), with protective and preventive effects due to the induced regulation via the Keap1/NRF2/ARE pathway [100]. Phase II enzymes expressed by ARE include UDP-glucuronosyl transferases, sulfotransferases, N-acetyl transferases, glutathione S-transferases, and methyltransferases (mainly thiopurine S-methyl transferase and catechol O-methyl transferase). Impaired phase II enzyme activity can also lead to toxic effects of unmetabolized drugs [101].
Some specific enzymes produced by ARE genes are ferritin, heme oxygenase 1 (HO-1), NAD(P)H quinone oxidoreductase (NQO1), thioredoxin reductase (TrxR), and glutathione-metabolizing genes (glutathione peroxidase (GPx), glutathione s-transferase (GST), γ-glutamyl transpeptidase (GGT), glutathione-disulfide reductase (GSR), and glutamyl cysteine ligase (GCL)) [102].
NAD(P)H quinone oxidoreductase 1 (NQO1) catalyzes the 2e− reduction of quinones to hydroquinones, catechols, and a broad range of organic compounds [103]. Its physiological function is to reduce the free radical load in cells and the detoxification of xenobiotics [104][105].
Polyphenols possess a nucleophilic catechol moiety and their oxidized form (as o-quinone), formed after 2H+ and 2e− donation, induces the NRF2 factor due to the influence of its electrophilic character [106]. Indeed, when the Keap1 protein senses electrophilic stress, NRF2 is released from Keap1-mediated repression and accumulates in the nucleus, strongly inducing the expression of the cytoprotective gene set [107].
Keap1 is an intracellular protein sensor endowed with 27 reactive cysteine residues that act as receptors for electrophiles and oxidizing agents such as ROS [108], and 10 of these cysteines are adjacent to positively charged amino acids (maintaining the cysteine sulfhydryl group in a reactive state). The Keap1 protein acts as a sensor endowed with 27 reactive cysteine residues that function as receptors for electrophilic compounds and ROS agents [108]. Under OS, the cysteine residues of Keap1 are modified, causing a conformational change that prevents NRF2 ubiquitination [107]. The IVR domain of Keap1 contains reactive cysteine residues key to regulating NRF2 activity, including Cys226, Cys257, Cys273, and Cys288. So, NRF2 dissociation from Keap1 is mediated by oxidative modification of specific cysteine residues [109]. Cys273 and Cys288 are required for OS detection under both basal and stress conditions, whereas Cys151 (in the BTB domain of Keap1, surrounded by a cluster of positively charged amino acids) may be required only during OS conditions [110]. Cys226, Cys613, Cys622, and Cys624 specifically detect H2O2 through a mechanism different than that used to detect other electrophilic NRF2 inducers [111]. Tests on NRF2-null mice have revealed the contribution of NRF2 to genetic protection against various diseases caused by electrophilic and oxidative stress [112].
The thiol group of cysteine (Cys-SH) has a high reactivity with •O2− and H2O2 species [113], NO-derived species [114], and electrophilic byproducts of redox reactions [115], modifying the thiol group to sulfide derivatives (sulfenic (Cys-SO−), sulfinic acid (Cys-SO2−), sulfonic acid (Cys-SO32−), S-nitrosothiol (Cys-S-NO), and disulfides) [115]. Most of the sulfide derivatives come from the oxidation of the thiol group of cysteine, for example, by the effect of quinones, acting as oxidants [116].
Cysteine is the most nucleophilic amino acid of proteins and readily undergoes Michael addition with electrophiles [117]. A donation and acceptance of electrons takes place between a nucleophile and an electrophile [118]. A nucleophile contains an excess of electrons in the form of a negative charge (as the phenoxyl radical of polyphenols) and an electrophile may be inherently electron-deficient. The thiol group of the cysteine reacts by donating a pair of electrons to the electrophile, resulting in the formation of a covalent bond [119].
Quinones are Michael acceptors and readily react with nucleophiles like the sulfhydryl group on cysteine [120]. O-quinones generally undergo 1,6-reductive addition reactions with thiol nucleophiles, although 1,4-reductive addition is often observed as a minor product [121] (Figure 17).
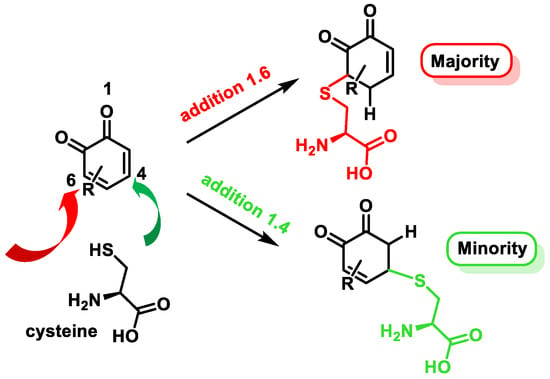
Figure 17. Michael addition products of cysteine to o-quinones.
The transcription factor BTB and CNC homology 1 (Bach1) is widely expressed in most mammalian tissues and its function is to act as a transcriptional suppressor [122]. Bach1 plays a key regulatory role in ROS production, cell cycle, heme homeostasis, hematopoiesis, and immunity, suppresses ischemic angiogenesis, and promotes breast cancer metastasis [123]. Bach1 inhibits the transcription of many OS response genes, including HO-1 and NQO1 [124]. Bach1, like Keap1, is a negative regulator of NRF2 that controls the ARE-dependent gene expressions [125]. Chuanyang Su et al. found that the electrophilic character of quinones is essential for the suppression of Bach1. Fe3+ enhanced NRF2 activation and ARE-driven gene expressions, suggesting quinones rather than catechol activate NRF2 through Bach1 arylation. The electrophilic character of quinones ensures their conjugation with Bach1 [126]. The Fenton reaction occurs in the presence of Fe2+ and H2O2, yielding Fe3+ and •OH + OH− (Figure 18).
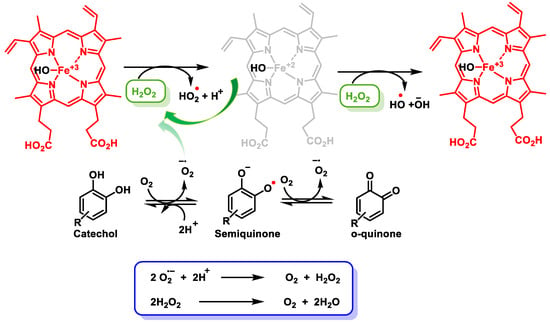
Figure 18. Reduction and oxidation of Fe and formed radicals. Oxidation of catechol to semiquinones and quinones, yielding H2O2.
Alternatively, the activation of NRF2 also occurs by H2O2 release, which is generated during the oxidation of polyphenols in aqueous media in the presence of oxygen or •O2− [127]. Polyphenols, in the oral human cavity, generate considerable levels of H2O2, involving interactions with transition metal ions [128]. Oxidation of polyphenols produces•O2−, H2O2, and a complex mixture of semiquinones and quinones [128].
6. Inhibitory Capacity of Polyphenols on Transcription Factors and on Enzymatic Mechanisms Involved in Several Diseases
Polyphenols are capable of modulating numerous processes, both at the level of transcription factors and cellular enzyme metabolism. Polyphenols are inhibitors of a variety of enzymes, including oxidative enzymes (such as XO and lipoxygenase), protease enzymes (which are involved in the breakdown of proteins), and inflammatory enzymes (such as COX-2) [129].
Dietary polyphenols also possess potential inhibitory activity against α-amylase (hydrolyzes α-1,4-glycosidic bonds of α-linked polysaccharides) and α-glucosidase (catalyzes the hydrolysis of non-reducing α-1,4 glucose molecules of disaccharides or oligosaccharides), the enzymes that convert the starch present in food into glucose. Hyperglycemia is one of the most common nutritional disorders in the world today [130]. Starch is a polysaccharide found in cereals, tubers, legumes, and some fruits and vegetables. It is the main source of energy in the human diet and the inhibition of the two enzymes described above contributes to the control of diabetes [131].
6.1. Polyphenols Are Inhibitors of NF-κB Transcription Factor
NF-κB is a family of five transcription factors involved in various physiological and pathological processes, such as immune function, apoptosis, carcinogenesis, and inflammatory diseases [132]. In cancer cells, NF-κB provides the ability to survive by upregulating anti-apoptotic genes, such as those in the BCL-2 family [133], and enhances drug resistance through the expression of resistance genes, such as resistance gene 1 (mdr1) [134].
Dysregulation of NF-κB signaling is implicated in several chronic diseases, such as inflammatory disorders, autoimmune diseases, and cancers [135]. Numerous studies have investigated the ability of polyphenols to modulate NF-κB signaling and have shown that they exert inhibitory effects on NF-κB activation through a variety of mechanisms [136]. (i) Polyphenols can inhibit the activity of IKK, the enzyme that phosphorylates IκB proteins. Polyphenols can block IKK activity and thus prevent translocation of NF-κB to the nucleus [137]. (ii) They indirectly inhibit NF-κB activation, due to its antioxidant properties [138]. (iii) They influence the composition of NF-κB subunits, altering the activity of the NF-κB complex (as the p65 subunit, a key component of the NF-κB complex) [139]. (iv) They disrupt upstream signaling pathways of NF-κB activation, such as receptors like Toll-like receptors (TLRs) or cytokine receptors, thereby inhibiting the initiation of NF-κB signaling cascades [140]. (v) They can modify epigenetic markers, such as DNA methylation [141] and histone modifications [142], altering the accessibility of NF-κB target genes. Examples of polyphenols that have been studied for their NF-κB-inhibitory effects include curcumin [143], resveratrol [144], quercetin [145], and epigallocatechin gallate (EGCG) [146].
6.2. Polyphenols Are Inhibitors of AP-1 Transcription Factor
AP-1 is a dimeric transcription factor, composed of proteins from the c-Fos, c-Jun, ATF, and JDP families, that is involved in the regulation of a wide range of cellular processes, including cell growth, differentiation, and apoptosis [147]. It is activated by a variety of stimuli, including OS, viral or bacterial infections, inflammation, and DNA damage [148]. AP-1 activation involves the following steps:
-
Phosphorylation of the transcription factors c-Jun and c-Fos, initially inactive, as they are bound to a protein called c-Jun-associated protein (JUN-AP). Phosphorylation of c-Jun and c-Fos by protein kinases separates them from JUN-AP and allows them to dimerize.
-
Once phosphorylated, c-Jun and c-Fos can dimerize to form the active AP-1 complex, which can then bind to specific DNA sequences, called AP-1 binding sites, activating the transcription of genes involved in differentiation, proliferation, and apoptosis.
Growth factors activate AP-1 through the MAP kinase pathway [149], whereas cytokines activate AP-1 through the NF-κB pathway [150]. OS can activate AP-1 through a variety of signaling pathways, including the cAMP-dependent protein kinase (PKA) pathway, the JNK pathway, and the p38 MAPK pathway, which ultimately lead to the phosphorylation and activation of c-Jun and c-Fos [151]. DNA damage can activate AP-1 through the p53 pathway, activating a series of genes that are involved in DNA repair and cell death, as c-Jun [152]. The activation of AP-1 is a tightly regulated process that is essential for many cellular processes [153]. However, the dysregulation of AP-1 can lead to a variety of diseases, such as cancer, inflammation, and neurodegenerative disorders [154].
Polyphenols can inhibit AP-1 activity by (i) blocking the binding of AP-1 to DNA, (ii) inactivating the AP-1 proteins, and (iii) interfering with the signal transduction pathways that activate AP-1 [155]. The inhibition of AP-1 activity has a number of beneficial effects, including reduced cell proliferation, increased cell differentiation, increased apoptosis, reduced inflammation, and increased antioxidant activity [156].
These findings suggest that polyphenols may have potential therapeutic applications for the prevention and treatment of a variety of diseases, including cancer [157], cardiovascular disease [158], and neurodegenerative diseases [159]. Some of the specific polyphenols that have been shown to inhibit AP-1 activity include EGCG, resveratrol, quercetin, luteolin, kaempferol, etc.
6.3. Polyphenols Are Inhibitors of NADPH Oxidases (NOX)
NOX, located in the cell membrane, are the main source of •O2− in non-phagocytic cells [160], and catalyze the transfer of an electron from the NADPH complex to FAD, leading to the first step in the reduction of O2 to superoxide anion [161]. The only function of NOX1-5 and DUOX1-2 is to produce •O2−, while other sources produce ROS as a byproduct (mitochondrial ETC, uncoupled NO synthase, or biochemical damage) [161]. In the particular case of NOX2, it has been associated with breast, colorectal, gastric, and prostate cancer, as well as myelomonocytic leukemia, among others. Extracellular ROS contribute to cancer, facilitating spread by creating an oncogenic environment that favors the formation of new micro-tumors and acts as a starting point for cell metastasis [162].
To remove extracellular •O2−, antioxidant enzymes include the superoxide dismutase SOD3 (Cu/Zn-SOD), which catalyzes its dismutation into H2O2 [163]. Subsequently, catalase reduces H2O2 to H2O. In cancer patients, there is a relationship between low SOD3 expression and NOX overproduction, favoring cancer progression [164]. Conversely, the reduction of extracellular ROS inhibits tumor growth and metastasis [165].
Several studies have investigated the potential of polyphenols to inhibit NOX activity and reduce ROS production and it has been observed that these natural compounds prevent NOX expression [14]. Several compounds that have been studied are resveratrol [166], quercetin [167], EGCG [168], and curcumin [169].
6.4. Polyphenols Are Inhibitors of the PI3K/AkT Axis
The critical PI3K/Akt axis is involved in various cellular pathway processes, including cell growth, proliferation, survival, and metabolism. Its deregulation is implicated in many diseases, including cancer, diabetes, and cardiovascular diseases [170]. Akt is a key regulator of cell survival and apoptosis (programmed cell death) [171].
Inhibition of PI3K limits downstream Akt activation. Several polyphenols, such as quercetin, resveratrol, and curcumin have been tested for inhibiting PI3K by phosphorylating phosphatidylinositol lipids [172]. These compounds have been examined in Akt inhibition, and their interference with Akt activation, by inhibiting its phosphorylation, has been observed.
The PI3K/Akt pathway also interacts with the mammalian target of the rapamycin pathway (mTOR), with a central role in cell growth and metabolism. Resveratrol and curcumin have been explored for their ability to inhibit mTOR signaling, even downstream of Akt [173].
6.5. Polyphenols Are Inhibitors of Anti-Apoptotic BCL-2 Family
The BCL-2 family of proteins (which includes both pro- and anti-apoptotic members) [174] regulates apoptosis and mediates the process by which mitochondria contribute to this pathway. The anti-apoptotic members (BCL-2 itself, BCL-XL, and MCL-1) prevent apoptosis by inhibiting the activation of pro-apoptotic proteins [175]. Disruption of the balance between pro- and anti-apoptotic members of the BCL-2 family is a common feature of many cancers, contributing to the survival and growth of cancer cells. Elevated levels of BCL-2 are related to cancer progression and resistance to chemotherapeutic treatments [176]. Certain polyphenols can modulate the BCL-2 activity, supporting apoptosis and inhibiting cancer cell growth [177]. Some polyphenols have been shown to down-regulate anti-apoptotic BCL-2 family members, tipping the balance in favor of pro-apoptotic proteins and sensitizing cancer cells to apoptosis [178]. This can lead to decreased cancer cell survival and enhanced response to anticancer therapies.
References
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2012, 18, 1818–1892.
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25.
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251.
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233.
- Tobin, I.; Zhang, G. Regulation of Host Defense Peptide Synthesis by Polyphenols. Antibiotics 2023, 12, 660.
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189.
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047.
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886.
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831.
- Ratnasari, N.; Walters, M.; Tsopmo, A. Antioxidant and lipoxygenase activities of polyphenol extracts from oat brans treated with polysaccharide degrading enzymes. Heliyon 2017, 3, e00351.
- Owczarek, K.; Lewandowska, U. The Impact of Dietary Polyphenols on COX-2 Expression in Colorectal Cancer. Nutr. Cancer 2017, 69, 1105–1118.
- Yeoh, B.S.; Aguilera Olvera, R.; Singh, V.; Xiao, X.; Kennett, M.J.; Joe, B.; Lambert, J.D.; Vijay-Kumar, M. Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am. J. Pathol. 2016, 186, 912–926.
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213.
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470.
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078.
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220.
- Jenifer, V.R.; Muthuvel, P.; Das, T.M. Rational Design of Heterocyclic Moieties Incorporated in Sugar-Triazole Derivatives for Antioxidant Studies. ChemistrySelect 2021, 6, 9955–9959.
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A Natural Arsenal against Bacterial Pathogens. Antibiotics 2020, 9, 336.
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122.
- Robb, E.L.; Page, M.M.; Wiens, B.E.; Stuart, J.A. Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD. Biochem. Biophys. Res. Commun. 2008, 367, 406–412.
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506.
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive tree derivatives and hydroxytyrosol: Their potential effects on human health and its use as functional ingredient in meat. Foods 2021, 10, 2611.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04728.
- Hu, T.; He, X.-W.; Jiang, J.-G.; Xu, X.-L. Hydroxytyrosol and Its Potential Therapeutic Effects. J. Agric. Food Chem. 2014, 62, 1449–1455.
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health: A critical review. Circ. Res. 2019, 124, 779–798.
- Marcelino, G.; Hiane, P.A.; Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826.
- Watson, R.R.; Preedy, V.R. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: Cambridge, MA, USA, 2019.
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224.
- Asakura, H.; Kitahora, T. Polyphenols: Prevention and Treatment of Human Disease; Academic Press: Cambridge, MA, USA, 2018.
- Hsueh, C.C.; Wu, C.C.; Chen, B.Y. Polyphenolic compounds as electron shuttles for sustainable energy utilization. Biotechnol. Biofuels 2019, 12, 271.
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Structures and Energy Content. Stresses 2022, 2, 213–230.
- Bonechi, M.; Innocenti, M.; Vanossi, D.; Fontanesi, C. The Fundamental and Underrated Role of the Base Electrolyte in the Polymerization Mechanism. The Resorcinol Case Study. J. Phys. Chem. A 2021, 125, 34–42.
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical Reactivities of ortho-Quinones Produced in Living Organisms: Fate of Quinonoid Products Formed by Tyrosinase and Phenoloxidase Action on Phenols and Catechols. Int. J. Mol. Sci. 2020, 21, 6080.
- Lin, D.; Jiang, S.; Zhang, A.; Wu, T.; Qian, Y.; Shao, Q. Structural derivatization strategies of natural phenols by semi-synthesis and total-synthesis. Nat. Prod. Bioprospect. 2022, 12, 8.
- Hawsawi, M.; Wickramasinghe, A.; Crich, D. Use of Phenols as Nucleophiles in the Zbiral Oxidative Deamination of N-Acetyl Neuraminic Acid: Isolation and Characterization of Tricyclic 3-Keto-2-deoxy-nonulosonic Acid (KDN) Derivatives via an Intermediate Vinyl Diazonium Ion. J. Org. Chem. 2019, 84, 14688–14700.
- Toteva, M.M.; Richard, J.P. The generation and reactions of quinone methides. In Advances in Physical Organic Chemistry; Richard, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 45, pp. 39–91.
- Fisher, A.A.; Labenski, M.T.; Malladi, S.; Gokhale, V.; Bowen, M.E.; Milleron, R.S.; Bratton, S.B.; Monks, T.J.; Lau, S.S. Quinone Electrophiles Selectively Adduct “Electrophile Binding Motifs” within Cytochrome c. Biochemistry 2007, 46, 11090–11100.
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol Oxidases in Crops: Biochemical, Physiological and Genetic Aspects. Int. J. Mol. Sci. 2017, 18, 377.
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M.; et al. Novel Roles for the Polyphenol Oxidase Enzyme in Secondary Metabolism and the Regulation of Cell Death in Walnut. Plant Physiol. 2014, 164, 1191–1203.
- Nogales-Delgado, S. Polyphenoloxidase (PPO): Effect, Current Determination and Inhibition Treatments in Fresh-Cut Produce. Appl. Sci. 2021, 11, 7813.
- Ramirez, E.; Whitaker, J.; Virador, V. Polyphenol oxidase. In Food Enzymes: Structure and Mechanism; Springer: Boston, MA, USA, 2003; pp. 509–524.
- Lerch, K. Tyrosinase: Molecular and Active-Site Structure. In Enzymatic Browning and Its Prevention; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1995; Volume 600, pp. 64–80.
- Mayer, A.; Harel, E. Phenoloxidases and their significance in fruit and vegetables. Food Enzymol. 1991, 1, 373–398.
- Ishii, T.; Ishikawa, M.; Miyoshi, N.; Yasunaga, M.; Akagawa, M.; Uchida, K.; Nakamura, Y. Catechol Type Polyphenol Is a Potential Modifier of Protein Sulfhydryls: Development and Application of a New Probe for Understanding the Dietary Polyphenol Actions. Chem. Res. Toxicol. 2009, 22, 1689–1698.
- Bruins, J.J.; Albada, B.; van Delft, F. ortho-Quinones and Analogues Thereof: Highly Reactive Intermediates for Fast and Selective Biofunctionalization. Chem.-A Eur. J. 2018, 24, 4749–4756.
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37.
- Penning, T.M. Genotoxicity of ortho-quinones: Reactive oxygen species versus covalent modification. Toxicol. Res. 2017, 6, 740–754.
- Curieses Andrés, C.M.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. From reactive species to disease development: Effect of oxidants and antioxidants on the cellular biomarkers. J. Biochem. Mol. Toxicol. 2023, e23455.
- Bolton, J.L.; Thatcher, G.R.J. Potential Mechanisms of Estrogen Quinone Carcinogenesis. Chem. Res. Toxicol. 2008, 21, 93–101.
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876.
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200.
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274.
- Testa, B.; Pedretti, A.; Vistoli, G. Reactions and enzymes in the metabolism of drugs and other xenobiotics. Drug Discov. Today 2012, 17, 549–560.
- Brunmark, A.; Cadenas, E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic. Biol. Med. 1989, 7, 435–477.
- Colucci, M.A.; Couch, G.D.; Moody, C.J. Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1: From synthetic organic chemistry to compounds with anticancer potential. Org. Biomol. Chem. 2008, 6, 637–656.
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of Quinones in Toxicology. Chem. Res. Toxicol. 2000, 13, 135–160.
- Satoh, T.; Stalder, R.; McKercher, S.R.; Williamson, R.E.; Roth, G.P.; Lipton, S.A. Nrf2 and HSF-1 Pathway Activation via Hydroquinone-Based Proelectrophilic Small Molecules is Regulated by Electrochemical Oxidation Potential. ASN Neuro 2015, 7.
- Kalgutkar, A.S.; Gardner, I.; Obach, R.S.; Shaffer, C.L.; Callegari, E.; Henne, K.R.; Mutlib, A.E.; Dalvie, D.K.; Lee, J.S.; Nakai, Y.; et al. A comprehensive listing of bioactivation pathways of organic functional groups. Curr. Drug Metab. 2005, 6, 161–225.
- Gant, T.W.; Ramakrishna Rao, D.N.; Mason, R.P.; Cohen, G.M. Redox cycling and sulphydryl arylation; Their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem.-Biol. Interact. 1988, 65, 157–173.
- Lin, Q.; Li, Q.; Batchelor-McAuley, C.; Compton, R.G. Two-Electron, Two-Proton Oxidation of Catechol: Kinetics and Apparent Catalysis. J. Phys. Chem. C 2015, 119, 1489–1495.
- Ngamchuea, K.; Tharat, B.; Hirunsit, P.; Suthirakun, S. Electrochemical oxidation of resorcinol: Mechanistic insights from experimental and computational studies. RSC Adv. 2020, 10, 28454–28463.
- Nasr, B.; Abdellatif, G.; Canizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical Oxidation of Hydroquinone, Resorcinol, and Catechol on Boron-Doped Diamond Anodes. Environ. Sci. Technol. 2005, 39, 7234–7239.
- Bonechi, M.; Giurlani, W.; Stefani, A.; Marchetti, A.; Innocenti, M.; Fontanesi, C. Resorcinol electropolymerization process obtained via electrochemical oxidation. Electrochim. Acta 2022, 428, 140928.
- Worsfold, P.; Townshend, A.; Poole, C.F.; Miró, M. Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019.
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841.
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014.
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736.
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909.
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141.
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66.
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357.
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809.
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary Polyphenols in Metabolic and Neurodegenerative Diseases: Molecular Targets in Autophagy and Biological Effects. Antioxidants 2021, 10, 142.
- Van Acker, S.A.B.E.; Van Den Berg, D.-j.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342.
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123.
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure-Activity Relationship-A Quantum Chemical Study. Antioxid 2020, 9, 461.
- Sahu, S.C.; Gray, G.C. Pro-oxidant activity of flavonoids: Effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cancer Lett. 1996, 104, 193–196.
- Cadenas, E. Basic mechanisms of antioxidant activity. Biofactors 1997, 6, 391–397.
- Jia, Y.; Alayash, A.I. Effects of (-)-epigallocatechin gallate on the redox reactions of human hemoglobin. Free Radic. Biol. Med. 2008, 45, 659–666.
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354.
- Joyner, P.M. Protein Adducts and Protein Oxidation as Molecular Mechanisms of Flavonoid Bioactivity. Molecules 2021, 26, 5102.
- Lee-Hilz, Y.Y.; Boerboom, A.-M.J.F.; Westphal, A.H.; van Berkel, W.J.H.; Aarts, J.M.M.J.G.; Rietjens, I.M.C.M. Pro-Oxidant Activity of Flavonoids Induces EpRE-Mediated Gene Expression. Chem. Res. Toxicol. 2006, 19, 1499–1505.
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11.
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60.
- Dryden, G.W.; Song, M.; McClain, C. Polyphenols and gastrointestinal diseases. Curr. Opin. Gastroenterol. 2006, 22, 165–170.
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342.
- Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797.
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; Al-Farga, A.M. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644.
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380.
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732.
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208.
- Cerón, I.; Higuita, J.; Cardona, C. Capacidad antioxidante y contenido fenólico total de tres frutas cultivadas en la región andina. Vector 2010, 5, 5.
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547.
- Tian, W.; de la Vega, M.R.; Schmidlin, C.J.; Ooi, A.; Zhang, D.D. Kelch-like ECH-associated protein 1 (KEAP1) differentially regulates nuclear factor erythroid-2–related factors 1 and 2 (NRF1 and NRF2). J. Biol. Chem. 2018, 293, 2029–2040.
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022, 291, 120111.
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218.
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47.
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2010, 154, 103–116.
- Higgins, L.G.; Kelleher, M.O.; Eggleston, I.M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol. Appl. Pharmacol. 2009, 237, 267–280.
- Siegel, D.; Kepa, J.K.; Ross, D. NAD(P)H:quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in human cells. PLoS ONE 2012, 7, e44861.
- Anusevičius, Ž.; Šarlauskas, J.; Narimantas, Č. Two-electron reduction of quinones by rat liver NAD(P)H:quinone oxidoreductase: Quantitative structure–activity relationships. Arch. Biochem. Biophys. 2002, 404, 254–262.
- Onyenwoke, R.U.; Wiegel, J. Iron (III) reduction: A novel activity of the human NAD(P)H:oxidoreductase. Biochem. Biophys. Res. Commun. 2007, 353, 389–393.
- Sirota, R.; Gibson, D.; Kohen, R. The role of the catecholic and the electrophilic moieties of caffeic acid in Nrf2/Keap1 pathway activation in ovarian carcinoma cell lines. Redox Biol. 2015, 4, 48–59.
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and Electrophilic Stresses Activate Nrf2 through Inhibition of Ubiquitination Activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229.
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93.
- Miseta, A.; Csutora, P. Relationship Between the Occurrence of Cysteine in Proteins and the Complexity of Organisms. Mol. Biol. Evol. 2000, 17, 1232–1239.
- Cleasby, A.; Yon, J.; Day, P.J.; Richardson, C.; Tickle, I.J.; Williams, P.A.; Callahan, J.F.; Carr, R.; Concha, N.; Kerns, J.K.; et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS ONE 2014, 9, e98896.
- Larraufie, P.; Roberts, G.P.; McGavigan, A.K.; Kay, R.G.; Li, J.; Leiter, A.; Melvin, A.; Biggs, E.K.; Ravn, P.; Davy, K.; et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019, 26, 1399–1408.
- Uruno, A.; Motohashi, H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 2011, 25, 153–160.
- Ying, J.; Clavreul, N.; Sethuraman, M.; Adachi, T.; Cohen, R.A. Thiol oxidation in signaling and response to stress: Detection and quantification of physiological and pathophysiological thiol modifications. Free Radic. Biol. Med. 2007, 43, 1099–1108.
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls.: The cytotoxic potential of superoxide and nitric oxide*. J. Biol. Chem. 1991, 266, 4244–4250.
- Ghezzi, P.; Bonetto, V. Redox proteomics: Identification of oxidatively modified proteins. PROTEOMICS Int. Ed. 2003, 3, 1145–1153.
- Iciek, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Kwiecień, I.; Górny, M.; Włodek, L. S-sulfhydration as a cellular redox regulation. Biosci. Rep. 2015, 36.
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003, 111, 583–593.
- LoPachin, R.M.; Gavin, T. Reactions of electrophiles with nucleophilic thiolate sites: Relevance to pathophysiological mechanisms and remediation. Free Radic. Res. 2016, 50, 195–205.
- Netto, L.E.S.; de Oliveira, M.A.; Monteiro, G.; Demasi, A.P.D.; Cussiol, J.R.R.; Discola, K.F.; Demasi, M.; Silva, G.M.; Alves, S.V.; Faria, V.G.; et al. Reactive cysteine in proteins: Protein folding, antioxidant defense, redox signaling and more. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 180–193.
- Iverson, S.L.; Hu, L.Q.; Vukomanovic, V.; Bolton, J.L. The Influence of the p-Alkyl Substituent on the Isomerization of o-Quinones to p-Quinone Methides: Potential Bioactivation Mechanism for Catechols. Chem. Res. Toxicol. 1995, 8, 537–544.
- Bolton, J.L.; Acay, N.M.; Vukomanovic, V. Evidence That 4-Allyl-o-quinones Spontaneously Rearrange to Their More Electrophilic Quinone Methides: Potential Bioactivation Mechanism for the Hepatocarcinogen Safrole. Chem. Res. Toxicol. 1994, 7, 443–450.
- Zhang, X.; Guo, J.; Wei, X.; Niu, C.; Jia, M.; Li, Q.; Meng, D. Bach1: Function, Regulation, and Involvement in Disease. Oxid. Med. Cell Longev. 2018, 2018, 1347969.
- Igarashi, K.; Nishizawa, H.; Saiki, Y.; Matsumoto, M. The transcription factor BACH1 at the crossroads of cancer biology: From epithelial-mesenchymal transition to ferroptosis. J. Biol. Chem. 2021, 297, 101032.
- Dhakshinamoorthy, S.; Jain, A.K.; Bloom, D.A.; Jaiswal, A.K. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J. Biol. Chem. 2005, 280, 16891–16900.
- MacLeod, A.K.; McMahon, M.; Plummer, S.M.; Higgins, L.G.; Penning, T.M.; Igarashi, K.; Hayes, J.D. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: Demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis 2009, 30, 1571–1580.
- Su, C.; Liu, Z.; Wang, Y.; Wang, Y.; Song, E.; Song, Y. The electrophilic character of quinones is essential for the suppression of Bach1. Toxicology 2017, 387, 17–26.
- Fourquet, S.; Guerois, R.; Biard, D.; Toledano, M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010, 285, 8463–8471.
- Ofoedu, C.E.; You, L.; Osuji, C.M.; Iwouno, J.O.; Kabuo, N.O.; Ojukwu, M.; Agunwah, I.M.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Hydrogen Peroxide Effects on Natural-Sourced Polysacchrides: Free Radical Formation/Production, Degradation Process, and Reaction Mechanism—A Critical Synopsis. Foods 2021, 10, 699.
- Sandra, G.; Anabela, R. Inhibitory Properties of Phenolic Compounds Against Enzymes Linked with Human Diseases. In Phenolic Compounds; Marcos, S.-H., Mariana, P.-T., Maria del Rosario, G.-M., Eds.; IntechOpen: Rijeka, Yugoslavia, 2017; Chapter 6.
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692.
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253.
- Jimi, E.; Huang, F.; Nakatomi, C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int. J. Mol. Sci. 2019, 20, 6275.
- Mahoney, D.J.; Cheung, H.H.; Mrad, R.L.; Plenchette, S.; Simard, C.; Enwere, E.; Arora, V.; Mak, T.W.; Lacasse, E.C.; Waring, J.; et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc. Natl. Acad. Sci. USA 2008, 105, 11778–11783.
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002, 180, 91–101.
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618.
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467.
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell Longev. 2012, 2012, 914273.
- da Cunha, L.R.; Muniz-Junqueira, M.I.; Dos Santos Borges, T.K. Impact of polyphenols in phagocyte functions. J. Inflamm. Res. 2019, 12, 205–217.
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10, 1000.
- Fang, M.; Chen, D.; Yang, C.S. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007, 137, 223s–228s.
- Yamaguchi, K.; Itakura, M.; Tsukamoto, M.; Lim, S.Y.; Uchida, K. Natural polyphenols convert proteins into histone-binding ligands. J. Biol. Chem. 2022, 298, 102529.
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) . J. Biol. Chem. 1995, 270, 24995–25000.
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-κB signaling through suppression of p65 and IB kinase activities. Die Pharm.-Int. J. Pharm. Sci. 2013, 68, 689–694.
- Ruiz, P.A.; Braune, A.; Hölzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 2007, 137, 1208–1215.
- Lakshmi, S.P.; Reddy, A.T.; Kodidhela, L.D.; Varadacharyulu, N.C. The tea catechin epigallocatechin gallate inhibits NF-κB-mediated transcriptional activation by covalent modification. Arch. Biochem. Biophys. 2020, 695, 108620.
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136.
- Kyriakis, J.M. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr. 1999, 7, 217–231.
- Gannett, P.M.; Ye, J.; Ding, M.; Powell, J.; Zhang, Y.; Darian, E.; Daft, J.; Shi, X. Activation of AP-1 through the MAP kinase pathway: A potential mechanism of the carcinogenic effect of arenediazonium ions. Chem. Res. Toxicol. 2000, 13, 1020–1027.
- Fujioka, S.; Niu, J.; Schmidt, C.; Sclabas, G.M.; Peng, B.; Uwagawa, T.; Li, Z.; Evans, D.B.; Abbruzzese, J.L.; Chiao, P.J. NF-kappaB and AP-1 connection: Mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol. Cell Biol. 2004, 24, 7806–7819.
- Chiu, W.C.; Chen, C.J.; Lee, T.S.; Chen, Z.J.; Ke, P.H.; Chiang, A.N. Oxidative stress enhances AP-1 and NF-κB-mediated regulation of β(2)-glycoprotein I gene expression in hepatoma cells. J. Cell Biochem. 2010, 111, 988–998.
- Schreiber, M.; Kolbus, A.; Piu, F.; Szabowski, A.; Möhle-Steinlein, U.; Tian, J.; Karin, M.; Angel, P.; Wagner, E.F. Control of cell cycle progression by c-Jun is p53 dependent. Genes. Dev. 1999, 13, 607–619.
- Balli, M.; Chui, J.S.; Athanasouli, P.; Abreu de Oliveira, W.A.; El Laithy, Y.; Sampaolesi, M.; Lluis, F. Activator Protein-1 Transcriptional Activity Drives Soluble Micrograft-Mediated Cell Migration and Promotes the Matrix Remodeling Machinery. Stem Cells Int. 2019, 2019, 6461580.
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers 2019, 11, 1037.
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes. Nutr. 2007, 2, 257–273.
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868.
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515.
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131.
- Yan, L.; Guo, M.S.; Zhang, Y.; Yu, L.; Wu, J.M.; Tang, Y.; Ai, W.; Zhu, F.D.; Law, B.Y.; Chen, Q.; et al. Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities. Oxid. Med. Cell Longev. 2022, 2022, 5288698.
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735.
- Andersson, K.E. Oxidative Stress and Its Relation to Lower Urinary Tract Symptoms. Int. Neurourol. J. 2022, 26, 261–267.
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192.
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928.
- Griess, B.; Tom, E.; Domann, F.; Teoh-Fitzgerald, M. Extracellular superoxide dismutase and its role in cancer. Free Radic. Biol. Med. 2017, 112, 464–479.
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857.
- Jang, J.Y.; Min, J.H.; Wang, S.B.; Chae, Y.H.; Baek, J.Y.; Kim, M.; Ryu, J.S.; Chang, T.S. Resveratrol inhibits collagen-induced platelet stimulation through suppressing NADPH oxidase and oxidative inactivation of SH2 domain-containing protein tyrosine phosphatase-2. Free Radic. Biol. Med. 2015, 89, 842–851.
- Sul, O.-J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949.
- Zhu, W.; Oteiza, P.I. NADPH oxidase 1: A target in the capacity of dimeric ECG and EGCG procyanidins to inhibit colorectal cancer cell invasion. Redox Biol. 2023, 65, 102827.
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698.
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635.
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128.
- Arena, A.; Romeo, M.A.; Benedetti, R.; Masuelli, L.; Bei, R.; Gilardini Montani, M.S.; Cirone, M. New Insights into Curcumin- and Resveratrol-Mediated Anti-Cancer Effects. Pharmaceuticals 2021, 14, 1068.
- Tamaddoni, A.; Mohammadi, E.; Sedaghat, F.; Qujeq, D.; As’Habi, A. The anticancer effects of curcumin via targeting the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway. Pharmacol. Res. 2020, 156, 104798.
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5.
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363.
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619.
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008, 9, 213–228.
- Thyagarajan, A.; Forino, A.S.; Konger, R.L.; Sahu, R.P. Dietary Polyphenols in Cancer Chemoprevention: Implications in Pancreatic Cancer. Antioxidants 2020, 9, 651.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
2 times
(View History)
Update Date:
19 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





