Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Przemysław Zalewski | -- | 2227 | 2023-09-14 11:31:37 | | | |
| 2 | Sirius Huang | Meta information modification | 2227 | 2023-09-15 04:14:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kulawik, A.; Cielecka-Piontek, J.; Zalewski, P. Health-Promoting Effect of Lycopene. Encyclopedia. Available online: https://encyclopedia.pub/entry/49164 (accessed on 07 February 2026).
Kulawik A, Cielecka-Piontek J, Zalewski P. Health-Promoting Effect of Lycopene. Encyclopedia. Available at: https://encyclopedia.pub/entry/49164. Accessed February 07, 2026.
Kulawik, Anna, Judyta Cielecka-Piontek, Przemysław Zalewski. "Health-Promoting Effect of Lycopene" Encyclopedia, https://encyclopedia.pub/entry/49164 (accessed February 07, 2026).
Kulawik, A., Cielecka-Piontek, J., & Zalewski, P. (2023, September 14). Health-Promoting Effect of Lycopene. In Encyclopedia. https://encyclopedia.pub/entry/49164
Kulawik, Anna, et al. "Health-Promoting Effect of Lycopene." Encyclopedia. Web. 14 September, 2023.
Copy Citation
Lycopene is a compound of colored origin that shows strong antioxidant activity. The positive effect of lycopene is the result of its pleiotropic effect. The ability to neutralize free radicals via lycopene is one of the foundations of its pro-health effect, including the ability to inhibit the development of many civilization diseases.
lycopene
neuroinflammation
1. Lycopene
Lycopene is a compound of red and orange fruit and vegetables, like tomato, watermelon, papaya, pink guava, carrot, rosehip, apricot, pink grapefruit, and pumpkin [1][2][3]. It is responsible for their red and orange color due to light absorption with a maximum wavelength of λ = 444, 470, and 502 nm [2]. It takes part in the process of photosynthesis and protects plants from damage caused by overexposure to light. It is also an essential intermediate in the synthesis of beta-carotene and xanthophylls [4]. However, lycopene is also found in some plants that have other colors, such as parsley and asparagus [5].
The most common source of lycopene in diets are tomatoes and products containing tomatoes [1][2][3]. More than 85% of this ingredient in our diet comes from these sources [6]. Tomatoes are also the cheapest source for lycopene production [7]. The tomato-based products are a better source of this compound than raw tomatoes [1]. Different varieties of tomatoes, as well as other fruits and vegetables, contain different amounts of this ingredient [3].
The amount of this carotenoid is affected by various factors, such as the degree of maturity of the plant material, fruit variety, light, temperature, climate, irrigation, location of plantation, soil quality, processing, and conditions of storage [3][8]. When the temperature exceeds 35 °C, the amount of lycopene decreases because it is converted to beta-carotene. The lycopene content can increase by about 36% when grown in soil containing the necessary microorganisms [2][3]. The amount of this carotenoid is higher in ripe fruits because they contain less chlorophyll [8]. Light and oxygen have the greatest impact on storage and processing. Its synthesis in tomatoes is promoted via supplementation with red, far-red, and blue light [9][10]. The content of lycopene in tomato puree shows the highest stability (with a total loss of 20%). It has high stability in comparison with other substances, such as ascorbic acid, kaempferol, and quercetin, and also after multiple sterilization and evaporation cycles. Its stability may be related to the presence of, e.g., ascorbic acid and phenolic compounds in tomatoes, and also to the influence of these compounds on the inhibition of the process of isomerization and autooxidation of lycopene. They ensure greater stability compared to pure lycopene [8].
The human body cannot synthesize lycopene. It must be supplied with the diet [11]. Its intake varies by region of the world. In Europe, its consumption from natural sources is 0.5–5 mg/day. In the United States, its daily supply is over 7 mg/day. It is possible to consume 20 mg of this compound daily with a higher consumption of vegetables, fruits, and tomato-based products. Lycopene from natural sources accounts for 50 to 65% of the total intake of this ingredient [3]. Chewing and peristalsis are needed to increase the bioavailability of this carotenoid. These processes mechanically disturb the food. This enables the lycopene in the food matrix to be released [12][13]. In the stomach, it is subjected to the action of digestive enzymes, gastric acid, and the mechanical movements of the stomach. These factors will also contribute to its release from the food matrix. Lycopene can be incorporated into lipid droplets and pass into the small intestine. There, the next stage of decomposition of the food matrix takes place with the help of bile acids and enzymes [13]. Lycopene can be incorporated into lipid micelles and then absorbed by enterocytes [14][15]. Absorption of this compound can occur via passive diffusion. Its absorption may be mediated by the class B scavenger receptor type 1 (SR-B1), which is involved in the absorption of other compounds from the group of carotenoids, such as lutein and beta-carotene [14][16][17]. Due to the high expression of ß-carotene oxygenase 1 (BCO1) and ß-carotene oxygenase 2 (BCO2) in the intestine, partial cleavage of this carotenoid may occur in the enterocytes [18]. Most of the lycopene is incorporated into the chylomicrons unchanged and then passes into the lymphatic system [14][19]. The microsomal triglyceride transfer protein (MTTP) may be involved in this process. It is an enzyme that delivers lipids to the chylomicrons that are formed [20]. They pass from the lymph into the portal circulation. There, extrahepatic lipoprotein lipases break them down to chylomicron residues. The liver removes remnants of chylomicrons from the portal system. Lycopene is incorporated into very low-density lipoproteins that are carried into the bloodstream [21]. Many lifestyle and biological factors affect the absorption of this carotenoid. These are factors such as age, gender, blood lipid concentrations, hormonal status, body mass index and composition, the content of other carotenoids in food, and smoking and alcohol consumption [22]. Its bioavailability decreases with age and in the case of certain diseases [23]. Lycopene is a lipophilic compound, so the presence of fat in food enhances its absorption [24]. Its absorption is hampered by dietary fiber and beta-carotene [25]. After absorption from food, it is mainly stored in the liver, prostate, and adrenals. It is also present in lower concentrations, e.g., in the brain and skin [3][26]. The adipose tissue contains more lycopene than serum because it is a lipophilic compound [25]. The average serum concentration is 0.2–1.0 µmol/L [27]. The half-life of lycopene in human plasma is 12–33 days [1]. Its metabolism occurs in the liver [16]. Oxidative and enzymatic degradation leads to the formation of its metabolites. Biologically active metabolites include apo-lycopenals, apo-lycopenones, apo-carotenedials, epoxides, and carboxylic acids [28][29]. The main metabolites of lycopene are lycopene 1,2-epoxide and lycopene 5,6-epoxide, along with other minor metabolites like lycopene 1,2;5,6-diepoxide, lycopene 1,2;50,60-diepoxide, lycopene 5,6;50,60-diepoxide, and lycopene 1,2;10,20-diepoxide (Figure 1) [30].
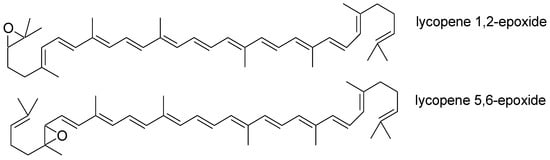
Figure 1. Chemical structure of the main lycopene metabolites.
Lycopene is classified as a carotenoid [31]. It is not classified as a precursor of vitamin A, due to the lack of the final b-ion ring, which is present in the structure of the vitamin A core. The molecular formula of lycopene is C40H56 [32]. Its molecular weight is 536.85 Da [33]. Chemically, this molecule is a linear hydrocarbon with 2 non-conjugated and 11 conjugated double bonds [34]. These bonds may undergo isomerization under the influence of factors such as temperature, light, and chemical reactions [2][35]. The isomerization leads to structures such as 5-cis, 9-cis, 13-cis, and 15-cis (Figure 2) [2][3][36]. With so many double bonds, it can theoretically exist in 1056 cis-trans configurations [37]. However, there are 72 isoforms found in nature [38]. Lycopene is sensitive to oxygen, light, heat, acids, metal ions, and catalysts [33][37]. The most stable form is 5-cis lycopene. The stability of the other isomers decreases in the following order: all-trans, 9-cis, 13-cis, 15-cis, 7-cis, and 11-cis [37]. Its isomerization affects its activity and bioavailability [39]. Lycopene isomers differ in their physical and chemical properties. They have a different melting point, polarization, color intensity, solubility, and ability to crystallize [40].
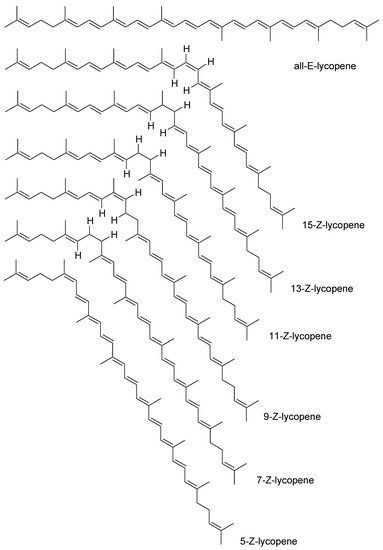
Figure 2. Various trans-cis isomeric forms of lycopene [36].
Lycopene of natural origin occurs as trans isomers [41]. However, in the human body, it exists as cis isomers. Isomerization conversion occurs during the storage, processing, and transport of food products and during metabolic processes in the human body [3]. The heat treatment process of food increases the bioavailability of lycopene. This is related to the transformation of its trans form into the cis form [42]. These processes also help to release this carotenoid from the plant matrix, which also improves its absorption [24]. The cis isomers have greater solubility in bile acids and are more readily absorbed in the colon [42][43]. They are also better absorbed into the bloodstream due to their smaller crystal sizes [44].
The acyclic structure of lycopene affects its solubility [2]. It shows good solubility in organic solvents like acetone, chloroform, benzene, hexane, methylene chloride, and petroleum ether [2][33]. It also has good solubility in carbon disulfide [33]. It is slightly soluble in ethanol and methanol [2]. However, it is insoluble in water [45].
Lycopene in the aqueous environment accumulates and precipitates in the form of crystals. It occurs in the chromoplasts of tomatoes in the form of elongated needle-shaped crystals. It is also found deep within the polysaccharides membrane structure [40].
Lycopene has a wide biological activity. Many studies show that this carotenoid and tomato-based products containing lycopene protect work against many chronic diseases and alleviate their effects [46]. Studies show its positive effect on cardiovascular diseases, including the regulation of blood lipid levels [8][23][47][48][49]. Lycopene also showed antidiabetic properties [37][50]. Research proves its beneficial effects in nervous system disorders, including neurodegenerative diseases such as Parkinson′s disease and Alzheimer′s disease [51][52][53][54]. The positive effect of lycopene has also been observed in liver diseases and ulcerative colitis [16][55].
2. Antioxidant Effects of Lycopene
Oxidative stress is characterized by an imbalance between the amount of reactive oxygen species (ROS) produced and the amount eliminated by antioxidants [56]. Endogenous reactive oxygen and nitrogen species (RONS) are produced in the physiological state by enzymes, including nitric oxide synthase (NOS) and NADPH oxidase. They are a by-product of oxidation reactions catalyzed via metals or mitochondrial electron transport chain processes. Free radical peroxide (O2•−) is the primary precursor to other reactive oxygen and nitrogen species. It can react with other particles to generate other free radicals such as hydroxyl (OH•), peroxyl (ROO−), and alkoxy (RO−), as well as H2O2. During the reaction of nitric oxide with free radical peroxide, the free radical peroxynitrite (ONOO−) is formed [57]. Antioxidant defense systems control reactions that produce reactive oxygen and nitrogen species. It deactivates free radicals and molecules that can turn into RONS [58]. Enzymes such as glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) are essential to this process [59]. Other endogenous antioxidants are also glutathione (GSH), melatonin, lipoic acid, uric acid, and bilirubin [60]. Oxidative stress contributes to cell and tissue damage via the multiple pathways. It is one of the main causes of chronic diseases. These include atherosclerosis, liver diseases, ulcerative colitis, and cardiovascular and nervous system disorders, including neurodegenerative disorders [61][62][63][64][65][66][67][68].
Lycopene is a molecule that most effectively quenches singlet oxygen from carotenoids [1]. Lycopene quenches singlet oxygen twice as much as beta-carotene and 10 times as α-tocopherol [69]. These properties result from its chemical structure-especially the system of conjugated double bonds. And to a lesser extent, it is affected by cyclic or acyclic end groups [1]. Among all the lycopene isomers, the greatest antioxidant properties are shown by the 5-cis form, followed by 9-cis, 7-cis, 13-cis, 11-cis and all-trans [37]. This is probably related to the better solubility and lower self-aggregation in the polar environment by cis forms [28]. The antioxidant activity of lycopene is greater in the case of tomato extracts than in the case of pure lycopene. This is due to its synergistic effect with other compounds such as beta-carotene, phytofluene, and phytoene [70]. Lycopene has the ability to reduce reactive oxygen species (ROS) and eliminate singlet oxygen, nitrogen dioxide, hydroxyl radicals, and hydrogen peroxide [70][71]. Its effect on reactive oxygen species includes radical attachment, electron transfer, and allylic hydrogen abstraction [70]. Lycopene can react with free radicals in more than one way [70][72]. The mechanisms of the antioxidant action of lycopene is presented in Figure 3 [37]. Many factors influence the reactivity of this compound in biological systems. These include the physical and molecular structures, concentration of lycopene, possibility of interaction with other antioxidants, partial pressure of oxygen, and place of action in the cell [73]. Adduct formation and allylic abstraction of hydrogen mainly occur in a non-polar environment. Electron transfer occurs in a polar medium [70]. Lycopene can act as a superoxide radical scavenger (LOO•) and as a singlet oxygen (1O2) [74]. It increases the levels of enzymatic antioxidants such as catalase, superoxide dismutase, and glutathione peroxidase. This is due to the activation of the antioxidant response element, which is associated with nuclear factor E2 (NFE2L2) [75]. Lycopene also has the ability to regenerate non-enzymatic antioxidants such as vitamin C and E. This has a positive effect on the cellular antioxidant defense system [71]. Thanks to its antioxidant properties, lycopene has the ability to protect structures important for the body, such as DNA and lipids [76].
Figure 3. Mechanisms of lycopene′s antioxidant effect [37].
The studies showed the antioxidant activity of lycopene. Pataro et al. [77] tested its activity using the FRAP (ferric reducing antioxidant power) method. They used lycopene extracted from tomato peels with ethyl lactate and acetone. The pulsed electric fields pre-treatment increased the antioxidant activity of all-trans form [77]. Amorim et al. [78] showed in the ORAC (oxygen radical absorbance capacity) test that there are more antioxidants in red guava than in tomatoes. Extracts from these plants were characterized by higher antioxidant activity than pure lycopene. This may be due to the content of other compounds with antioxidant potential in the extract. Lycopene and plant extracts’ Trolox equivalent antioxidant capacity (TEAC) values showed good correlations via simple linear regression analyses [78]. Stinco et al. [79], based on TEAC values, showed that lycopene may have a good ability to capture ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) [79]. Alvi et al. [80] showed in the DPPH (2,2-diphenyl-1-picrylhydrazyl) test that this carotenoid has a high free radical scavenging activity. It was IC50 = 4.57 ± 0.23 μg/mL, and for vitamin C, IC50 = 9.82 ± 0.42 μg/mL. [80]. Wang et al. [81] compared the antioxidant activity of lycopene samples with different contents of Z isomers (5%, 30% and 55% of Z isomers). DPPH and ABTS tests showed that the antioxidant activity of lycopene increases with the content of Z isomers. In the DDPH test, for a sample containing 55% Z isomers, the IC50 value was 80 μg/mL; for a sample containing 30% Z isomers, it was 110 μg/mL; and for a sample containing 5% Z isomers, it was 140 μg/mL. In the ABTS assay, the IC50 values for the samples with 55%, 30%, and 5% Z isomers were 35, 60, and 80 μg/mL, respectively [81].
References
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232.
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let Food Be Your Medicine: Nutraceutical Properties of Lycopene. Food Funct. 2019, 10, 3090–3102.
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706.
- Numan, N.; Jeyaram, S.; Kaviyarasu, K.; Neethling, P.; Sackey, J.; Kotsedi, C.L.; Akbari, M.; Morad, R.; Mthunzi-Kufa, P.; Sahraoui, B.; et al. On the Remarkable Nonlinear Optical Properties of Natural Tomato Lycopene. Sci. Rep. 2022, 12, 9078.
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Wallace Hayes, A.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective Effect of Lycopene against Chemical and Natural Toxins: A Review: Lycopene against Chemical and Natural Toxins. BioFactors 2019, 45, 5–23.
- Park, H.; Kim, Y.-J.; Shin, Y. Estimation of Daily Intake of Lycopene, Antioxidant Contents and Activities from Tomatoes, Watermelons, and Their Processed Products in Korea. Appl. Biol. Chem. 2020, 63, 50.
- Chen, J.; Cao, X.; Huang, Z.; Chen, X.; Zou, T.; You, J. Research Progress on Lycopene in Swine and Poultry Nutrition: An Update. Animals 2023, 13, 883.
- Hsieh, M.-J.; Huang, C.-Y.; Kiefer, R.; Lee, S.-D.; Maurya, N.; Velmurugan, B.K. Cardiovascular Disease and Possible Ways in Which Lycopene Acts as an Efficient Cardio-Protectant against Different Cardiovascular Risk Factors. Molecules 2022, 27, 3235.
- Xie, B.; Wei, J.; Zhang, Y.; Song, S.; Su, W.; Sun, G.; Hao, Y.; Liu, H. Supplemental Blue and Red Light Promote Lycopene Synthesis in Tomato Fruits. J. Integr. Agric. 2019, 18, 590–598.
- Song, Y.; Teakle, G.; Lillywhite, R. Unravelling Effects of Red/Far-Red Light on Nutritional Quality and the Role and Mechanism in Regulating Lycopene Synthesis in Postharvest Cherry Tomatoes. Food Chem. 2023, 414, 135690.
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and Health in Older People. Maturitas 2015, 80, 63–68.
- Low, D.Y.; D’Arcy, B.; Gidley, M.J. Mastication Effects on Carotenoid Bioaccessibility from Mango Fruit Tissue. Food Res. Int. 2015, 67, 238–246.
- Cervantes-Paz, B.; de Jesús Ornelas-Paz, J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Ibarra-Junquera, V.; Yahia, E.M.; Gardea-Béjar, A.A. Effects of Pectin on Lipid Digestion and Possible Implications for Carotenoid Bioavailability during Pre-Absorptive Stages: A Review. Food Res. Int. 2017, 99, 917–927.
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342.
- Borel, P.; Desmarchelier, C.; Dumont, U.; Halimi, C.; Lairon, D.; Page, D.; Sébédio, J.L.; Buisson, C.; Buffière, C.; Rémond, D. Dietary Calcium Impairs Tomato Lycopene Bioavailability in Healthy Humans. Br. J. Nutr. 2016, 116, 2091–2096.
- Ibrahim, I.M.; Althagafy, H.S.; Abd-alhameed, E.K.; Al-Thubiani, W.S.; Hassanein, E.H.M. Promising Hepatoprotective Effects of Lycopene in Different Liver Diseases. Life Sci. 2022, 310, 121131.
- Rowles, J.L.; Erdman, J.W. Carotenoids and Their Role in Cancer Prevention. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158613.
- Raghuvanshi, S.; Reed, V.; Blaner, W.S.; Harrison, E.H. Cellular Localization of β-Carotene 15,15’ Oxygenase-1 (BCO1) and β-Carotene 9’,10’ Oxygenase-2 (BCO2) in Rat Liver and Intestine. Arch. Biochem. Biophys. 2015, 572, 19–27.
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid Metabolism at the Intestinal Barrier. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158580.
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. Lycopene Bioavailability Is Associated with a Combination of Genetic Variants. Free Radic. Biol. Med. 2015, 83, 238–244.
- Srivastava, S.; Srivastava, A.K. Lycopene; Chemistry, Biosynthesis, Metabolism and Degradation under Various Abiotic Parameters. J. Food Sci. Technol. 2015, 52, 41–53.
- van Steenwijk, H.P.; Bast, A.; de Boer, A. The Role of Circulating Lycopene in Low-Grade Chronic Inflammation: A Systematic Review of the Literature. Molecules 2020, 25, 4378.
- Petyaev, I.M. Lycopene Deficiency in Ageing and Cardiovascular Disease. Oxidative Med. Cell. Longev. 2016, 2016, 3218605.
- Doyle, L.M. Lycopene: Implications for Human Health–A Review. Adv. Food Technol. Nutr. Sci. Open J. 2020, 6, 1–12.
- Wu, S.; Guo, X.; Shang, J.; Li, Y.; Dong, W.; Peng, Q.; Xie, Z.; Chen, C. Effects of Lycopene Attenuating Injuries in Ischemia and Reperfusion. Oxidative Med. Cell. Longev. 2022, 2022, 9309327.
- Macar, O.; Kalefetoğlu Macar, T.; Çavuşoğlu, K.; Yalçın, E.; Yapar, K. Lycopene: An Antioxidant Product Reducing Dithane Toxicity in Allium cepa L. Sci. Rep. 2023, 13, 2290.
- Wang, Y.-H.; Zhang, R.-R.; Yin, Y.; Tan, G.-F.; Wang, G.-L.; Liu, H.; Zhuang, J.; Zhang, J.; Zhuang, F.-Y.; Xiong, A.-S. Advances in Engineering the Production of the Natural Red Pigment Lycopene: A Systematic Review from a Biotechnology Perspective. J. Adv. Res. 2023, 46, 31–47.
- Abenavoli, L.; Procopio, A.C.; Paravati, M.R.; Costa, G.; Milić, N.; Alcaro, S.; Luzza, F. Mediterranean Diet: The Beneficial Effects of Lycopene in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 3477.
- Martínez, A.; Melendez-Martínez, A.J. Lycopene, Oxidative Cleavage Derivatives and Antiradical Activity. Comput. Theor. Chem. 2016, 1077, 92–98.
- Cámara, M.; De Cortes Sánchez-Mata, M.; Fernández-Ruiz, V.; Cámara, R.M.; Manzoor, S.; Caceres, J.O. Lycopene. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 40, pp. 383–426. ISBN 978-0-444-59603-1.
- Przybylska, S. Lycopene—A Bioactive Carotenoid Offering Multiple Health Benefits: A Review. Int. J. Food Sci. Technol. 2020, 55, 11–32.
- Song, X.; Luo, Y.; Ma, L.; Hu, X.; Simal-Gandara, J.; Wang, L.-S.; Bajpai, V.K.; Xiao, J.; Chen, F. Recent Trends and Advances in the Epidemiology, Synergism, and Delivery System of Lycopene as an Anti-Cancer Agent. Semin. Cancer Biol. 2021, 73, 331–346.
- Carvalho, G.C.; Sábio, R.M.; Chorilli, M. An Overview of Properties and Analytical Methods for Lycopene in Organic Nanocarriers. Crit. Rev. Anal. Chem. 2020, 51, 674–686.
- Campos-Lozada, G.; Pérez-Marroquín, X.A.; Callejas-Quijada, G.; Campos-Montiel, R.G.; Morales-Peñaloza, A.; León-López, A.; Aguirre-Álvarez, G. The Effect of High-Intensity Ultrasound and Natural Oils on the Extraction and Antioxidant Activity of Lycopene from Tomato (Solanum lycopersicum) Waste. Antioxidants 2022, 11, 1404.
- Pu, C.; Tang, W. Encapsulation of Lycopene in Chlorella Pyrenoidosa: Loading Properties and Stability Improvement. Food Chem. 2017, 235, 283–289.
- Kessy, H.N.; Zhang, L.; Zhang, H. Lycopene (Z)—Isomers Enrichment and Separation. Int. J. Food Sci. Technol. 2013, 48, 2050–2056.
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335.
- Li, Y.; Cui, Z.; Hu, L. Recent Technological Strategies for Enhancing the Stability of Lycopene in Processing and Production. Food Chem. 2023, 405, 134799.
- Liang, X.; Ma, C.; Yan, X.; Liu, X.; Liu, F. Advances in Research on Bioactivity, Metabolism, Stability and Delivery Systems of Lycopene. Trends Food Sci. Technol. 2019, 93, 185–196.
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural Origin Lycopene and Its “Green” Downstream Processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709.
- Górecka, D.; Wawrzyniak, A.; Jędrusek-Golińska, A.; Dziedzic, K.; Hamułka, J.; Kowalczewski, P.Ł.; Walkowiak, J. Lycopene in Tomatoes and Tomato Products. Open Chem. 2020, 18, 752–756.
- Ozkan, G.; Günal-Köroğlu, D.; Karadag, A.; Capanoglu, E.; Cardoso, S.M.; Al-Omari, B.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. A Mechanistic Updated Overview on Lycopene as Potential Anticancer Agent. Biomed. Pharmacother. 2023, 161, 114428.
- Martínez-Hernández, G.B.; Boluda-Aguilar, M.; Taboada-Rodríguez, A.; Soto-Jover, S.; Marín-Iniesta, F.; López-Gómez, A. Processing, Packaging, and Storage of Tomato Products: Influence on the Lycopene Content. Food Eng. Rev. 2016, 8, 52–75.
- Ashraf, W.; Latif, A.; Lianfu, Z.; Jian, Z.; Chenqiang, W.; Rehman, A.; Hussain, A.; Siddiquy, M.; Karim, A. Technological Advancement in the Processing of Lycopene: A Review. Food Rev. Int. 2022, 38, 857–883.
- Amorim, A.D.G.N.; Vasconcelos, A.G.; Souza, J.; Oliveira, A.; Gullón, B.; de Souza de Almeida Leite, J.R.; Pintado, M. Bio-Availability, Anticancer Potential, and Chemical Data of Lycopene: An Overview and Technological Prospecting. Antioxidants 2022, 11, 360.
- Mehta, N.; Patani, P.; Singhvi, I. A Review on Tomato Lycopene. Int. J. Pharm. Sci. Res. 2018, 9, 916–923.
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases—A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879.
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can Lycopene Be Considered an Effective Protection against Cardiovascular Disease? Food Chem. 2018, 245, 1148–1153.
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and Lycopene Supplementation and Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Atherosclerosis 2017, 257, 100–108.
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; van der A, D.L.; Spijkerman, A.M.W.; van der Schouw, Y.T. Dietary Intake of Carotenoids and Risk of Type 2 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381.
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Di Santo, S.G. A Narrative Review on the Potential of Tomato and Lycopene for the Prevention of Alzheimer’s Disease and Other Dementias. Crit. Rev. Food Sci. Nutr. 2022, 62, 4970–4981.
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Justin Thenmozhi, A. Neuroprotective Effect of Lycopene against MPTP Induced Experimental Parkinson’s Disease in Mice. Neurosci. Lett. 2015, 599, 12–19.
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective Effects of Lycopene in Cancer, Cardiovascular, and Neurodegenerative Diseases: An Update on Epidemiological and Mechanistic Perspectives. Pharmacol. Res. 2020, 155, 104730.
- Chen, D.; Huang, C.; Chen, Z. A Review for the Pharmacological Effect of Lycopene in Central Nervous System Disorders. Biomed. Pharmacother. 2019, 111, 791–801.
- Kashef, S.M.; Yassien, R.I.; El-Ghazouly, D.E.-S. The Possible Effect of Lycopene in Ameliorating Experimentally Induced Ulcerative Colitis in Adult Male Albino Rats (A Histological, Immunohistochemical, and Ultrastructural Study). Ultrastruct. Pathol. 2023, 47, 172–187.
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131.
- Varela, E.L.P.; Gomes, A.R.Q.; da Silva Barbosa dos Santos, A.; de Carvalho, E.P.; Vale, V.V.; Percário, S. Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases. Nutrients 2022, 14, 5303.
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293.
- York-Duran, M.J.; Godoy-Gallardo, M.; Jansmanung, M.M.T.; Hosta-Rigau, L. A Dual-Component Carrier with Both Non-Enzymatic and Enzymatic Antioxidant Activity towards ROS Depletion. Biomater. Sci. 2019, 7, 4813–4826.
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704.
- Rives, C.; Fougerat, A.; Ellero-Simatos, S.; Loiseau, N.; Guillou, H.; Gamet-Payrastre, L.; Wahli, W. Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants. Biomolecules 2020, 10, 1702.
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of Beneficial Effects of Exercise Training on Non-Alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003.
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709.
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417.
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601.
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583.
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938.
- Wu, H.; Wu, Y.; Cui, Z.; Hu, L. Nutraceutical Delivery Systems to Improve the Bioaccessibility and Bioavailability of Lycopene: A Review. Crit. Rev. Food Sci. Nutr. 2023; 1–19, online ahead of print.
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323.
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511.
- Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules 2021, 26, 5333.
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957.
- Joshi, B.; Kar, S.K.; Yadav, P.K.; Yadav, S.; Shrestha, L.; Bera, T.K. Therapeutic and Medicinal Uses of Lycopene: A Systematic Review. Int. J. Res. Med. Sci. 2020, 8, 1195.
- Sun, X.; Jia, H.; Xu, Q.; Zhao, C.; Xu, C. Lycopene Alleviates H2O2 -Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Food Funct. 2019, 10, 6276–6285.
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. Food Chem. 2021, 343, 128396.
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of Lycopene from Industrially Derived Tomato Processing By-Products by Pulsed Electric Fields-Assisted Extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369.
- Amorim, A.G.N.; Souza, J.M.T.; Santos, R.C.; Gullón, B.; Oliveira, A.; Santos, L.F.A.; Virgino, A.L.E.; Mafud, A.C.; Petrilli, H.M.; Mascarenhas, Y.P.; et al. HPLC-DAD, ESI–MS/MS, and NMR of Lycopene Isolated From P. Guajava L. and Its Biotechnological Applications. Eur. J. Lipid Sci. Technol. 2018, 120, 1700330.
- Stinco, C.M.; Heredia, F.J.; Vicario, I.M.; Meléndez-Martínez, A.J. In Vitro Antioxidant Capacity of Tomato Products: Relationships with Their Lycopene, Phytoene, Phytofluene and Alpha-Tocopherol Contents, Evaluation of Interactions and Correlation with Reflectance Measurements. LWT-Food Sci. Technol. 2016, 65, 718–724.
- Alvi, S.S.; Iqbal, D.; Ahmad, S.; Khan, M.S. Molecular Rationale Delineating the Role of Lycopene as a Potent HMG-CoA Reductase Inhibitor: In Vitro and in Silico Study. Nat. Prod. Res. 2016, 30, 2111–2114.
- Wang, H.; Lin, Y.; Liu, Q.; Zhou, A.; Bian, H.; Zhang, W.; Hui, A.; Wu, Z. Antioxidant, Anticancer Activity and Molecular Docking Study of Lycopene with Different Ratios of Z-Isomers. Curr. Res. Food Sci. 2023, 6, 100455.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
628
Revisions:
2 times
(View History)
Update Date:
15 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




