Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Riad Farajallah Nasr | -- | 3290 | 2023-09-14 17:28:29 | | | |
| 2 | Jason Zhu | Meta information modification | 3290 | 2023-09-15 03:40:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nasr, S.; Borges, A.; Sahyoun, C.; Nasr, R.; Roufayel, R.; Legros, C.; Sabatier, J.; Fajloun, Z. Antimicrobial Peptides from Scorpion Venom. Encyclopedia. Available online: https://encyclopedia.pub/entry/49189 (accessed on 07 February 2026).
Nasr S, Borges A, Sahyoun C, Nasr R, Roufayel R, Legros C, et al. Antimicrobial Peptides from Scorpion Venom. Encyclopedia. Available at: https://encyclopedia.pub/entry/49189. Accessed February 07, 2026.
Nasr, Sara, Adolfo Borges, Christina Sahyoun, Riad Nasr, Rabih Roufayel, Christian Legros, Jean-Marc Sabatier, Ziad Fajloun. "Antimicrobial Peptides from Scorpion Venom" Encyclopedia, https://encyclopedia.pub/entry/49189 (accessed February 07, 2026).
Nasr, S., Borges, A., Sahyoun, C., Nasr, R., Roufayel, R., Legros, C., Sabatier, J., & Fajloun, Z. (2023, September 14). Antimicrobial Peptides from Scorpion Venom. In Encyclopedia. https://encyclopedia.pub/entry/49189
Nasr, Sara, et al. "Antimicrobial Peptides from Scorpion Venom." Encyclopedia. Web. 14 September, 2023.
Copy Citation
Scorpion venoms have long captivated scientific researchers, primarily due to the potency and specificity of the mechanism of action of their derived components. Among other molecules, these venoms contain highly active compounds, including antimicrobial peptides (AMPs) and ion channel-specific components that selectively target biological receptors with remarkable affinity. Some of these receptors have emerged as prime therapeutic targets for addressing various human pathologies, including cancer and infectious diseases, and have served as models for designing novel drugs. Consequently, extensive biochemical and proteomic investigations have focused on characterizing scorpion venoms.
scorpion venom
antimicrobial peptides
proteomic
separation techniques
1. Implementation of Separation Methods for Scorpion Venoms
1.1. Bioassay-Guided Fractionation
Bioassay-guided fractionation involves the separation of the crude scorpion venoms primarily through chromatographic techniques. The resulting fractions are subjected to the desired functional assay to identify the active fractions of interest. Eventually, an additional fractionation step might be required to obtain a pure active fraction [1][2]. Depending on the characteristics of the target molecule, different biological assays might be used. Scorpion venom could be submitted to a bioassay-guided fractionation in the pursuit of isolating biomolecules of interest. The newly discovered and purified molecules from scorpion venom can be thus used for drug design owing to their beneficial pharmacological potential. A bioassay-guided fractionation of Hemiscorpius lepturus scorpion venom allowed the purification of a novel anticancer protein known as Leptulipin. The process first included fractionation by SEC, using a Sephadex G-50 column, followed by purification of the fraction with the highest anticancer activity using a C18 RP-HPLC column. The final isolated fraction with distinctive cytotoxic activity was then identified by 2-DGE. The bioactivities of Hemiscorpius lepturus peptides discovered in specific fractions constitute novel biomolecules with potential pharmacological use [3]. Different neurotoxins in scorpion venom are accountable for the various envenomation effects in humans, especially cardiotoxic peptides that cause severe cardiorespiratory complications to envenomation victims [4]. To identify the cardiotoxic component of Hemiscorpuis lepturus venom, bioassay-guided fractionation was employed. SEC using Sephadex G-50 was applied, and fractionation was followed by measurement of optical density, allowing the collection of six peaks. Subsequently, protein profiles were acquired by subjecting the six resulting peaks to 12% SDS-PAGE, which were used to measure levels of specific biochemical cardiac-related enzymes after injection of each fraction. Fraction IV and the whole crude venom were selected for having the highest levels of cardiotoxicity and were then subjected to a histopathological examination of damaged heart tissues. The series of bioassays identified fraction IV of Hemiscorpius lepturus, containing low molecular mass peptides, as the fraction responsible for the cardiotoxic effects [5]. Aside from the highly studied neurotoxins, non-disulfide-bridged peptides (NDBPs) were recently put under the spotlight for their important pharmacological activities. In this context, three Iranian scorpion species were subjected to separation by RP-HPLC using C18 columns to isolate fractions with bradykinin potentiating effect. The collected fractions were evaluated for their smooth muscle contracting ability on the guinea pig ileum and rat uterus. Thus, results confirmed the presence of bradykinin potentiating peptides in the venom of the three scorpions, illustrating the use of the above-mentioned fractionation scheme for making such peptides available in the quantities needed for further molecular and structural studies [6]. Interestingly, the implementation of bioassay-guided fractionation has underscored its effectiveness in isolating and characterizing biologically significant compounds present in scorpion venom. Figure 1 presents a selection of essential bioassays employed to isolate and purify molecules with noteworthy biological activity. Given the known presence of analgesic compounds in scorpion venoms, the analgesic assay conducted on mice proved instrumental in assessing the analgesic potential of fractions obtained from Mesobuthus martensii venom through a sequence of five successive chromatographic separations. Consequently, this systematic approach facilitated the identification of active analgesic fractions, ultimately leading to identification of the BmKAGAP-SYPU2 component. Similarly, diverse assays such as insect toxicity evaluations contributed to the understanding of the mechanism of action of β-insect depressant toxins derived from the venom of Isometrus maculatus, which underwent purification via a two-stage RP-HPLC process. Conversely, a murine toxicity assay applied to fractions derived from Androctonus australis venom through four consecutive chromatographic steps led to the identification of AaTx1, a potassium channel blocker belonging to the alpha-KTx family [7][8][9]. Table 1 displays some examples of bioassay-guided fractionation of different peptides contained in scorpion venom using modern separation techniques. In a recent study, transcriptomic analysis through RNA-seq followed by sequence assembly and search in BLAST provided annotation regarding the studied peptide structure contained in Liocheles australasiae venom. Bioassay-guided fractionation followed, in order to isolate and characterize venom peptides exhibiting biological activity. First, Liocheles australasiae venom was separated by RP-HPLC using a C4 column, while each obtained fraction was subjected to an anti-viral activity test against hepatitis C virus, which allowed identification of the fraction of interest. Further RP-HPLC purification of this fraction using a C18 column was performed. The resulting fractions were also tested for anti-viral activity, and the eluted fraction with the highest activity was identified as containing phospholipase A2 (LaPLA2-1). This process allowed isolation of a newly detected phospholipase A2 in scorpion venom and its characterization for the first time [10]. In the framework of bioassay-guided fractionation, obtaining credible biological results remains the crucial and indispensable element for the success of this experimental approach. This method is not currently widespread compared to conventional analytical techniques. Some limitations and technical difficulties may be at the origin of its slow progress in the field of identification of drug candidates from natural extracts, specifically from scorpion venoms.
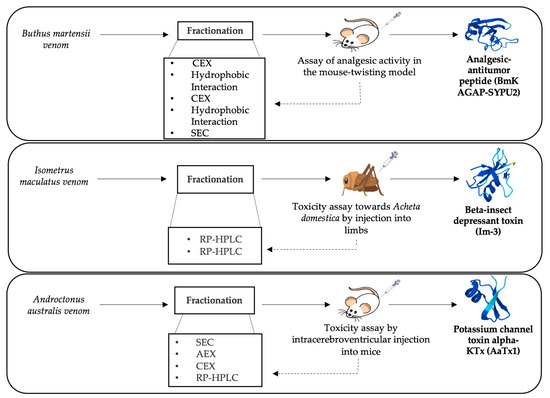
Figure 1. Schematic representation of bioassay-guided fractionation examples for isolation of three scorpion neurotoxins [7][8][9].
Table 1. Different separation strategies used to isolate enzyme components from scorpion venoms. Da: Dalton.
| Purified Molecule | Scorpion Species |
Molecular Mass (Da) |
Separation Process | Column Used | References |
|---|---|---|---|---|---|
| Phospholipase A2 |
Liocheles australasiae | 13,079.8 | RP-HPLC RP-HPLC LC/MS |
C4 C18 C18 |
[10] |
| Hemiscorpius lepturus | 14,000 | SEC RP-HPLC RP-HPLC |
Sephadex G-50 Semi preparative C8 Analytical C8 |
[11] | |
| Scorpio maurus | 17,000 | SEC AEX Hydrophobic Interaction HPLC |
Sephadex G-100 Q-Sepharose Phenyl-Sepharose Nucleogel GFC 300-8 |
[12] | |
| Heterometrus laoticus | 14,018.4 | SEC CEX RP-HPLC |
Sephadex G-50 CM-650 M C4 |
[13] | |
| Hyaluronidase | Rhopalurus junceus | 45,000–60,000 | SEC | Superdex 75 | [14] |
| Tityus serrulatus | 49,312 | SEC RP-HPLC |
Sephadex G-50 Analytical C8 |
[15] | |
| Palamneus gravimanus | 52,000 | SEC IEX SEC |
Sephadex G-75 DEAE-cellulose |
[16] | |
| Metalloproteinase | Tityus serrulatus | 22,000 24,000 |
AEX SEC |
DEAE Diol-300 |
[17] |
| 25,500 | SEC RP-HPLC |
Sephadex G-50 C18 |
[18] | ||
| Serine proteinase | Mesobuthus martensii | 33,000 | SEC CEX RP-HPLC |
Superdex G-75 UNO-Q C8 |
[19] |
| Scorpio maurus | 25,000 | CEX SECFP AEXSEC |
DEAE-SephadexSephadex G-100SP-SepharoseSephadex G-50 | [20] | |
| Neurotoxins | Mesobuthus martensii | 7246.40 | CEX Hydrophobic Interaction CEX Hydrophobic Interaction SEC |
SP-Sepharose Phenyl Sepharose 4 SP-Sepharose Phenyl Sepharose 4 Superdex Peptide HR 10/30 |
[7] |
| Centruroides suffusus suffusus | 7524.9 7537.6 7588.6 13,596 |
RP-HPLC CEX RP-HPLC |
C18 TSK-gel sulfopropyl C18 |
[21] | |
| Isometrus maculatus | 6894 | RP-HPLC RP-HPLC |
C4 C18 |
[8] | |
| Androctonus australis | 3849.5 | SEC SEC Exchange FPLC RP-HPLC |
Sephadex G-50 Resource S C18 |
[9] | |
| Hemiscorpius lepturus | 4874 5107 |
SEC AEX CEX RP-HPLC |
Sephadex G-50 DEAE-Sepharose CM-Sepharose C8 |
[22] | |
| AMPs | Heterometrus laoticus (Heteroscorpine-1) | 8293 | SEC CEX |
Sephadex G-50 CM-Sepharose |
[23] |
| Pandinus imperator (Scorpine) | 8350 | SEC CEX RP-HPLC |
Sephadex G-50 CM-Cellulose C18 |
[24] | |
| Hoffmannihadrurus aztecus (Hadrurin) | 4436 | SEC HPLC HPLC |
Sephadex G-50 C18 C18 |
[25] | |
| Pandinus imperator (Pandinin-1) | 4799 | RP-HPLC CEX RP-HPLC |
C18 TSK-gel sulphopropyl C4 |
[26] |
1.2. Whole Proteome Characterization
In the quest to analyze the complete composition of various venoms, “omics” technologies have been employed. The advent of these state-of-the-art technologies has facilitated the decomplexation of venom’s composition and the discovery of hidden biological effects. Additionally, they have become crucial tools in the development of antivenoms, specifically targeting the most toxic components found in animal venoms [27]. The large-scale proteomic investigation of venoms is currently known as venomics. This branch of proteomics helps understand venom’s evolution and diversity, facilitating, therefore, the profiling and characterization of venoms [28]. To date, despite the discovery of approximately 2700 scorpion species, the percentage of manually annotated (Swiss-Prot) venom proteins constitute only 14.9% of all the scorpion venom-derived peptide and protein recorded in the Uni-Prot database. This highlights the need for more efficient methods of venom analysis in order to gain more knowledge on scorpion venom components. In scorpion venomics, the transcriptomic approach has played a crucial role in deciphering the expression levels of individual components produced within venom glands, therefore guiding the identification of novel structures and functions of venom proteins and peptides [29][30]. Recently, a new transcriptomic strategy has emerged, focusing on genomics analysis that is still in its early stages and requires further development and expansion [31]. Genomic analysis of scorpion venom now includes full genome sequencing by implementing high-throughput sequencing techniques named Next Generation Sequencing (NGS), revealing the genes encoding venom proteins in a more efficient manner while also enabling the detection of low-abundance components [1]. The implementation of new high-throughput technologies such as 454 pyrosequencing and Illumina sequencing has allowed quantitative and qualitative inter-specific comparisons and also unveiled differential mechanisms of toxin evolution between species [32][33]. The combination of transcriptomic and proteomic approaches increased the protein and peptide coverage in scorpion venom analysis and gave insights about protein composition from the gene sequences identified by transcriptomic analysis [34][35][36]. Collectively, these approaches provide a more comprehensive characterization of scorpion venoms, thereby enhancing the clinical assessment of scorpion envenomation [30][37]. Proteomics has emerged as a key strategy for analyzing scorpion venom, offering a fundamental breakdown of the complex mixture and enabling the profiling and characterization of its components [38][39]. Two approaches can be employed to perform proteomic analysis: bottom-up (BU) and top-down (TD). Unlike BU, where protease-digested proteins are analyzed, in the TD analysis, intact proteins are studied. In recent years, a novel approach known as the middle-down strategy has emerged as a middle ground between bottom-up (BU) and top-down (TD) approaches. The BU approach involves a crucial digestion step, generally using trypsin, to simplify the identification process. In contrast, the middle-down strategy offers a compromise by retaining larger peptide fragments for analysis. These digested peptides are subsequently identified using tandem mass spectrometry (MS/MS) [40]. The implementation of high-throughput mass spectrometry for sequencing enhanced venom analysis, covering a larger number of protein sequences in a faster time, making LC-MS/MS the most applied approach in BU analysis [41][42]. The inclusion of a pre-fractionation/decomplexation step prior to digestion is crucial in facilitating the identification of proteins. This step allows for the detection of low-abundant proteins that are often overlooked, thereby improving the overall protein identification process [43]. Only in shotgun BU analysis a decomplexation step prior to protein digestion is not required, avoiding the possible loss of peptides. Other BU workflows depend on a separation step prior to protein digestion. Both gel-based and chromatographic separation approaches might be used [44].
Workflow 1 (Figure 2) involves the separation of crude scorpion venom using RP-HPLC followed by in-solution trypsin digestion of the obtained fractions. The resulting digested peptides are then subjected to LC-MS/MS analysis. This approach is commonly used particularly for comparing scorpion venom composition. For instance, a study compared venoms from Chactas reticulatus, Opisthacanthus elatus, Centruroides edwardsii, and Tityus asthenes. It was observed that compounds from Centruroides edwardsii and Tityus asthenes eluted below 38% acetonitrile, while those from Chactas reticulatus and Opisthacanthus elatus eluted at higher acetonitrile percentages (50% and 60%, respectively). This revealed similarities among Buthidae scorpion venoms and differences in comparison to non-Buthidae scorpion venoms. However, all four venoms contained potassium channel-active and sodium channel-active neurotoxins, AMPs, metalloproteinase-like proteins, and phospholipase-like proteins [18]. Another example involves the determination of primary structures of four inhibitory proteins (NaTx-22, NaTx-4, NaTx-36, and NaTx-13) from Centruroides sculpturatus venom. These proteins were found to specifically inhibit a sodium channel isoform (Nav 1.8) associated with inflammation and nociception [45]. This workflow is favorable for detecting low-abundance peptides [46]. In workflow 2, the crude venom is separated by a gel-based (SDS-PAGE) technique, followed by in-gel digestion of the separated bands, as shown in Figure 2B. Protein bands marked by Coomassie Blue or silver staining are then analyzed using LC-MS/MS [47]. Prior to gel separation, the use of RP-HPLC on C18 columns can enhance fraction resolution, as demonstrated in the analysis of Heterometrus longimanus venom [44][48]. Workflow 3, shown in Figure 2C, involves first a separation of the crude venom using 2-DGE followed by in-gel digestion of protein spots isolated from the gel and the analysis of their protein content by LC-MS/MS. This workflow provides information about peptide mass and pI, enriching the identification of venom proteins [49]. It can be preceded by chromatographic techniques such as SEC. For example, SEC fractions of Centruroides limpidus venom were separated by 2-DGE, followed by tryptic digestion of spots and LC-MS/MS analysis. This enabled amino acid sequencing of peptides and a comparison of venoms from female and male scorpions [50]. As previously mentioned, the 2-DGE approach can be utilized for the isolation and identification of specific venom proteins and peptides [13]. One limitation of this approach is the potential loss of proteins during gel separation, making SDS-PAGE a more favorable option. However, despite this limitation, the fourth workflow is generally preferred due to its capability to achieve high proteome coverage. The last workflow (workflow 4) is known as the shotgun approach. In this workflow, scorpion venom is directly digested by trypsin, followed by analysis using a C18 RP-HPLC column and LC-MS/MS identification. This method provides a fast, qualitative analysis that offers a general understanding of venom composition and diversity. For example, when Serradigitus gertschi and Centruroides hentzi venoms were analyzed using the shotgun approach, 204 and 59 proteins and peptides were identified, respectively [36][51].
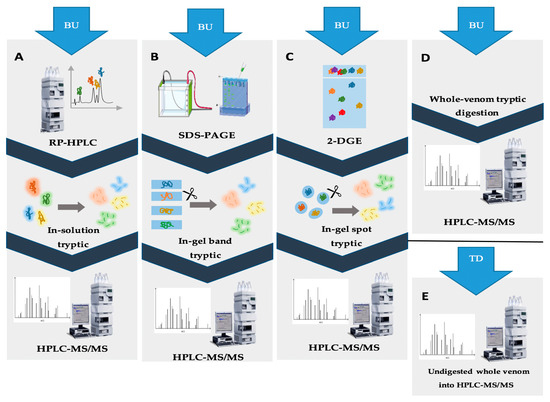
Figure 2. Illustration of various proteomic approaches for scorpion venom analysis. (A) The second workflow in the BU approach involves RP-HPLC fractionation, followed by in-solution tryptic digestion of each peak. The resulting digested peptides are then analyzed using HPLC-MS/MS for protein identification. (B) The third workflow in the BU approach includes the fractionation of crude venom using SDS-PAGE, followed by tryptic digestion of the protein bands. These bands are subsequently analyzed using HPLC-MS/MS for identification. (C) The fourth workflow in the BU approach starts with a first fractionation of crude venom by 2-DGE, followed by spot tryptic digestion. Protein identification is then performed using HPLC-MS/MS. (D) The shotgun approach involves direct digestion of crude venom by trypsin, followed by analysis using HPLC-MS/MS. (E) The TD approach entails the direct analysis of intact proteins in crude venom using HPLC separation, followed by tandem mass spectrometry (MS/MS). These different approaches provide a range of options for analyzing scorpion venom, allowing researchers to choose the most suitable method based on their specific objectives and requirements.
In contrast, the TD strategy focuses on analyzing proteins in their intact, native form without a digestion step. This approach overcomes the challenges faced in BU, particularly in terms of misidentification of isoforms and proteoforms. Additionally, it enables the analysis of post-translation modifications, large proteins, and protein-protein interactions [52][53]. Depending on the protein size, TD requires either a denaturation or alkylation step before LC-MS/MS analysis for proteins below 30 kDa or a direct application of native proteins for those above 50 kDa. Despite providing more comprehensive results, this method is less commonly used due to the challenges associated with its execution and the interpretation of the large amount of generated data [53]. Typically, preceding separation methods, particularly RP-HPLC, are employed in the TD process [54]. For example, TD analysis of Buthus occitanus venom resulted in the identification of 68 peptides, compared to 36 for in-gel BU and 37 for in-solution BU approaches. To overcome limitations in protein identification, a combination of BU and TD methods has been proposed to ensure a more thorough analysis and a deeper understanding of all venom components. This approach was employed in the analysis of Buthus occitanus venom, leading to the identification of a total of 102 proteins [55].
The emergence of a new analysis concept named middle-down (MD) was influenced by the former two approaches. It includes the digestion of peptides like in BU but with different proteases that generate longer truncated peptides. Following that, the MD approach allows an improvement in protein sequence coverage [56].
2. Scorpion Venom Antimicrobial Peptides (AMPs)
Recent proteomic analyses have illuminated the highly diversified composition of scorpion venoms, uncovering a link to the realm of AMPs. While these venoms were initially found to contain only small amounts of AMPs, the UniProt database now showcases an impressive collection of over 200 distinct scorpion venom AMPs—a significant advancement from the mere 50 peptides documented a decade ago.
Notably, a significant portion of these identified peptides exhibit remarkable efficacy against multidrug-resistant (MDR) bacteria, which holds great promise in combating the growing threat of antibiotic resistance. Additionally, these scorpion venom-derived AMPs, especially those derived from the Buthidae family, exhibit minimal hemolytic activity, thereby minimizing potential harm to healthy cells [57]. A distinguishing feature of scorpion venom AMPs is their discerning approach to microbial targets. Unlike their counterparts from spider venoms, scorpion venom AMPs display a remarkable specificity towards particular microorganisms rather than exhibiting a broad-spectrum anti-bacterial activity [58]. As seen, three AMPs derived from Urodacus yaschenkoi venom recorded high antimicrobial activity against eight MDR bacterial strains, mostly inhibiting Streptococcus strains. The minimum bacterial concentration for Uy234, Uy17 and Uy192 against SP10 was 2.9 µM, 23.2 µM and 10.6 µM, respectively, while the minimum bacterial concentration against ST9 was 5.9 µM, 11.6 µM and 15.9 ± 7 µM, respectively. In addition, Uy17 and Uy192 exhibited a lower hemolytic activity (<6%) compared to Uy234 (26.18%) at 380 μM. Despite this, these AMPs displayed a lower hemolytic activity than most scorpion venom AMPs. Collectively, these three peptides exhibit distinct action against MDR bacteria while also showing a low cytotoxic effect. This combination makes them a valuable asset in addressing the increasing prevalence of antibiotic-resistant bacterial strains. Similar findings continue to pave the way for the discovery and development of novel antibiotic-active biomolecules such as Uy17, Uy192, and Uy234 [59].
In scorpions, AMPs are short cationic amphipathic peptides divided into three categories according to their structure: (1) the first group contains peptides with cysteine residues and disulfide bridges; (2) the second group lacking cysteine residues contains members with amphipathic α-helix and (3) the third group encompasses members rich in proline and glycine residues. The cysteine-containing AMPs are formed by three to four disulfide bridges. For example, Heteroscorpine-1 (HS-1), from Heterometrus laoticus scorpion venom, contains ninety-five amino acids and three disulfide bonds [60]. HS-1 possesses a broad anti-bacterial spectrum, affecting both Gram-negative and Gram-positive strains. The purified fraction of HS-1 scored 300 times higher inhibition activity on Bacillus subtilis, Klebsiella pneumoniae and Pseudomonas aeruginosa compared to the whole crude venom of Heterometrus laoticus [23]. Scorpine is also a scorpion venom-derived AMP from Pandinus imperator with three disulfide bonds that constitute only 1.4% of the crude venom. This 75 amino acid-long AMP inhibited both Bacillus subtilis and Klebsiella pneumoniae strains, recording a MIC of 1 and 10 µM, respectively [24].
The non-disulfide bridged AMPs can be long, intermediate or short AMPs. Long-chain non-disulfide bridged AMPs vary in size, with an average of around 40 amino acids. For instance, Hadurin, isolated from Hoffmannihadrurus aztecus scorpion venom, is a 41 amino acid-long AMP that constitutes 1.7% of the total venom content. Antimicrobial activity was mainly detected against Escherichia coli, Serratia marscencens and Enterococcus cloacae with MICs lower than 10 µM while the hemolytic activity was significant [25]. Additionally, Pandinus imperator venom contains an AMP identified as pandinin-1, which comprises 44 amino acids. The application of pandinin-1 demonstrated notable sensitivity against Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis, with minimum inhibitory concentrations (MICs) of 1.3 µM, 5.2 µM, 2.6 µM, and 5.2 µM, respectively. Moreover, pandinin-1 displayed minimal hemolytic effects, with only 1.4% hemolysis observed at the highest concentration tested (44.5 µM). Another polycationic, α-helical peptide was purified from the venom of Pandinus imperator venom, pandinin-2, with 24 amino acids, making it an intermediate-chain AMP. This peptide acted mostly on Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus and Staphylococcus epidermidis strains with corresponding MICs of 2.4 µM, 4.8 µM, 2.4 µM and 4.8 µM, respectively. Nevertheless, pandinin-2 had a significant hemolytic activity [26].
Intermediate-chain AMPs constitute only 9% of scorpion AMPs described to date. On the other hand, short-chain AMPs are the most commonly found AMPs in scorpion venoms, representing 46% of reported scorpion antimicrobial peptides [60]. Amphipathic peptide CT2 from Scorpiops tibetanus venom falls among the short-chain, cationic, non-disulfide bridged AMPs. This AMP has 14 amino acids and inhibits mainly Gram-positive bacteria, especially Staphylococcus aureus, with a minimal inhibition concentration (MIC) of 6.25 μg/mL. CT2 was also effective against methicillin-resistant bacterial strains and had a low hemolytic activity even at high concentrations, showing major promise in drug development [61]. A second short-chain AMP, the 13 residue-long cytotoxic linear peptide (IsCT) isolated from Opisthacanthus madagascariensis scorpion venom, is also a potential compound for novel antimicrobial drug development. This AMP was significantly more active against Gram-positive bacteria, scoring MICs varying from 0.7 µM to 16.6 µM. Hemolytic activity of IsCT was relatively low, not exceeding 30% at 200 µM [62].
References
- Phillipson, D.W.; Milgram, K.E.; Yanovsky, A.I.; Rusnak, L.S.; Haggerty, D.A.; Farrell, W.P.; Greig, M.J.; Xiong, X.; Proefke, M.L. High-Throughput Bioassay-Guided Fractionation: A Technique for Rapidly Assigning Observed Activity to Individual Components of Combinatorial Libraries, Screened in HTS Bioassays. J. Comb. Chem. 2002, 4, 591–599.
- Malviya, N.; Malviya, S. Bioassay guided fractionation-an emerging technique influence the isolation, identification and characterization of lead phytomolecules. Int. J. Hosp. Pharm. 2017, 2, 1–6.
- Rezaei, A.; Asgari, S.; Komijani, S.; Sadat, S.N.; Sabatier, J.-M.; Nasrabadi, D.; Bagheri, K.P.; Shahbazzadeh, D.; Eidgahi, M.R.A.; De Waard, M.; et al. Discovery of Leptulipin, a New Anticancer Protein from the Iranian Scorpion, Hemiscorpius lepturus. Molecules 2022, 27, 2056.
- Abdel-Rahman, M.A.; Ayed, A.S.; Abdel-Mottaleb, Y.; Omran, M.A.A.; Nabil, Z.I. Cardiac disorders and mode of action of the Egyptian scorpion venom Androctonus bicolor on isolated toad’s heart. J. Basic Appl. Zool. 2015, 72, 137–144.
- Yazdkhasti, M.; Jalali, M.R.; Khadjeh, G.H.; Jafari, H.; Rezaie, A. Cardiotoxic Effects of Hemiscorpius lepturus Scorpion Venom Fractions in Rats. Iran. J. Toxicol. 2021, 15, 27–36.
- Goudarzi, H.R.; Nazari, A.; Noofeli, M.; Samiani, M. Bioassay of derived components from venom of Iranian medically important scorpions to identify the bradykinin potentiating factors. Pharmacol. Toxicol. 2017, preprint.
- Shao, J.-H.; Cui, Y.; Zhao, M.-Y.; Wu, C.-F.; Liu, Y.-F.; Zhang, J.-H. Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides 2014, 53, 89–96.
- Kawachi, T.; Miyashita, M.; Nakagawa, Y.; Miyagawa, H. Isolation and Characterization of an Anti-Insect β-Toxin from the Venom of the Scorpion Isometrus maculatus. Biosci. Biotechnol. Biochem. 2013, 77, 205–207.
- Mlayah-Bellalouna, S.; Dufour, M.; Mabrouk, K.; Mejdoub, H.; Carlier, E.; Othman, H.; Belghazi, M.; Tarbe, M.; Goaillard, J.M.; Gigmes, D.; et al. AaTX1, from Androctonus australis scorpion venom: Purification, synthesis and characterization in dopaminergic neurons. Toxicon 2014, 92, 14–23.
- Miyashita, M.; Mitani, N.; Kitanaka, A.; Yakio, M.; Chen, M.; Nishimoto, S.; Uchiyama, H.; Sue, M.; Hotta, H.; Nakagawa, Y.; et al. Identification of an antiviral component from the venom of the scorpion Liocheles australasiae using transcriptomic and mass spectrometric analyses. Toxicon 2021, 191, 25–37.
- Jridi, I.; Catacchio, I.; Majdoub, H.; Shahbazzadeh, D.; El Ayeb, M.; Frassanito, M.A.; Solimando, A.G.; Ribatti, D.; Vacca, A.; Borchani, L. The small subunit of Hemilipin2, a new heterodimeric phospholipase A2 from Hemiscorpius lepturus scorpion venom, mediates the antiangiogenic effect of the whole protein. Toxicon 2017, 126, 38–46.
- Louati, H.; Krayem, N.; Fendri, A.; Aissa, I.; Sellami, M.; Bezzine, S.; Gargouri, Y. A thermoactive secreted phospholipase A2 purified from the venom glands of Scorpio maurus: Relation between the kinetic properties and the hemolytic activity. Toxicon 2013, 72, 133–142.
- Incamnoi, P.; Patramanon, R.; Thammasirirak, S.; Chaveerach, A.; Uawonggul, N.; Sukprasert, S.; Rungsa, P.; Daduang, J.; Daduang, S. Heteromtoxin (HmTx), a novel heterodimeric phospholipase A2 from Heterometrus laoticus scorpion venom. Toxicon 2013, 61, 62–71.
- Díaz-García, A.; Ruiz-Fuentes, J.L.; Yglesias-Rivera, A.; Rodríguez-Sánchez, H.; Garlobo, Y.R.; Martinez, O.F.; Castro, J.A.F. Enzymatic analysis of venom from Cuban scorpion Rhopalurus junceus. J. Venom Res. 2015, 6, 11–18.
- Abreu, C.B.; Bordon, K.C.F.; Cerni, F.A.; Oliveira, I.S.; Balenzuela, C.; Alexandre-Silva, G.M.; Zoccal, K.F.; Reis, M.B.; Wiezel, G.A.; Peigneur, S.; et al. Pioneering Study on Rhopalurus crassicauda Scorpion Venom: Isolation and Characterization of the Major Toxin and Hyaluronidase. Front. Immunol. 2020, 11, 2011.
- Morey, S.S.; Kiran, K.M.; Gadag, J.R. Purification and properties of hyaluronidase from Palamneus gravimanus (Indian black scorpion) venom. Toxicon 2006, 47, 188–195.
- Cajado-Carvalho, D.; da Silva, C.C.F.; Kodama, R.T.; Mariano, D.O.C.; Pimenta, D.C.; Duzzi, B.; Kuniyoshi, A.K.; Portaro, F.V. Purification and Biochemical Characterization of TsMS 3 and TsMS 4: Neuropeptide-Degrading Metallopeptidases in the Tityus serrulatus Venom. Toxins 2019, 11, 194.
- Paul, L.F.; Fletcher, M.D.; Weninger, K.; Anderson, T.E.; Martin, B.M. Vesicle-associated Membrane Protein (VAMP) Cleavage by a New Metalloprotease from the Brazilian Scorpion Tityus serrulatus. J. Biol. Chem. 2010, 285, 7405–7416.
- Gao, R.; Zhang, Y.; Gopalakrishnakone, P. A Novel Trypsin-like Serine Proteinase from the Venom of the Chinese Scorpion Buthus martensii Karsch. In Proceedings of the 4th Kuala Lumpur International Conference on Biomedical Engineering 2008, Kuala Lumpur, Malaysia, 25–28 June 2008; Abu Osman, N.A., Ibrahim, F., Wan Abas, W.A.B., Abdul Rahman, H.S., Ting, H.-N., Eds.; IFMBE Proceedings 21. Springer: Berlin/Heidelberg, Germany, 2008; pp. 829–832.
- Louati, H.; Zouari, N.; Miled, N.; Gargouri, Y. A new chymotrypsin-like serine protease involved in dietary protein digestion in a primitive animal, Scorpio maurus: Purification and biochemical characterization. Lipids Health Dis. 2011, 10, 121.
- Espino-Solis, G.P.; Estrada, G.; Olamendi-Portugal, T.; Villegas, E.; Zamudio, F.; Cestele, S.; Possani, L.D.; Corzo, G. Isolation and molecular cloning of beta-neurotoxins from the venom of the scorpion Centruroides suffusus suffusus. Toxicon 2011, 57, 739–746.
- Maleki, M.; Dounighi, N.M. Purification and characterization of a novel type of neurotoxic peptides from the venom of the Iranian scorpion Hemiscorpius lepturus. Iran. J. Basic Med. Sci. 2019, 23, 195–201.
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Arkaravichien, T.; Bunyatratchata, W.; Ruangjirachuporn, W.; Jearranaiprepame, P.; Nakamura, T.; Matsuda, M.; Kobayashi, M.; et al. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon 2007, 49, 19–29.
- Conde, R.; Zamudio, F.Z.; Rodríguez, M.H.; Possani, L.D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168.
- Torres-Larios, A.; Gurrola, G.B.; Zamudio, F.Z.; Possani, L.D. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus: Scorpion antimicrobial peptide. Eur. J. Biochem. 2000, 267, 5023–5031.
- Corzo, G.; Escoubas, P.; Villegas, E.; Barnham, K.J.; He, W.; Norton, R.S.; Nakajima, T. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem. J. 2001, 359, 35–45.
- Vetter, I.; Davis, J.L.; Rash, L.D.; Anangi, R.; Mobli, M.; Alewood, P.F.; Lewis, R.J.; King, G.F. Venomics: A new paradigm for natural products-based drug discovery. Amino Acids 2011, 40, 15–28.
- Wilson, D.; Daly, N. Venomics: A Mini-Review. High-Throughput 2018, 7, 19.
- Schwartz, E.F.; Diego-Garcia, E.; de la Vega, R.C.R.; Possani, L.D. Transcriptome analysis of the venom gland of the Mexican scorpion Hadrurus gertschi (Arachnida: Scorpiones). BMC Genom. 2007, 8, 119.
- Abdel-Rahman, M.A.; Quintero-Hernandez, V.; Possani, L.D. Venom proteomic and venomous glands transcriptomic analysis of the Egyptian scorpion Scorpio maurus palmatus (Arachnida: Scorpionidae). Toxicon 2013, 74, 193–207.
- Cao, Z.; Yu, Y.; Wu, Y.; Hao, P.; Di, Z.; He, Y.; Chen, Z.; Yang, W.; Shen, Z.; He, X.; et al. The genome of Mesobuthus martensii reveals a unique adaptation model of arthropods. Nat. Commun. 2013, 4, 2602.
- Rendón-Anaya, M.; Delaye, L.; Possani, L.D.; Herrera-Estrella, A. Global Transcriptome Analysis of the Scorpion Centruroides noxius: New Toxin Families and Evolutionary Insights from an Ancestral Scorpion Species. PLoS ONE 2012, 7, e43331.
- Luna-Ramírez, K.; Quintero-Hernández, V.; Juárez-González, V.R.; Possani, L.D. Whole Transcriptome of the Venom Gland from Urodacus yaschenkoi Scorpion. PLoS ONE 2015, 10, e0127883.
- Abdel-Rahman, M.A.; Harrison, P.L.; Strong, P.N. Snapshots of scorpion venomics. J. Arid. Environ. 2015, 112, 170–176.
- Rokyta, D.R.; Ward, M.J. Venom-gland transcriptomics and venom proteomics of the black-back scorpion (Hadrurus spadix) reveal detectability challenges and an unexplored realm of animal toxin diversity. Toxicon 2017, 128, 23–37.
- Ward, M.J.; Ellsworth, S.A.; Rokyta, D.R. Venom-gland transcriptomics and venom proteomics of the Hentz striped scorpion (Centruroides hentzi; Buthidae) reveal high toxin diversity in a harmless member of a lethal family. Toxicon 2018, 142, 14–29.
- De Oliveira, U.C.; Nishiyama, M.Y.J.; Dos Santos, M.B.V.; Santos-Da-Silva, A.D.P.; Chalkidis, H.D.M.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, V.A.C.; Junqueira-De-Azevedo, I.D.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS ONE 2018, 13, e0193739.
- Díaz, C.; Chang-Castillo, A.; Lomonte, B.; Bonilla, F.; Víquez, C.; Alfaro-Chinchilla, A.; Triana, F.; Sasa, M. Venomics of the scorpion Tityus ocelote (Scorpiones, Buthidae): Understanding venom evolution in the subgenus Archaeotityus. Int. J. Pept. Res. Ther. 2022, 29, 2.
- Ghezellou, P.; Jakob, K.; Atashi, J.; Ghassempour, A.; Spengler, B. Mass-Spectrometry-Based Lipidome and Proteome Profiling of Hottentotta saulcyi (Scorpiones: Buthidae) Venom. Toxins 2022, 14, 370.
- Slagboom, J.; Kaal, C.; Arrahman, A.; Vonk, F.J.; Somsen, G.W.; Calvete, J.J.; Wüster, W.; Kool, J. Analytical strategies in venomics. Microchem. J. 2022, 175, 107187.
- Solovyeva, E.M.; Lobas, A.A.; Kopylov, A.T.; Ilina, I.Y.; Levitsky, L.I.; Moshkovskii, S.A.; Gorshkov, M.V. FractionOptimizer: A method for optimal peptide fractionation in bottom-up proteomics. Anal. Bioanal. Chem. 2018, 410, 3827–3833.
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Expert Rev. Proteom. 2020, 17, 67–83.
- Matallana-Surget, S.; Leroy, B.; Wattiez, R. Shotgun proteomics: Concept, key points and data mining. Expert Rev. Proteom. 2010, 7, 5–7.
- Lomonte, B.; Calvete, J.J. Strategies in ‘snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 26.
- El-Aziz, T.M.A.; Xiao, Y.; Kline, J.; Gridley, H.; Heaston, A.; Linse, K.D.; Ward, M.J.; Rokyta, D.R.; Stockand, J.D.; Cummins, T.R.; et al. Identification and Characterization of Novel Proteins from Arizona Bark Scorpion Venom That Inhibit Nav1.8, a Voltage-Gated Sodium Channel Regulator of Pain Signaling. Toxins 2021, 13, 501.
- Kim, B.; Araujo, R.; Howard, M.; Magni, R.; Liotta, L.A.; Luchini, A. Affinity enrichment for mass spectrometry: Improving the yield of low abundance biomarkers. Expert Rev. Proteom. 2018, 15, 353–366.
- Magalhães, A.C.M.; de Santana, C.J.C.; Melani, R.D.; Domont, G.B.; Castro, M.S.; Fontes, W.; Roepstorff, P.; Júnior, O.R.P. Exploring the biological activities and proteome of Brazilian scorpion Rhopalurus agamemnon venom. J. Proteom. 2021, 237, 104119.
- Bringans, S.; Eriksen, S.; Kendrick, T.; Gopalakrishnakone, P.; Livk, A.; Lock, R.; Lipscombe, R. Proteomic analysis of the venom of Heterometrus longimanus (Asian black scorpion). Proteomics 2008, 8, 1081–1096.
- Rogowska-Wrzesinska, A.; Le Bihan, M.-C.; Thaysen-Andersen, M.; Roepstorff, P. 2D gels still have a niche in proteomics. J. Proteom. 2013, 88, 4–13.
- Uribe, J.I.C.; Vargas, J.M.J.; Batista, C.V.F.; Zuñiga, F.Z.; Possani, L.D. Comparative proteomic analysis of female and male venoms from the Mexican scorpion Centruroides limpidus: Novel components found. Toxicon 2017, 125, 91–98.
- Romero-Gutiérrez, M.; Santibáñez-López, C.; Jiménez-Vargas, J.; Batista, C.; Ortiz, E.; Possani, L. Transcriptomic and Proteomic Analyses Reveal the Diversity of Venom Components from the Vaejovid Scorpion Serradigitus gertschi. Toxins 2018, 10, 359.
- Verano-Braga, T.; Dutra, A.A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.C.; Kjeldsen, F. Moving Pieces in a Venomic Puzzle: Unveiling Post-translationally Modified Toxins from Tityus serrulatus. J. Proteome Res. 2013, 12, 3460–3470.
- Melani, R.D.; Nogueira, F.C.S.; Domont, G.B. It is time for top-down venomics. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 44.
- Melani, R.D.; Nogueira, F.C.S.; Domont, G.B. Female-biased population divergence in the venom of the Hentz striped scorpion (Centruroides hentzi). Toxicon 2018, 152, 137–149.
- Daoudi, K.; Malosse, C.; Lafnoune, A.; Darkaoui, B.; Chakir, S.; Sabatier, J.; Chamot-Rooke, J.; Cadi, R.; Oukkache, N. Mass spectrometry-based top-down and bottom-up approaches for proteomic analysis of the Moroccan Buthus occitanus scorpion venom. FEBS Open Bio 2021, 11, 1867–1892.
- Pandeswari, P.B.; Sabareesh, V. Middle-down approach: A choice to sequence and characterize proteins/proteomes by mass spectrometry. RSC Adv. 2019, 9, 313–344.
- Zeng, X.-C.; Corzo, G.; Hahin, R. Scorpion Venom Peptides without Disulfide Bridges. Int. Union Biochem. Mol. Biol. Life 2005, 57, 13–21.
- Liu, G.; Yang, F.; Li, F.; Li, Z.; Lang, Y.; Shen, B.; Wu, Y.; Li, W.; Harrison, P.L.; Strong, P.N.; et al. Therapeutic Potential of a Scorpion Venom-Derived Antimicrobial Peptide and Its Homologs Against Antibiotic-Resistant Gram-Positive Bacteria. Front. Microbiol. 2018, 9, 1159.
- Cesa-Luna, C.; Muñoz-Rojas, J.; Saab-Rincon, G.; Baez, A.; Morales-García, Y.E.; Juárez-González, V.R.; Quintero-Hernández, V. Structural characterization of scorpion peptides and their bactericidal activity against clinical isolates of multidrug-resistant bacteria. PLoS ONE 2019, 14, e0222438.
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137.
- Cao, L.; Li, Z.; Zhang, R.; Wu, Y.; Li, W.; Cao, Z. StCT2, a new antibacterial peptide characterized from the venom of the scorpion Scorpiops tibetanus. Peptides 2012, 36, 213–220.
- Dai, L.; Corzo, G.; Naoki, H.; Andriantsiferana, M.; Nakajima, T. Purification; structure–function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2002, 293, 1514–1522.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
877
Revisions:
2 times
(View History)
Update Date:
19 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




