Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel Chiu | -- | 1164 | 2023-09-16 00:54:49 | | | |
| 2 | Camila Xu | Meta information modification | 1164 | 2023-09-18 02:44:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chiu, D.; Rhee, J.; Gonzalez Castro, L.N. Epidemiology and Pathophysiology of Paraneoplastic Neurologic Syndromes. Encyclopedia. Available online: https://encyclopedia.pub/entry/49257 (accessed on 07 February 2026).
Chiu D, Rhee J, Gonzalez Castro LN. Epidemiology and Pathophysiology of Paraneoplastic Neurologic Syndromes. Encyclopedia. Available at: https://encyclopedia.pub/entry/49257. Accessed February 07, 2026.
Chiu, Daniel, John Rhee, L. Nicolas Gonzalez Castro. "Epidemiology and Pathophysiology of Paraneoplastic Neurologic Syndromes" Encyclopedia, https://encyclopedia.pub/entry/49257 (accessed February 07, 2026).
Chiu, D., Rhee, J., & Gonzalez Castro, L.N. (2023, September 15). Epidemiology and Pathophysiology of Paraneoplastic Neurologic Syndromes. In Encyclopedia. https://encyclopedia.pub/entry/49257
Chiu, Daniel, et al. "Epidemiology and Pathophysiology of Paraneoplastic Neurologic Syndromes." Encyclopedia. Web. 15 September, 2023.
Copy Citation
Paraneoplastic neurologic syndromes are defined and characterized by an inappropriate immune response targeting native nervous system antigens that are ectopically expressed by a systemic tumor. The first reported case of a possible paraneoplastic neurologic disorder was documented in 1888 by Hermann Oppenheim, a young neurologist working at the Charité Hospital in Berlin.
paraneoplastic neurologic syndrome
onconeural antibodies
autoimmune
1. Overview and History
Paraneoplastic neurologic syndromes are defined and characterized by an inappropriate immune response targeting native nervous system antigens that are ectopically expressed by a systemic tumor. The first reported case of a possible paraneoplastic neurologic disorder was documented in 1888 by Hermann Oppenheim, a young neurologist working at the Charité Hospital in Berlin [1][2]. He described a case of a 54-year-old female patient presenting with a variety of neurocognitive abnormalities, including agnosia, mood changes and aphasia. She died a few days after her presentation and was found to have a large gastric cancer at autopsy. The careful micro- and macroscopic inspection of the brain, however, did not reveal any pathologic changes to explain the neurologic symptoms. Oppenheim hypothesized “… that the toxic focal neurological symptoms of the brain are in fact caused by the presence of a carcinoma”, thus establishing the idea that cancer can mediate distant neurologic effects through as yet unclear “toxic products”, even in the absence of direct tumor infiltration or neuronal death in the brain [1].
Subsequently, the French physician M. Auché described in 1890 the first case of a paraneoplastic syndrome affecting the peripheral nervous system (neuropathy) associated with cancer [3]. This was then followed almost 60 years later by a report from Dr. Derek Denny-Brown describing two cases of neuromyopathy associated with lung cancer [4].
In the 1960s, Drs. Wilkinson and Zeromski at the Western Infirmary in Glasgow described their own cases of patients with neuromyopathy associated with bronchial carcinoma; when they examined the sera of these patients, they found circulating antibodies directed against neurons in a perinuclear pattern [5], thus providing support for Oppenheim’s hypothesis regarding elusive “toxic products” that are able to mediate neurologic effects from a distance. In the 1980s, Graus and colleagues identified and characterized these anti-neuronal nuclear antibodies as anti-Hu [6]. In the following years, additional autoantibodies with characteristic oncologic associations and prototypical neurologic manifestations were identified, thus laying the groundwork for the modern field of paraneoplastic neurologic disorders. Oppenheim’s initial hypothesis regarding the presence of “toxic products’’ proved to be prescient and can at present be understood to represent components of the humoral and cell-mediated immune system (i.e., autoantibodies and cytotoxic T cells) that are generated in the presence of cancer and inappropriately target the nervous system.
2. Epidemiology
Paraneoplastic neurologic syndromes (PNSs) are rare, affecting less than 1% of cancer patients overall [7]. The incidence of neurologic paraneoplastic syndromes varies with the specific syndrome and the type of primary tumor. Overrepresented tumors frequently associated with PNSs tend to either: express neuroendocrine proteins (e.g., small-cell lung cancer and neuroblastoma), involve immunoregulatory organs (e.g., thymoma), contain neuronal components (e.g., teratomas), or affect immunoglobulin production (e.g., myeloma) [8]. The most common PNSs are: Lambert–Eaton Myasthenic Syndrome (LEMS), which affects 3.8% of small-cell lung cancer (SCLC) patients [9], and myasthenia gravis (MG), which affects approximately 39% of patients with thymoma [10]. One prospective study found that 9.4% of patients with SCLC have one or more paraneoplastic syndromes, most commonly LEMS, sensory neuropathy, and limbic encephalitis [9]. The incidence of PNSs is much lower (~1%) for other solid tumors [11]. Between 5–15% of patients with plasma cell dyscrasias develop paraneoplastic peripheral neuropathies [12]. The likelihood that a given neurologic disorder can be attributed to a paraneoplastic process varies widely depending on the syndrome, ranging from approximately 60% for LEMS vs. approximately 10% for encephalomyelitis [13].
3. Pathophysiology
PNSs are autoimmune disorders. The pathophysiology of these syndromes is based on an immune response generated against CNS antigens that are normally expressed exclusively in the nervous system, but which are aberrantly and ectopically expressed by tumor cells. The tumor antigen and the neural antigen are identical, but for reasons that are still unclear, the immune system identifies it as foreign and mounts an attack [14]. Tumor-associated intracellular and cell-surface proteins are phagocytosed by dendritic cells and subsequently presented to lymphocytes in regional lymph nodes (Figure 1). There, they activate antigen-specific CD8+ cytotoxic T cells and antibody-producing B cells [14]. The antigen-specific antibodies and cytotoxic T cells that comprise this immune response can then trigger a PNS affecting the peripheral or central nervous system; the latter can be affected if they are able to cross the blood–brain barrier and react with neurons expressing these antigens [14]. From a pathophysiologic standpoint, PNSs can be classified into two groups: (1) PNSs with antibodies directed against intracellular neuronal proteins (the so-called “classical” or “onconeural” proteins) and (2) PNSs with antibodies directed against synaptic or cell membrane proteins. Note that antibodies help to characterize and diagnose the syndrome, but they are not necessarily pathogenic.
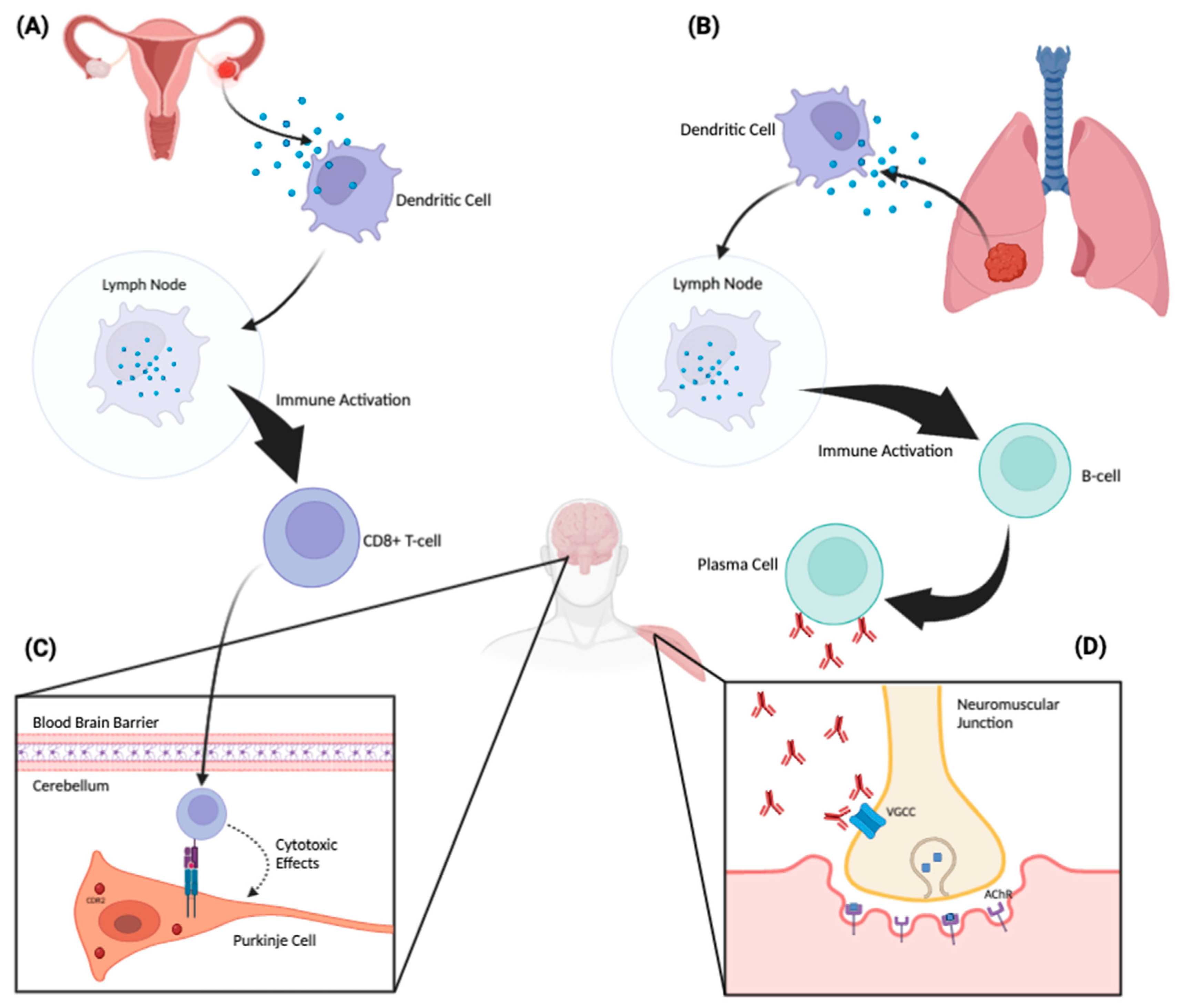
Figure 1. Proposed pathophysiologic mechanism of paraneoplastic neurologic disorders. A tumor aberrantly expresses a neuronal protein (antigen) that the immune system recognizes as non-self. An ovarian tumor (A) and a lung tumor (B) are depicted as examples. These tumor-associated proteins are phagocytosed by dendritic cells and subsequently presented to lymphocytes in regional lymph nodes. There, they activate antigen-specific CD8+ cytotoxic T cells and antibody-producing B cells. These antibodies and cytotoxic T cells can then trigger an antigen-specific PNS affecting the peripheral or central nervous system. When the corresponding neuronal antigen is located intracellularly, CD8+ cytotoxic T cells can recognize and bind to these antigens when they are presented on the cell surface by MHC-1 molecules (C). Through this binding, the T cell can then exert cytotoxic effects on the target neuronal cell. Panel C demonstrates a CDR2-specific T-cell response against a Purkinje cell in the cerebellum. On the other hand, when the corresponding neuronal antigen is located on the cell surface, the antibodies themselves can be pathogenic (D). Panel (D) depicts antibodies acting directly against voltage-gated calcium channels located on the surface of a presynaptic nerve terminal at the neuromuscular junction. This inhibits the influx of calcium into the nerve, which, in turn, attenuates the release of acetylcholine into the neuromuscular junction, producing the Lambert–Eaton myasthenic syndrome.
If identified, onconeural antibodies directed against intracellular neuronal proteins are suggestive, though not definitely indicative, of an underlying tumor. These antibodies—also termed “high-risk” because of their frequent association with malignancy—are associated with tumors in >70% of cases [15]. They include the following fully characterized antibodies: anti-Hu (a.k.a., anti-ANNA-1), anti-Ri (a.k.a., anti-ANNA-2), anti-Yo (a.k.a., anti-PCA-1), anti-amphiphysin, anti-Ma2, anti-Tr, anti-CRMP-5 and anti-recoverin antibodies. These antibodies are associated with specific PNSs, but they are not directly pathogenic; neurologic effects and neuronal loss are, instead, mediated by the cytotoxic effects of T lymphocytes, although the antibodies may induce or enhance the T-cell response [16]. On the other hand, PNSs with antibodies directed against synaptic or cell membrane proteins both establish the diagnosis and are also directly pathogenic. Examples include anti-NMDAR, anti-AMPAR, anti-P/Q VGCC, anti-AChR, anti-LGI1, anti-GABA-A, anti-GABA-B, anti-CASPR2, anti-GAD65, anti-GlyR, anti-mGluR1 and anti-mGluR5 antibodies.
References
- Oppenheim, H. Über Hirnsymptome bei Carcinomatose ohne nachweisbare Veränderungen im Gehirn. Charité-Annalen 1888, 13, 335–344.
- Schulz, P.; Prüss, H. “Hirnsymptome bei Carcinomatose”—Hermann Oppenheim an Early Description of a Paraneoplastic Neurological Syndrome. J. Hist. Neurosci. 2015, 24, 371–377.
- Auché, M. Des névrites périphériques chez les cancéreux. Rev. Méd. 1890, 10, 785–807.
- Denny-Brown, D. Primary sensory neuropathy with muscular changes associated with carcinoma. J. Neurol. Neurosurg. Psychiatry 1948, 11, 73–87.
- Wilkinson, P.C.; Zeromski, J. Immunofluorescent detection of antibodies against neurones in sensory carcinomatous neuropathy. Brain J. Neurol. 1965, 88, 529–583.
- Graus, F.; Cordon-Cardo, C.; Posner, J.B. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology 1985, 35, 538–543.
- Pelosof, L.C.; Gerber, D.E. Paraneoplastic syndromes: An approach to diagnosis and treatment. Mayo Clin. Proc. 2010, 85, 838–854.
- Rosenfeld, M.R.; Dalmau, J. Update on Paraneoplastic Neurologic Disorders. Oncologist 2010, 15, 603–617.
- Gozzard, P.; Woodhall, M.; Chapman, C.; Nibber, A.; Waters, P.; Vincent, A.; Lang, B.; Maddison, P. Paraneoplastic neurologic disorders in small cell lung carcinoma: A prospective study. Neurology 2015, 85, 235–239.
- Safieddine, N.; Liu, G.; Cuningham, K.; Ming, T.; Hwang, D.; Brade, A.; Bezjak, A.; Fischer, S.; Xu, W.; Azad, S.; et al. Prognostic Factors for Cure, Recurrence and Long-Term Survival After Surgical Resection of Thymoma. J. Thorac. Oncol. 2014, 9, 1018–1022.
- Rudnicki, S.A.; Dalmau, J. Paraneoplastic syndromes of the spinal cord, nerve, and muscle. Muscle Nerve 2000, 23, 1800–1818.
- Dumitru, D.; Amato, A.A.; Zwarts, M.J. Electrodiagnostic Medicine, 2nd ed.; Hanley & Belfus: Philadelphia, PA, USA, 2002.
- Darnell, R.B.; Darnell, R.; Posner, J.B. Paraneoplastic Syndromes, Contemporary Neurology Series; Oxford University Press: Los Angeles, CA, USA, 2011.
- Darnell, R.B.; Posner, J.B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003, 349, 1543–1554.
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.-C.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol.—Neuroimmunol. Neuroinflammation 2021, 8, e1014.
- Blachère, N.E.; Orange, D.E.; Santomasso, B.D.; Doerner, J.; Foo, P.K.; Herre, M.; Fak, J.; Monette, S.; Gantman, E.C.; Frank, M.O.; et al. T cells targeting a neuronal paraneoplastic antigen mediate tumor rejection and trigger CNS autoimmunity with humoral activation. Eur. J. Immunol. 2014, 44, 3240–3251.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
536
Revisions:
2 times
(View History)
Update Date:
18 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




