
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kuo-Hu Chen | -- | 1547 | 2023-09-16 16:26:33 | | | |
| 2 | Catherine Yang | Meta information modification | 1547 | 2023-09-18 10:23:03 | | |
Video Upload Options
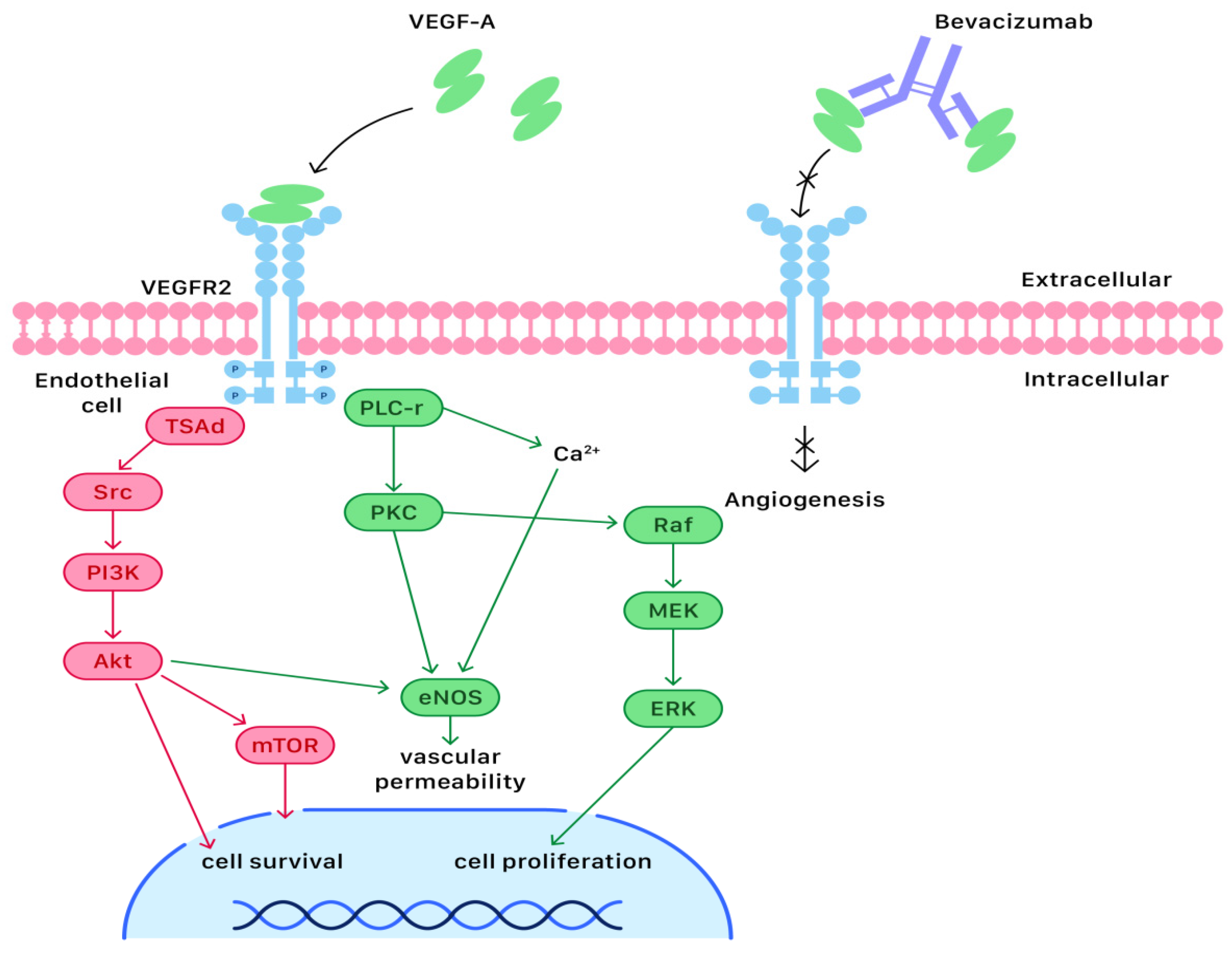
Most ovarian cancer cases are diagnosed at an advanced stage (III or IV), in which a primary debulking surgery combined with adjuvant systemic chemotherapy is the standard management. Since targeted therapy is less toxic to human cells than systemic chemotherapy, it has drawn much attention and become more popular. Angiogenesis is a critical process during the proliferation of ovarian cancer cells. Currently, many studies have put emphases on anti-angiogenetic medication, such as bevacizumab, the first and most investigated angiogenesis inhibitor that can exert anti-neoplastic effects. Bevacizumab is a recombinant humanized monoclonal antibody that has been approved for first-line maintenance treatment of advanced ovarian cancer.
1. Introduction
2. Molecular and Cellular Mechanisms of Bevacizumab Actions
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296.
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2020, 137, 108–121.
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3460–3473.
- Marchetti, C.; Muzii, L.; Romito, A.; Panici, P.B. First-line treatment of women with advanced ovarian cancer: Focus on bevacizumab. OncoTargets Ther. 2019, 12, 1095–1103.
- Arora, S.; Balasubramaniam, S.; Zhang, H.; Berman, T.; Narayan, P.; Suzman, D.; Bloomquist, E.; Tang, S.; Gong, Y.; Sridhara, R.; et al. FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist 2020, 26, e164–e172.
- Stacker, S.A.; Achen, M.G. The VEGF signaling pathway in cancer: The road ahead. Chin. J. Cancer 2013, 32, 297–302.
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2015, 139, 10–16.
- Aravantinos, G.; Pectasides, D. Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: A systematic review. J. Ovarian Res. 2014, 7, 57.
- Fleming, N.D.; Coleman, R.L.; Tung, C.; Westin, S.N.; Hu, W.; Sun, Y.; Bhosale, P.; Munsell, M.F.; Sood, A.K. Phase II trial of bevacizumab with dose-dense paclitaxel as first-line treatment in patients with advanced ovarian cancer. Gynecol. Oncol. 2017, 147, 41–46.
- Paris, I.; Cianci, S.; Vizzielli, G.; Fagotti, A.; Ferrandina, G.; Alletti, S.G.; Costantini, B.; Cosentino, F.; Capoluongo, E.D.; Pasqualoni, M.; et al. Upfront HIPEC and bevacizumab-containing adjuvant chemotherapy in advanced epithelial ovarian cancer. Int. J. Hyperth. 2018, 35, 370–374.
- Vergote, I.; Ray-Coquard, I.; Anderson, D.M.; Cantuaria, G.; Colombo, N.; Garnier-Tixidre, C.; Gilbert, L.; Harter, P.; Hettle, R.; Lorusso, D.; et al. Population-adjusted indirect treatment comparison of the SOLO1 and PAOLA-1/ENGOT-ov25 trials evaluating maintenance olaparib or bevacizumab or the combination of both in newly diagnosed, advanced BRCA-mutated ovarian cancer. Eur. J. Cancer 2021, 157, 415–423.
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428.
- Ellis, L.M. Mechanisms of Action of Bevacizumab as a Component of Therapy for Metastatic Colorectal Cancer. Semin. Oncol. 2006, 33, S1–S7.
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Physiol. 2002, 282, C947–C970.
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H. Bevacizumab. Oncologist 2010, 15, 819–825.
- Aziz, A.U.R.; Farid, S.; Qin, K.; Wang, H.; Liu, B. PIM Kinases and Their Relevance to the PI3K/AKT/mTOR Pathway in the Regulation of Ovarian Cancer. Biomolecules 2018, 8, 7.
- Van der Ploeg, P.; van der Ploeg, P.; Uittenboogaard, A.; Thijs, A.M.J.; Westgeest, H.M.; Boere, I.A.; Lambrechts, S.; van de Stolpe, A.; Bekkers, R.L.M.; Piek, J.M.J. The effectiveness of monotherapy with PI3K/AKT/mTOR pathway inhibitors in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2021, 163, 433–444.
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92.
- Dzhalilova, D.S.; Makarova, O.V. HIF-Dependent Mechanisms of Relationship between Hypoxia Tolerance and Tumor Development. Biochemie 2021, 86, 1163–1180.
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2009, 29, 625–634.
- Shih, T.; Lindley, C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin. Ther. 2006, 28, 1779–1802.
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121.




