Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Saleem Jaffar | -- | 3267 | 2023-09-15 08:36:55 | | | |
| 2 | Fanny Huang | Meta information modification | 3267 | 2023-09-18 07:27:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jaffar, S.; Rizvi, S.A.H.; Lu, Y. Understanding Invasion, Ecological Adaptations, Management of Bactrocera dorsalis. Encyclopedia. Available online: https://encyclopedia.pub/entry/49230 (accessed on 08 February 2026).
Jaffar S, Rizvi SAH, Lu Y. Understanding Invasion, Ecological Adaptations, Management of Bactrocera dorsalis. Encyclopedia. Available at: https://encyclopedia.pub/entry/49230. Accessed February 08, 2026.
Jaffar, Saleem, Syed Arif Hussain Rizvi, Yongyue Lu. "Understanding Invasion, Ecological Adaptations, Management of Bactrocera dorsalis" Encyclopedia, https://encyclopedia.pub/entry/49230 (accessed February 08, 2026).
Jaffar, S., Rizvi, S.A.H., & Lu, Y. (2023, September 15). Understanding Invasion, Ecological Adaptations, Management of Bactrocera dorsalis. In Encyclopedia. https://encyclopedia.pub/entry/49230
Jaffar, Saleem, et al. "Understanding Invasion, Ecological Adaptations, Management of Bactrocera dorsalis." Encyclopedia. Web. 15 September, 2023.
Copy Citation
Bactrocera dorsalis (Hendel, 1912) (Diptera: Tephritidae), commonly known as the oriental fruit fly, is a highly destructive pest that globally infests fruits and vegetables, resulting in significant annual economic losses. Initially detected in Taiwan Island, it has rapidly expanded its distribution range to various regions in mainland China since the 1980s, with a continuous northward spread. To mitigate the damage caused by this pest, extensive efforts have been undertaken to comprehend its ecological and physiological adaptations and develop management strategies.
B. dorsalis
oriental fruit fly
pest management
fruit fly
Invasion and dispersion
tolerance
risistance
1. Introduction
Tephritid fruit flies are an economically significant pest species globally, including mainland China [1]. They exhibit endophagous feeding behavior, which causes both quantitative and qualitative yield reductions. As a result, they pose significant threats to global fruit and vegetable production [2][3]. The pest affects a broad array of fruit and fleshy vegetable crops in tropical and subtropical regions. The presence of these pests was first observed in Taiwan Island, China, in 1912 [4][5]. The genus Bactrocera, which comprises a minimum of 440 species [6], is primarily distributed throughout tropical Asia, Australia, and the South Pacific [7][8]. The wide host range, great climate tolerance, and strong dispersing capacities of these species have led to their spread over the Asia Pacific region in the last century, covering all of South-East Asia from India to Hawaii [7]. The oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), is recognized as a destructive and persistent fruit fly pest. B. dorsalis has been documented to infest over 250 host plant species [9][10], including mango (Mangifera indica L., Anacardiaceae), banana (Musa spp., Musaceae), guava (Psidium guajava L., Myrtaceae), orange (Citrus spp., Rutaceae), papaya (Carica papaya L., Caricaceae), peach (Prunus persica (L.) Batsch, Rosaceae), grape (Vitis spp., Vitaceae), pomegranate (Punica granatum L., Lythraceae), lychee (Litchi chinensis Sonn., Sapindaceae), and longan (Dimocarpus longan Lour., Sapindaceae) [11][12]. Numerous studies have documented the economic damage caused by B. dorsalis. For instance, a study carried out in Thailand found that B. dorsalis infestation in mango farms caused an average annual yield loss of 15.5% [13]. Similarly, in India, fruit fly infestation led to a reduction in the marketable yield of mango by 25–30% [14]. According to an estimate, guava, sapota, citrus fruits, and mango in India, incurred losses equivalent to USD 356 million [15]. This significant economic loss is attributed to the fruit damage caused by B. dorsalis, which can affect 30% to 100% of fruits, depending on the season [16].
In addition to yield reduction, B. dorsalis also leads to the quality degradation of fruits, causing phytosanitary issues and triggering trade restrictions, thereby aggravating economic losses. A study conducted in Taiwan revealed that the infestation of fruit flies resulted in trade restrictions on the export of guava to the United States and Japan, leading to an estimated economic loss of USD 2.5 million per year [17]. These studies demonstrate the substantial economic losses caused by B. dorsalis and emphasize the necessity for implementing effective management strategies to mitigate the impact of this insect pest on horticultural crops. In China, the economic losses caused by the fruit fly pest species in citrus orchards have been widely reported, especially in Guangdong [18] and Fujian Provinces of China [1]. B. dorsalis exhibits three to eleven generations per year in China, with the majority of areas experiencing four to eight generations [8][19]. In the near future, there is the potential for B. dorsalis to expand into temperate northern and southern areas of China [20] (Figure 1).
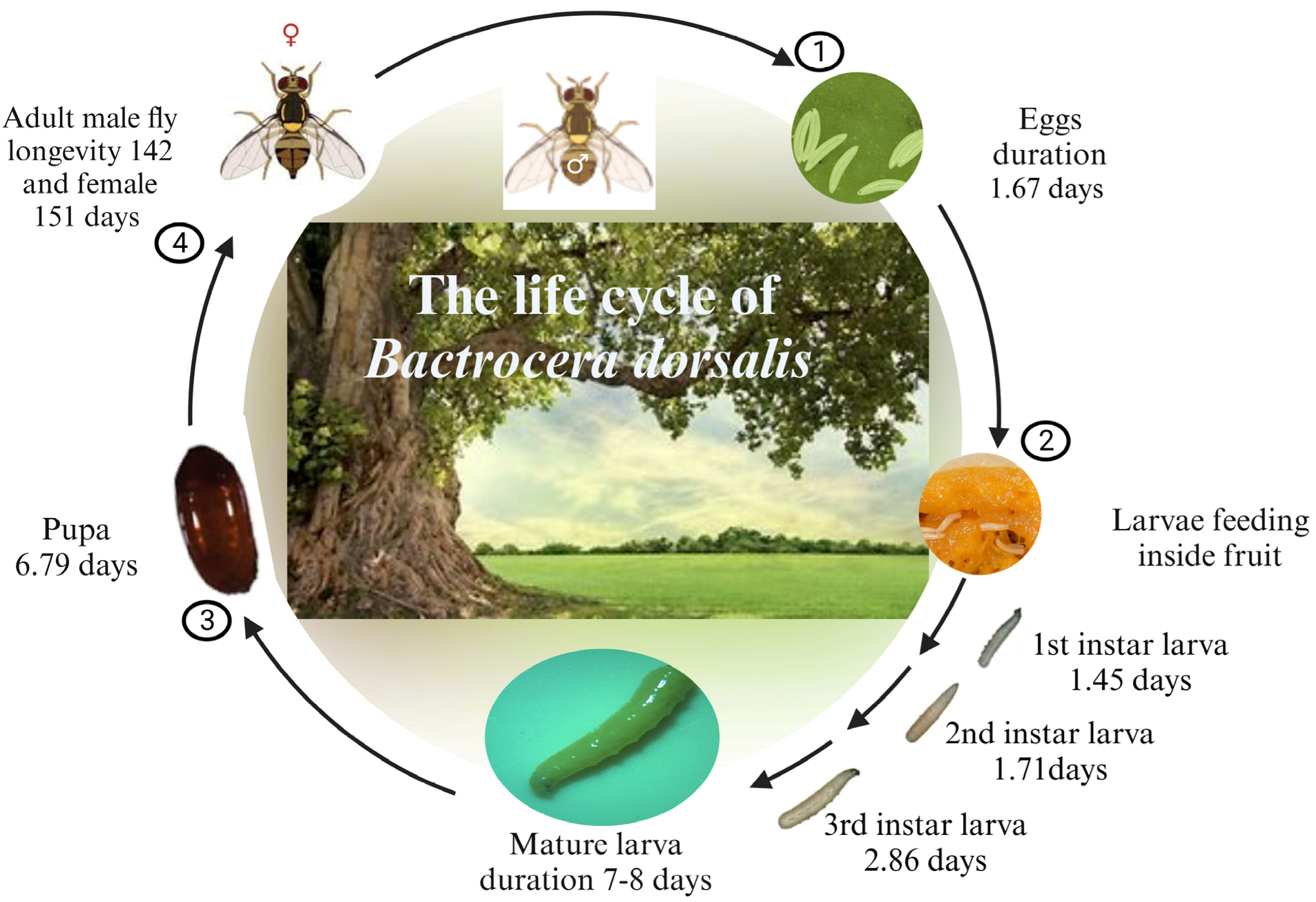
Figure 1. The life cycle of B. dorsalis.
2. Pest Management of Bactrocera dorsalis
2.1. Mass Trapping
B. dorsalis could be mass-trapped using pheromone and food-based baits. Various pheromones and scent-based compounds, including synthetic para-pheromones or male lures such as methyl eugenol (ME), have been developed to attract and control B. dorsalis. These compounds mimic the natural pheromones produced by melon and oriental fruit flies. These pheromones are used to attract and trap male flies, allowing for population monitoring and infestation assessment [21]. E-coniferyl alcohol (E-CF) has also been found to be effective in attracting female B. dorsalis [22]. A comprehensive investigation on current resistance and lure tolerance to fruit flies [23] assessed the response of B. dorsalis males to non-ME lures. The experiment evaluated the mating and lure response of non-ME-responding (NMR) and non-responding lines (NRLs) of B. dorsalis males. Results showed that NMR males had higher mating success rates compared to NRL males and exhibited a greater attraction to non-ME lures, which have been implicated in the development of tolerance mechanisms among B. dorsalis populations [23][24].
Another research revealed that B. dorsalis causes significant economic losses in the fruit and vegetable industry by laying eggs inside hosts. Chemical controls are not very effective due to the pest’s cryptic feeding habits, strong flight ability, and resistance to insecticides. Olfaction-based trapping using ME has been the most cost-effective tool for monitoring and controlling B. dorsalis populations for seven decades [22][25]. However, laboratory selection for ME responsiveness has resulted in the non-responsiveness of B. dorsalis, which may lead to the recolonization of the pest in some areas [26]. The study aimed to determine the levels of ME responsiveness in B. dorsalis field populations in China [23][24]. Results showed that the field populations had lower ME sensitivity compared to the susceptible strain, possibly due to odorant binding protein (BdorOBP2, BdorOBP83b), and P450 gene expressions in olfactory organs [24]. Protein-based baits and food odors, such as yeast, vinegar, and fermentation products, can also attract both male and female oriental fruit flies. These baits can be combined with pheromones to increase trap efficacy [27]. Visual attractants, such as brightly colored sticky traps, can also be used to attract oriental fruit flies, and they can complement other attractants for a more comprehensive monitoring and control approach [28][29]. It is important to note that attractants can be species-specific, and the most effective ones for B. dorsalis may vary based on environmental conditions and other factors. Following are the steps involved in bait-based physical control techniques for managing B. dorsalis infestations: (a) Monitoring: It includes observations and record-keeping of the presence, distribution, and abundance of B. dorsalis in affected areas. (b) Selection of bait material: It includes selection of appropriate bait material, such as food-based baits (fishmeal or yeast hydrolysates, ME, raspberry ketone, cue lure, honey, or molasses) that have been successful in attracting fruit fly species. (c) Formulation of bait: It includes formulating the selected bait material into an attractive and easily dispersible form by adding a food-grade preservative for shelf-life extension and a hydroscopic agent to maintain its moisture content. (d) Deployment of baits: It involves deployment of the baits using various methods, including bait stations, bait trees, or spray applications, depending on the specific circumstances of each situation. (e) Collection and disposal of captured fruit flies: It involves regular monitoring to assess the effectiveness of the bait and removing and disposing of captured fruit flies to prevent escape and further spread. (f) Evaluation: It includes assessing the success of the bait-based physical control technique by monitoring oriental fruit fly population levels over time and comparing pre- and post-treatment populations to determine the reduction in the number of fruit flies.
2.2. Biological Control
Parasitoids, hymenopteran wasps, lay their eggs inside hosts, consuming them from the inside and leading to their death. Fopius arisanus (Sonan), a species of egg parasitoid, targets B. dorsalis [8]. As a potential biological control agent, F. arisanus effectively parasitizes the host eggs and reduces the pest population [30]. It is well-adapted to tropical and subtropical environments, distributed throughout Asia, Africa, and the Pacific region [31]. Utilizing F. arisanus offers advantages over chemical pest control, including specificity to the target pest, conservation of beneficial insects, and long-term sustainability [32]. In order to effectively utilize F. arisanus for biological control, it is important to understand its biology, behavior, and life cycle, as well as its interactions with the host and other factors that may affect its efficacy. Researchers have also developed mass rearing for F. arisanus to produce large numbers of individuals for release into the field. F. arisanus is a promising biological control agent for the oriental fruit fly, offering a sustainable and environmentally friendly approach to managing this destructive pest [33]. Another parasitoid, Spalangia endius (Walker) (Hymenoptera: Pteromalidae) is a solitary endoparasitoid that attacks fruit fly pupae, including B. dorsalis. This wasp lays its eggs inside the pupae, and the emerging larvae consume the host pupae from within, killing the fruit fly [34]. Using S. endius for the biological control of B. dorsalis has advantages over other methods. It is highly specific in targeting fruit fly pests and does not harm beneficial insects. Field trials have shown that this parasitoid can effectively reduce the number of B. dorsalis adults, thereby minimizing crop damage [35][36]. To effectively use S. endius for biological control, understanding the biology and behavior of both the wasp and the fruit fly is crucial. The timing of wasp releases is critical in achieving maximum parasitism rates. In general, releases should coincide with the emergence of fruit fly pupae, which is the stage at which S. endius lays its eggs. Releasing large numbers of parasitoids can help control fruit fly populations in a targeted area [37][38] (Table 1). Viruses, bacteria, and fungi can also infect and be lethal to the fruit fly adult and larvae [39]. Among the pathogens studied for use against B. dorsalis, viruses, especially baculoviruses, have been found to be highly virulent to fruit fly species. They have demonstrated effectiveness in reducing fruit fly populations in both laboratory and field studies.
Baculoviruses are insect-specific viruses that replicate within the insect host and cause death. Among the baculovirus isolates identified and characterized, the nuclear polyhedrosis virus (NPV) has been found to be highly virulent to several insect species [40][41]. NPV studies have demonstrated its ability to reduce the number of fruit fly individuals in laboratory and field settings, thereby decreasing the damage caused by this pest. Moreover, NPV is safe for the environment and non-target organisms, making it a promising option for fruit fly management [42]. However, it is important to note that using pathogenic microorganisms, including viruses, for insect pest management is still in its early stages, and more research is needed to fully understand their potential and limitations. Baculoviruses, particularly NPV, have shown potential for controlling B. dorsalis, and further research is needed to integrate them into pest management programs effectively. The entomophagous fungus Beauveria bassiana (Sordariomycetes: Clavicipitaceae) is an entomopathogenic fungus that is known to be an effective biological control agent against B. cucurbitae. This fungus infects the insects and causes mortality [43]. In China, B. bassiana effectively controlled B. dorsalis, achieving a mortality rate of over 80% in laboratory experiments. Similarly, another study showed that B. bassiana effectively reduced the population density of B. dorsalis in the field [44][45]. B. bassiana can be used as a biological control agent in several ways: (1) Inoculative releases: This involves releasing large numbers of fungal spores (conidia) into the environment, which then infect the insects. This approach is most effective when used in conjunction with other management strategies, such as the use of pheromones or host-plant resistance. (2) Injection or spraying: This process involves injecting or spraying a suspension of conidia directly onto the insects, causing them to become infected. (3) Formulations: B. bassiana can also be formulated into granules or dusts that can be applied to the host plants or environment, where they will encounter the insects. The entomopathogenic bacterium Bacillus thuringiensis (Bt) is a naturally occurring soil bacterium that produces a toxic crystal protein effective against many insect pests, including B. dorsalis [46]. Entomopathogenic nematodes are parasitic roundworms that can infect and kill fruit fly larvae [47]. Further research and understanding of biology will improve their integration into pest management programs (Table 1).
Table 1. Natural enemies of B. dorsalis.
| Bio-Control Agents | Name of Species | Host Stages | Reference |
|---|---|---|---|
| Predator | Oecophylla longinoda | Pupa/larva | [48] |
| Pachycrepoideus vindemmiae | Larva/pupa | [49] | |
| Parasitoids | Fopius arisanus | Egg | [50] |
| Psyttalia cosyrae | Larva-pupal | [51] | |
| Diachasmimorpha longicaudata | Larva | [51] | |
| Nematodes | Heterorhabditis taysearae | Larva/pupa | [49] |
| H. indica | Larva/pupa | [52] | |
| Steinernema sp | Larva/pupa | [53] |
2.3. Sterile Insect Technique (SIT)
The sterile insect technique is a promising biological control for Bactrocera species, with a proven track record of success in various countries worldwide. It is a sustainable and eco-friendly method of pest management that complements other control strategies, providing long-term control of this economically important insect pest. The technique has been used for decades to manage various insect pests, including B. dorsalis, commonly known as the oriental fruit fly. SIT involves mass-rearing and sterilization of male insects, which are then released into the wild to mate with females. Mating with sterilized males leads to the laying of eggs by female insects that do not hatch, ultimately leading to a decline in the pest population [8][54]. This technique was first employed in the 1950s to control the screwworm, Cochliomyia hominivorax, in the southern United States and has since been effectively utilized against various other insect pests worldwide. Fruit flies, including B. dorsalis, have been successfully managed using SIT in several countries, such as China, Australia, and Hawaii [55][56]. For instance, in Hawaii, SIT was implemented in the early 2000s to manage oriental fruit fly outbreaks in the state’s agriculture industry. The program’s success resulted in a significant decline in the pest population [57]. In Australia, SIT has been incorporated into integrated pest management to control Mediterranean fruit fly C. capitata populations in the country’s horticulture industry [58]. The genetic sexing strain is a technique utilized to manipulate the sex ratios of a population, leading to more effective and efficient pest management. It has been successfully applied worldwide, including China, to manage B. dorsalis. This technique employs a genetic marker to distinguish between male and female fruit flies. By releasing only sterilized males into the environment, the population growth of the pest can be suppressed without the need for chemical insecticides [59].
In China, researchers have developed a genetic sexing strain for B. dorsalis using the temperature-sensitive lethal (tsl) mutation. This mutation causes the death of females at a certain temperature, enabling the separation of male and female fruit flies [60]. The genetic sexing strain has been proven effective in suppressing the population growth of several fruit fly species in field trials [61]. This technique has also been applied to manage fruit flies in other countries, including Australia and Thailand [13][58][62][63]. These studies demonstrate the potential of the genetic sexing strain as an integrated pest management tool for managing tephritid fruit flies.
2.4. Molecular Control
The management of B. dorsalis is challenging due to its high resistance to insecticides. To overcome this challenge, it is crucial to identify new targets for insect pest control. Transient receptor potential (TRP) channels play a crucial role in various physiological processes in insects, including nociception, thermo-sensation, and olfaction [64][65]. In recent years, there have been extensive studies on the identification and characterization of TRP channels in various insect species, including B. dorsalis. In one study [65], 15 TRP channel genes were identified in the genome of B. dorsalis. The expression patterns of these genes were analyzed in different tissues, such as the antennae, brain, midgut, Malpighian tubules, and fat body. The results revealed that TRP channels were differentially expressed across various tissues, with some TRP genes being predominantly expressed in specific tissues. Additionally, another study [66] investigated the role of TRP channels in insecticide resistance in insects. They used RNA interference (RNAi) to knock down the expression of TRP channels in B. dorsalis. The findings showed that knockdown of TRP channels significantly reduced insecticide resistance in B. dorsalis, suggesting the potential utilization of TRP channels as targets for insect pest control [67][68]. The identification, characterization, and expression analysis of TRP channel genes in the oriental fruit fly will provide crucial information for the development of new and effective strategies for the management and control of this pest.
2.5. RNA Interference (RNAi)
RNA interference (RNAi) is a highly effective technique for gene silencing through the use of double-stranded RNA (dsRNA) [69]. It has shown promise in knocking down insect pests as a more environmentally friendly option. Previous studies have demonstrated successful silencing of genes rpl19, v-ATPase-D, noa, and rab11 in adult B. dorsalis through the feeding of corresponding dsRNA. Other potential target genes involved in midgut digestion and detoxification have also been identified [70][71]. However, using RNAi for controlling the oriental fruit fly faces challenges, including effectively delivering dsRNA to the insect and potential risks to non-target organisms. The delivery of dsRNA has not been fully implemented yet, and the possible impacts on non-target organisms and host fruits and vegetables must be carefully considered. There is a risk of reducing the expression of genes in natural enemies and other beneficial insects due to the high similarity in rpl19 sequences between these insects and B. dorsalis. Therefore, minimizing the impact of dsRNA on non-target insects and host fruits and vegetables is a priority in ongoing efforts to use RNAi for controlling B. dorsalis. In a research article addressing the problem of insecticide resistance in B. dorsalis, a global pest affecting various crops, researchers focused on the role of UDP-glycosyltransferases (UGTs) in resistance development [72]. These enzymes are involved in metabolically processing both plant secondary metabolites and synthetic insecticides. The study identified 31 UGT genes in the genome of B. dorsalis, with 12 of them highly expressed in key tissues such as the antennae, midgut, Malpighian tubules, and fat body. Furthermore, exposure to four different insecticides caused a significant upregulation of 17 UGT genes. To investigate further, RNA interference was used to knock down five selected UGT genes, resulting in reduced oriental fruit fly mortality in response to insecticides from 9.29% to 27.22% [73].
2.6. CRISPR-Cas9
The clustered regularly interspaced palindromic repeat (CRISPR-Cas9) system is a revolutionary tool for precise and efficient genome editing in various organisms [74]. In a study of B. dorsalis, researchers targeted a specific gene known as the Sex Peptide Receptor (Bdspr) using CRISPR/Cas9 technology [75]. The Bdspr gene plays a critical role in the regulation of female reproduction, including ovary development and egg laying. By introducing mutations into this gene, the researchers aimed to examine its effects on female fecundity and reproductive functions in B. dorsalis. Several research experiments showed that CRISPR/Cas9-mediated disruption of the Bdspr gene, when the insects were fed with the ds-spr gene, led to significant changes in the number and size of ovarioles, a reduction in the number of eggs laid, and a decrease in overall female fecundity. This indicated the importance of the Bdspr gene in the normal functioning of the female reproductive system in B. dorsalis. The study also demonstrated that the CRISPR/Cas9 system is an effective tool for studying gene function and disrupting specific genes in insects. In the future, this information could potentially be used to develop new strategies for controlling the population of oriental fruit flies, a major agricultural pest causing significant damage to crops worldwide [75][76][77][78]. The CRISPR/Cas9-induced mutation of the Bdspr gene in the oriental fruit fly underscores the significance of this gene in female reproduction and highlights the potential of genome editing technology for advancing the field of insect pest management.
In another study focused on understanding the functional role of the white gene in pigmentation in B. dorsalis, the white gene was cloned, and knockout strains were created using the CRISPR/Cas9 genome editing system. The results revealed that the mutants lost pigmentation in the compound eye and their head spots. Further analysis using quantitative reverse-transcription PCR showed lower expression levels of the Bd-yellow1 gene in the head of mutants compared to the wild-type strain, while there were no significant differences in the expression of the other six genes. As the yellow gene is crucial for melanin biosynthesis, the reduced expression of Bd-yellow1 in mutants led to a decrease in dark pigmentation in the head spots. This study provides evidence for the first time that the white gene may play a role in cuticle pigmentation by affecting the expression of the yellow gene [79].
References
- He, Y.; Xu, Y.; Chen, X. Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review. Insects 2023, 14, 196.
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319.
- Hossain, M.; Sarkar, B.; Hossain, M.; Mian, M.; Rajotte, E.; Muniappan, R.; O’Rourke, M. Comparison of biorational management approaches against mango fruit fly (Bactrocera dorsalis Hendel) in Bangladesh. Crop Prot. 2020, 135, 104807.
- Jaffar, S.; Lu, Y. Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Insects 2022, 13, 954.
- Wan, X.; Liu, Y.; Zhang, B. Invasion history of the oriental fruit fly, Bactrocera dorsalis, in the Pacific-Asia region: Two main invasion routes. PLoS ONE 2012, 7, e36176.
- Drew, R.; Hancock, D. Biogeography, Speciation and Taxonomy within the genus Bactrocera Macquart with application to the Bactrocera dorsalis (Hendel) complex of fruit flies (Diptera: Tephritidae: Dacinae). Zootaxa 2022, 5190, 333–360.
- Zeng, Y.; Reddy, G.V.; Li, Z.; Qin, Y.; Wang, Y.; Pan, X.; Jiang, F.; Gao, F.; Zhao, Z.H. Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2019, 143, 165–176.
- Liu, H.; Zhang, D.; Xu, Y.; Wang, L.; Cheng, D.; Qi, Y.; Zeng, L.; Lu, Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Integr. Agric. 2019, 18, 771–787.
- Aketarawong, N.; Guglielmino, C.; Karam, N.; Falchetto, M.; Manni, M.; Scolari, F.; Gomulski, L.; Gasperi, G.; Malacrida, A. The oriental fruitfly Bactrocera dorsalis ss in East Asia: Disentangling the different forces promoting the invasion and shaping the genetic make-up of populations. Genetica 2014, 142, 201–213.
- Meng, L.W.; Yuan, G.R.; Lu, X.P.; Jing, T.X.; Zheng, L.S.; Yong, H.X.; Wang, J.J. Two delta class glutathione S-transferases involved in the detoxification of malathion in Bactrocera dorsalis (Hendel). Pest. Manag. Sci. 2019, 75, 1527–1538.
- Mutamiswa, R.; Nyamukondiwa, C.; Chikowore, G.; Chidawanyika, F. Overview of oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) in Africa: From invasion, bio-ecology to sustainable management. Crop Prot. 2021, 141, 105492.
- Zhu, Y.; Qi, F.; Tan, X.; Zhang, T.; Teng, Z.; Fan, Y.; Wan, F.; Zhou, H. Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China. Insects 2022, 13, 258.
- Orankanok, W.; Chinvinijkul, S.; Thanaphum, S.; Sitilob, P.; Enkerlin, W.R. Area-Wide Integrated Control of Oriental Fruit Fly Bactrocera dorsalis and Guava Fruit Fly Bactrocera correcta in Thailand. In Area-Wide Control of Insect Pests; Springer: Dordrecht, The Netherlands, 2007; pp. 517–526.
- Bana, J.; Sharma, H.; Kumar, S.; Singh, P. Impact of weather parameters on population dynamics of oriental fruit fly, Bactrocera dorsalis (Hendel)(Diptera: Tephritidae) under south Gujarat mango ecosystem. J. Agrometeorol. 2017, 19, 78–80.
- Jitendra, M.; Sandeep, S.; Akilesh, T.; Chaube, M. Population dynamics of oriental fruit fly, Bactrocera dorsalis (Hendel) in relation to abiotic factors. HortFlora Res. Spectr. 2012, 1, 187–189.
- Dhillon, M.; Singh, R.; Naresh, J.; Sharma, H. The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. J. Insect Sci. 2005, 5, 40.
- Coates, B.S.; Poelchau, M.; Childers, C.; Evans, J.D.; Handler, A.; Guerrero, F.; Skoda, S.; Hopper, K.; Wintermantel, W.M.; Ling, K.-S. Arthropod genomics research in the United States Department of Agriculture-Agricultural Research Service: Current impacts and future prospects. Trends Entomol. 2015, 11, 12–27.
- Liu, L.J.; Martinez-Sañudo, I.; Mazzon, L.; Prabhakar, C.S.; Girolami, V.; Deng, Y.L.; Dai, Y.; Li, Z.H. Bacterial communities associated with invasive populations of Bactrocera dorsalis (Diptera: Tephritidae) in China. Bull. Entomol. Res. 2016, 106, 718–728.
- Zhu, Y.-F.; Tan, X.-M.; Qi, F.-J.; Teng, Z.-W.; Fan, Y.-J.; Shang, M.-Q.; Lu, Z.-Z.; Wan, F.-H.; Zhou, H.-X. The host shift of Bactrocera dorsalis: Early warning of the risk of damage to the fruit industry in northern China. Entomol. Gen. 2022, 42, 691–699.
- Ullah, F.; Gul, H.; Hafeez, M.; Güncan, A.; Tariq, K.; Desneux, N.; Zhao, Z.; Li, Z. Impact of temperature stress on demographic traits and population projection of Bactrocera dorsalis. Entomol. Gen. 2022, 42, 949–957.
- Shelly, T. Effects of methyl eugenol and raspberry ketone/cue lure on the sexual behavior of Bactrocera species (Diptera: Tephritidae). Appl. Entomol. Zool. 2010, 45, 349–361.
- Liu, H.; Wang, D.D.; Wan, L.; Hu, Z.Y.; He, T.T.; Wang, J.B.; Deng, S.Z.; Wang, X.S. Assessment of attractancy and safeness of (E)-coniferyl alcohol for management of female adults of Oriental fruit fly, Bactrocera dorsalis (Hendel). Pest. Manag. Sci. 2022, 78, 1018–1028.
- Mandanayake, M.A.R.A.; Hee, A.K.W. Assessment of non-methyl eugenol-responding lines of Bactrocera dorsalis (Diptera: Tephritidae) males on lure response and mating. Bull. Entomol. Res. 2023, 113, 396–401.
- Fan, Y.; Zhang, C.; Qin, Y.; Yin, X.; Dong, X.; Desneux, N.; Zhou, H. Monitoring the Methyl Eugenol Response and Non-Responsiveness Mechanisms in Oriental Fruit Fly Bactrocera dorsalis in China. Insects 2022, 13, 1004.
- Liu, H.; Chen, Z.-S.; Zhang, D.-J.; Lu, Y.-Y. BdorOR88a Modulates the Responsiveness to Methyl Eugenol in Mature Males of Bactrocera dorsalis (Hendel). Front. Physiol. 2018, 9, 987.
- Mandanayake, M.A.R.A.; Shohaimi, S.; Ghani, N.I.A.; Hee, A.K.W. Establishment of non-methyl eugenol-responding lines from feral Oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera Tephritidae). Phytoparasitica 2023, 51, 425–436.
- Raza, A.B.M.; Majeed, M.Z.; Ismail, M.; Qasim, Z. Field evaluation of IPM strategies against Bactrocera dorsalis PUJZ. Punjab Univ. J. Zool. 2023, 38, 59–64.
- Kimbokota, F.; Hassanali, A.; Njagi, P.G.N. Potential attractants from three host plants of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2023, 43, 107–114.
- Tan, K.-H.; Wee, S.-L.; Nishida, R.; Shelly, T.E. Attraction of feral Bactrocera dorsalis males (Diptera: Tephritidae) to natural versus commercial sources of methyl eugenol. J. Asia-Pac. Entomol. 2021, 24, 1095–1100.
- Bautista, R.C.; Mochizuki, N.; Spencer, J.P.; Harris, E.J.; Ichimura, D.M. Mass-Rearing of the Tephritid Fruit Fly Parasitoid Fopius arisanus (Hymenoptera: Braconidae)1. Biol. Control 1999, 15, 137–144.
- Lahiri, S.; Orr, D. Chapter 11—Biological Control in Tomato Production Systems: Theory and Practice. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 253–267.
- Nanga Nanga, S.; Hanna, R.; Gnanvossou, D.; Fotso Kuate, A.; Fiaboe, K.K.M.; Djieto-Lordon, C. Fruit Preference, Parasitism, and Offspring Fitness of Fopius arisanus (Hymenoptera: Braconidae) Exposed to Bactrocera dorsalis’ (Diptera: Tephritidae) Infested Fruit Species. Environ. Entomol. 2019, 48, 1286–1296.
- Sime, K.R.; Daane, K.M.; Wang, X.G.; Johnson, M.W.; Messing, R.H. Evaluation of Fopius arisanus as a biological control agent for the olive fruit fly in California. Agric. For. Entomol. 2008, 10, 423–431.
- Zheng, Y.; Song, Z.-W.; Zhang, Y.-P.; Li, D.-S. Ability of Spalangia endius (Hymenoptera: Pteromalidae) to Parasitize Bactrocera dorsalis (Diptera: Tephritidae) after Switching Hosts. Insects 2021, 12, 613.
- Tang, L.-D.; Ji, X.-C.; Han, Y.; Fu, B.-L.; Liu, K. Parasitism, Emergence, and Development of Spalangia endius (Hymenoptera: Pteromalidae) in Pupae of Different Ages of Bactrocera cucurbitae (Diptera: Tephritidae). J. Insect Sci. 2015, 15, 15.
- Li, L.; Chen, J.; Niu, L.; Han, D.; Zhang, F.; Fu, Y. Field evaluation of Spalangia endius Walker in controlling Zeugodacus cucurbitae (Coquillett). Int. J. Pest. Manag. 2021, 67, 216–221.
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20.
- Liang, J.; Tang, S.; Cheke, R.A.; Wu, J. Adaptive release of natural enemies in a pest-natural enemy system with pesticide resistance. Bull. Math. Biol. 2013, 75, 2167–2195.
- Zeng, T.; Bai, X.; Liu, Y.L.; Li, J.F.; Lu, Y.Y.; Qi, Y.X. Intestinal responses of the oriental fruit fly Bactrocera dorsalis (Hendel) after ingestion of an entomopathogenic bacterium strain. Pest. Manag. Sci. 2020, 76, 653–664.
- Blissard, G.W.; Theilmann, D.A. Baculovirus Entry and Egress from Insect Cells. Annu. Rev. Virol. 2018, 5, 113–139.
- Prasad, V.; Srivastava, S. Chapter 13—Insect Viruses. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 411–442.
- Zhang, W.; Gu, Q.; Niu, J.; Wang, J.J. The RNA Virome and Its Dynamics in an Invasive Fruit Fly, Bactrocera dorsalis, Imply Interactions Between Host and Viruses. Microb. Ecol. 2020, 80, 423–434.
- Iqbal, M.; Gogi, M.D.; Atta, B.; Nisar, M.J.; Arif, M.J.; Javed, N. Assessment of pathogenicity of Beauveria bassiana, Metarhizium anisopliae, Verticillium lecanii and Bacillus thuringiensis var. kurstaki against Bactrocera cucurbitae Coquillett (Diptera: Tephritidae) via diet-bioassay technique under controlled conditions. Int. J. Trop. Insect Sci. 2021, 41, 1129–1145.
- Wang, D.; Liang, Q.; Chen, M.; Ye, H.; Liao, Y.; Yin, J.; Lü, L.; Lei, Y.; Cai, D.; Jaleel, W.; et al. Susceptibility of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) pupae to entomopathogenic fungi. Appl. Entomol. Zool. 2021, 56, 269–275.
- Martínez-Barrera, O.Y.; Toledo, J.; Cancino, J.; Liedo, P.; Gómez, J.; Valle-Mora, J.; Montoya, P. Interaction Between Beauveria bassiana (Hypocreales: Cordycipitaceae) and Coptera haywardi (Hymenoptera: Diapriidae) for the Management of Anastrepha obliqua (Diptera: Tephritidae). J. Insect Sci. 2020, 20, 1–10.
- Shishir, M.A.; Akter, A.; Bodiuzzaman, M.; Hossain, M.A.; Alam, M.M.; Khan, S.A.; Khan, S.N.; Hoq, M.M. Novel toxicity of Bacillus thuringiensis strains against the melon fruit fly, Bactrocera cucurbitae (Diptera: Tephritidae). Biocontrol Sci. 2015, 20, 115–123.
- Wakil, W.; Usman, M.; Piñero, J.C.; Wu, S.; Toews, M.D.; Shapiro-Ilan, D.I. Combined application of entomopathogenic nematodes and fungi against fruit flies, Bactrocera zonata and B. dorsalis (Diptera: Tephritidae): Laboratory cups to field study. Pest. Manag. Sci. 2022, 78, 2779–2791.
- Heve, W.K.; Adjadeh, T.A.; Billah, M.K. Overview and future research needs for development of effective biocontrol strategies for management of Bactrocera dorsalis Hendel (Diptera: Tephritidae) in sub-Saharan Africa. Pest. Manag. Sci. 2021, 77, 4224–4237.
- Godjo, A.; Chabi, N.; Zadji, L.; Dossou, P.; Batcho, O.; Baimey, H.; Bonou, W.; Sinzogan, A.A.C.; Bokonon-Ganta, A.; Decraemer, W.; et al. Evaluation of the ability of indigenous nematode isolates of Heterorhabditis taysearae and Steinernema kandii to control mango fruit fly Bactrocera dorsalis under laboratory, semi-field and field conditions in Northern Benin. Crop Prot. 2021, 149, 105754.
- Agboka, K.M.; Tonnang, H.E.Z.; Abdel-Rahman, E.M.; Kimathi, E.; Mutanga, O.; Odindi, J.; Niassy, S.; Mohamed, S.A.; Ekesi, S. A systematic methodological approach to estimate the impacts of a classical biological control agent’s dispersal at landscape: Application to fruit fly Bactrocera dorsalis and its endoparasitoid Fopius arisanus. Biol. Control 2022, 175, 105053.
- Ndlela, S.; Mohamed, S.A.; Azrag, A.G.A.; Ndegwa, P.N.; Ong’amo, G.O.; Ekesi, S. Interactions between Two Parasitoids of Tephritidae: Diachasmimorpha longicaudata (Ashmead) and Psyttalia cosyrae (Wilkinson) (Hymenoptera: Braconidae), under Laboratory Conditions. Insects 2020, 11, 671.
- Labaude, S.; Griffin, C.T. Transmission Success of Entomopathogenic Nematodes Used in Pest Control. Insects 2018, 9, 72.
- Toledo, J.; Morán-Aceves, B.M.; Ibarra, J.E.; Liedo, P. Can Entomopathogenic Nematodes and Their Symbiotic Bacteria Suppress Fruit Fly Pests? A Review. Microorganisms 2023, 11, 1682.
- Diouf, E.G.; Brévault, T.; Ndiaye, S.; Faye, E.; Chailleux, A.; Diatta, P.; Piou, C. An agent-based model to simulate the boosted Sterile Insect Technique for fruit fly management. Ecol. Model. 2022, 468, 109951.
- Pérez-Staples, D.; Díaz-Fleischer, F.; Montoya, P. The Sterile Insect Technique: Success and Perspectives in the Neotropics. Neotrop. Entomol. 2021, 50, 172–185.
- Cladera, J.L.; Vilardi, J.C.; Juri, M.; Paulin, L.E.; Giardini, M.C.; Gómez Cendra, P.V.; Segura, D.F.; Lanzavecchia, S.B. Genetics and biology of Anastrepha fraterculus: Research supporting the use of the sterile insect technique (SIT) to control this pest in Argentina. BMC Genet. 2014, 15 (Suppl. S2), S12.
- Mau, R.; Jang, E.; Vargas, R. The Hawaii area-wide fruit fly pest management programme. In Area-Wide Control of Insect Pests; Springer: Berlin/Heidelberg, Germany, 2007; pp. 671–683.
- Haq, I.U.; Abd-Alla, A.; Tomas, U.S.; Meza, J.S.; Bourtzis, K.; Cáceres, C. Cryopreservation of the Mediterranean fruit fly (Diptera: Tephritidae) VIENNA 8 genetic sexing strain: No effect on large scale production of high quality sterile males for SIT applications. PLoS ONE 2019, 14, e0211259.
- Ramírez-Santos, E.; Rendon, P.; Gouvi, G.; Zacharopoulou, A.; Bourtzis, K.; Cáceres, C.; Bloem, K. A Novel Genetic Sexing Strain of Anastrepha ludens for Cost-Effective Sterile Insect Technique Applications: Improved Genetic Stability and Rearing Efficiency. Insects 2021, 12, 499.
- Sollazzo, G.; Gouvi, G.; Nikolouli, K.; Cancio Martinez, E.I.; Schetelig, M.F.; Bourtzis, K. Temperature Sensitivity of Wild-Type, Mutant and Genetic Sexing Strains of Ceratitis capitata. Insects 2022, 13, 943.
- Robinson, A.S. Genetic sexing strains in medfly, Ceratitis capitata, sterile insect technique programmes. Genetica 2002, 116, 5–13.
- Aluja, M.; Norrbom, A. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; CRC Press: Boca Raton, FL, USA, 1999.
- Hendrichs, J.; Robinson, A.; Cayol, J.; Enkerlin, W. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Fla. Entomol. 2002, 85, 1–13.
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507.
- Su, H.; Bai, X.; Zeng, T.; Lu, Y.; Qi, Y. Identification, characterization and expression analysis of transient receptor potential channel genes in the oriental fruit fly, Bactrocera dorsalis. BMC Genom. 2018, 19, 674.
- Nesterov, A.; Spalthoff, C.; Kandasamy, R.; Katana, R.; Rankl, N.B.; Andrés, M.; Jähde, P.; Dorsch, J.A.; Stam, L.F.; Braun, F.-J.; et al. TRP Channels in Insect Stretch Receptors as Insecticide Targets. Neuron 2015, 86, 665–671.
- Zhang, Y.; Zhang, Y.-J.; Guo, D.; Wang, L.-X.; Niu, C.-D.; Wu, S.-F.; Zhang, Y.V.; Gao, C.-F. Function of Transient Receptor Potential-Like Channel in Insect Egg Laying. Front. Mol. Neurosci. 2022, 15, 823563.
- Wang, R.; Gao, B.; Zhang, Q.; Qu, C.; Luo, C. Knockdown of TRPV gene Nanchung decreases resistance to the novel pyropene insecticide, afidopyropen, in Bemisia tabaci. Int. J. Biol. Macromol. 2023, 224, 1566–1575.
- Xie, Y.F.; Niu, J.Z.; Jiang, X.Z.; Yang, W.J.; Shen, G.M.; Wei, D.; Smagghe, G.; Wang, J.J. Influence of various stressors on the expression of core genes of the small interfering RNA pathway in the oriental fruit fly, Bactrocera dorsalis. Insect Sci. 2017, 24, 418–430.
- Li, X.; Zhang, M.; Zhang, H. RNA interference of four genes in adult Bactrocera dorsalis by feeding their dsRNAs. PLoS ONE 2011, 6, e17788.
- Dong, Y.-C.; Wang, Z.-J.; Chen, Z.-Z.; Clarke, A.R.; Niu, C.-Y. Bactrocera dorsalis male sterilization by targeted RNA interference of spermatogenesis: Empowering sterile insect technique programs. Sci. Rep. 2016, 6, 35750.
- Zhou, Y.; Fu, W.-B.; Si, F.-L.; Yan, Z.-T.; Zhang, Y.-J.; He, Q.-Y.; Chen, B. UDP-glycosyltransferase genes and their association and mutations associated with pyrethroid resistance in Anopheles sinensis (Diptera: Culicidae). Malar. J. 2019, 18, 62.
- Chen, M.-L.; Zhang, S.-X.; Guo, P.-Y.; Qin, Q.-S.; Meng, L.-W.; Yuan, G.-R.; Wang, J.-J. Identification and characterization of UDP-glycosyltransferase genes and the potential role in response to insecticides exposure in Bactrocera dorsalis. Pest. Manag. Sci. 2023, 79, 666–677.
- Moon, T.T.; Maliha, I.J.; Khan, A.A.M.; Chakraborty, M.; Uddin, M.S.; Amin, M.R.; Islam, T. CRISPR-Cas Genome Editing for Insect Pest Stress Management in Crop Plants. Stresses 2022, 2, 493–514.
- Chen, H.; Sun, H.; Xie, J.; Yao, Z.; Zheng, W.; Li, Z.; Deng, Z.; Li, X.; Zhang, H. CRISPR/Cas9-induced Mutation of Sex Peptide Receptor Gene Bdspr Affects Ovary, Egg Laying, and Female Fecundity in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Sci. 2023, 23, 2.
- Wang, X.-F.; Chen, Z.; Wang, X.-B.; Xu, J.; Chen, P.; Ye, H. Bacterial-mediated RNAi and functional analysis of Natalisin in a moth. Sci. Rep. 2021, 11, 4662.
- Kim, Y.-J.; Bartalska, K.; Audsley, N.; Yamanaka, N.; Yapici, N.; Lee, J.-Y.; Kim, Y.-C.; Markovic, M.; Isaac, E.; Tanaka, Y.; et al. MIPs are ancestral ligands for the sex peptide receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 6520–6525.
- Zheng, W.; Liu, Y.; Zheng, W.; Xiao, Y.; Zhang, H. Influence of the silencing sex-peptide receptor on Bactrocera dorsalis adults and offspring by feeding with ds-spr. J. Asia-Pac. Entomol. 2015, 18, 477–481.
- Bai, X.; Zeng, T.; Ni, X.Y.; Su, H.A.; Huang, J.; Ye, G.Y.; Lu, Y.Y.; Qi, Y.X. CRISPR/Cas9-mediated knockout of the eye pigmentation gene white leads to alterations in colour of head spots in the oriental fruit fly, Bactrocera dorsalis. Insect Mol. Biol. 2019, 28, 837–849.
More
Information
Subjects:
Entomology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
21 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




