Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maryanne M Collinson | -- | 2426 | 2023-09-14 10:50:56 | | | |
| 2 | Sirius Huang | Meta information modification | 2426 | 2023-09-15 03:53:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Islam, M.S.; Banik, S.; Collinson, M.M. Fabrication Methods for Porous Gold Electrodes. Encyclopedia. Available online: https://encyclopedia.pub/entry/49159 (accessed on 07 February 2026).
Islam MS, Banik S, Collinson MM. Fabrication Methods for Porous Gold Electrodes. Encyclopedia. Available at: https://encyclopedia.pub/entry/49159. Accessed February 07, 2026.
Islam, Md. Shafiul, Subrata Banik, Maryanne M. Collinson. "Fabrication Methods for Porous Gold Electrodes" Encyclopedia, https://encyclopedia.pub/entry/49159 (accessed February 07, 2026).
Islam, M.S., Banik, S., & Collinson, M.M. (2023, September 14). Fabrication Methods for Porous Gold Electrodes. In Encyclopedia. https://encyclopedia.pub/entry/49159
Islam, Md. Shafiul, et al. "Fabrication Methods for Porous Gold Electrodes." Encyclopedia. Web. 14 September, 2023.
Copy Citation
Bimetallic nanocomposites and nanoparticles have received tremendous interest recently because they often exhibit better properties than single-component materials. Improved electron transfer rates and the synergistic interactions between individual metals are two of the most beneficial attributes of these materials.
nanoporous gold
bimetallic
electrochemistry
nanomaterials
nanoparticles
sensors
1. Introduction
Nanoporous materials have had an enormous impact in many scientific and technological applications, including the development of adsorbents for cleaning up toxic waste, filtration and separation media for separating complex samples, catalytic materials for speeding up slow reactions, and chemical sensors for detecting trace constituents in complex samples, among others. Important properties include high surface area, a high surface area-to-volume ratio, high number of catalytically active sites, confinement effects, and unique and tailorable pore morphology. Examples of commonly used nanoporous materials include zeolites and clays, sol–gel-derived materials (xerogels and aerogels), the M41S family of materials, inverse opals, covalent organic frameworks (COFs), metal–organic frameworks (MOF), and nanoporous metals and foams [1][2][3][4][5][6][7][8][9][10]. The pore size, geometry, and interconnectivity strongly depend on the method of fabrication. Nanoporous metals are particularly valuable, because they have high electron conductivity, which allows them to serve as scaffolds for electrochemical devices such as electrochemical sensors, electrocatalysts, batteries, super capacitors, and fuel cells [11][12][13][14][15][16]. More importantly, they can have unique catalytic and electrochemical properties [13] due to their nanosized pores, high surface area-to-volume ratio, and large number of functional sites. The chemical and physical properties of nanoporous metals are often much better than those of nonporous metals, and thus they present exciting opportunities in the fields of chemical sensing, electrocatalysis, and energy storage and delivery.
One popular nanoporous material is nanoporous gold (NPG) [16][17][18][19][20][21][22][23]. NPG has been widely used in the field of analytical chemistry because of its unique pore structure and the ease with which it can be fabricated [18]. When prepared via dealloying methods, NPG has a characteristic three-dimensional bicontinuous nanopore framework, which has proven to be very valuable in electrochemical sensing [18] and electrocatalysis [19]. Compared to planar gold, NPG has many unique features [19]: (1) a high surface area that provides more places for electron transfer; (2) tunable pore size, ranging from a few nanometers to tens of nanometers, which allows pore-size-dependent effects to be evaluated [24][25]; (3) when prepared by dealloying in concentrated HNO3 [26], NPG has very clean surfaces, thus eliminating the need for harsh cleaning methods before use; (4) improved accessibility to internal sites due to the continuity and interconnectivity of pores [27]; and (5) high density of low-coordination surface gold atoms, which can improve electrocatalytic properties [28][29].
The unique properties of NPG have been exploited in several different ways. For example, NPG is a significantly better electrocatalyst for the oxidation of methanol than planar gold due to the presence of low coordination sites [30][31]. NPG is also effective for enzyme immobilization due to its small pores, which help stabilize the macromolecule under otherwise denaturing environments [32]. The high surface area of NPG means a greater amount of an adsorbate (e.g., enzyme) can be immobilized on the gold surface, allowing for greater signals relative to planar gold [24][33]. The bicontinuous, porous framework provides an avenue for small substrate molecules to reach inner surfaces and at the same time can reduce the effect of biofouling on an electrochemical signal due to a unique biosieving mechanism [34]. Because gold is easily modified with thiol groups, NPG can be modified with thiolated probe DNA molecules (or aptamers), enabling their use as DNA/RNA/nucleic acid sensors [35]. Electron transfer rates are also improved at nanoporous electrodes because of nanoconfinement effects [13].
While gold is an important element and has many useful attributes, it also has limitations. One important example is that gold is not as catalytically active as other metals. However, by either alloying gold or decorating gold with more catalytically active elements, improvements in the physicochemical properties of the nanoporous material can be obtained. Bimetallic nanocomposites and nanoparticles, in particular, have shown better physicochemical properties compared to their single-metal counterpart, and thus have shown their usefulness in chemical sensing and electrocatalysis [36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51]. Studies have shown that nanoporous alloys exhibit better electrocatalytic properties and have improved electrochemical sensing compared to single-component metals [37][52]. In some cases, these enhancements have been attributed to the synergistic interactions between the individual metals [37]. In chemical sensing, improved selectivity and sensitivity can also result from improved electron transfer rates when a second metal is added to a nanoporous structure [53][54]. Decorating a NPG framework with a precious metal can also reduce the consumption of that precious metal and provide high accessibility to it as needed.
2. Fabrication Methods for Porous Gold Electrodes
NPG can be made in many different ways. How NPG is made ultimately determines the pore structure, roughness factor, surface area, pore size, pore-size distribution, level of interconnectivity of the pores, and accessibility to the inner surfaces. All these properties are important, as they collectively determine the overall performance of the device or application. For electrochemical sensing applications, accessibility, surface area, pore size, and connectivity are especially critical, as they influence analytical figures-of-merit such as sensitivity, detection limit, response time, and selectivity. This section will briefly describe the most common ways of fabricating the NPG scaffold.
2.1. Templating
Templating methods provide a simple approach for preparing porous gold electrodes with a wide range of pore sizes and/or unique nanostructures [3][4][8][55][56]. This approach involves the selection or fabrication of the template(s) and the formation of the metal (e.g., gold) framework around the template, which is then followed by template removal. For materials that require large pores in the 100+ nm range, hard templating methods are valuable. In this case, ‘hard’ sacrificial templates such as anodic aluminum oxide (AAO), large silica particles, colloidal crystals, or copolymer particles are used [8]. Gold (or another suitable metal) is electrochemically (or chemically) deposited in and around an assembly of hard templates, which is then subsequently removed by chemical means or via heat treatment [57][58]. Polystyrene templates can be easily removed in chloroform or toluene. An example of this approach is shown in Figure 1. The pores and the arrangement of the pores are dictated by the size of the template and how it is assembled on the surface. Because of the size of the templates (and ultimately the pores), the overall surface area of the electrodes is not as high as it is when smaller templates are used.
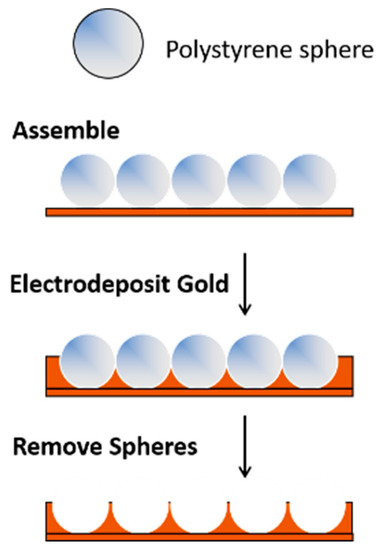
Figure 1. A templating approach used for the formation of porous gold electrodes.
Soft templates can be used to produce NPG with significantly smaller pore sizes, often in the sub-10 nm range, thus increasing the surface area of the electrode [4]. Examples of such ‘soft’ templates include surfactant micelles, amphiphilic block copolymers, and lyotropic liquid crystals [55]. Bimodal porosity can be achieved by combining templates with different sizes and types [7] or by using hierarchical templates [59]. Hierarchical porosity can also be achieved by combining different soft templates or hard templates with soft templates (e.g., large polystyrene or silica particles with lyotropic liquid crystals) [7][60].
Many templates and approaches have been developed to create nanoporous (meso- and microporous) gold electrodes with different pore arrangements. For instance, a one-step liquid-phase method using AgCl as a sacrificial template was developed for preparing zero-dimensional hollow NPG with adjustable particle size (150–1000 nm) and ligament thickness (21–54 nm) [61]. Strawberry- and raspberry-like hierarchical templates [62] were fabricated by linking different-sized polystyrene latex spheres together, and they were used to prepare hierarchical nanostructured gold electrodes with large outer pores (~μm in size) and ~10 s of nm sized inner pores [59]. In another example, Hsueh et al. prepared NPG with a bicontinuous morphology by using different types of block copolymer (BCP) [63]. Using electrodeposited silica as a template, NPG with a coral-like framework and high surface area was fabricated [64]. The one drawback of template-based methods for NPG formation is that the overall structure of the nanoporous matrix is dictated by the shape of the template and template assembly. Different structures typically require the design of corresponding templates [34][59].
2.2. Chemical Dealloying of a Pre-Formed Alloy
One common method for preparing NPG with a bicontinuous network of nanosized pores is via chemical or electrochemical dealloying of a pre-formed alloy [27][65][66]. In this approach, the least noble metal in a gold alloy is selectively removed, ultimately producing a nanopore structure containing a bicontinuous, well-connected network of ligands and pores. Examples of Au alloys that have been used include Au-Sn [67][68], Au-Zn [69][70], Au-Cu-Pd-Ag-Si [71][72], Au-Ni [73], Au-Cu [74], Au-Fe [75], Au-Al [76], and Au-Ag [65][77], with the latter alloy being the most common. White gold (~12K; Au-Ag alloy) has been widely used to form NPG [77], in part because it can be purchased commercially in the form of thin white gold leaves (~100 nm thick). Such materials are commonly used by artists, thus making this material popular and readily available. An Au-Ag alloy of the appropriate composition could also be electrochemically deposited from appropriate gold and silver salts on a conducting support [78]. It can also be sputtered or physically deposited under vacuum using appropriate targets [79].
A simple method for chemically dealloying white gold is to place it in nitric acid for a selected period of time [34][80]. This time can range from ~10 min to hours, depending on the thickness of the starting alloy [18]. During this corrosion process, the least noble metal (silver) is depleted, and the gold atoms diffuse at the electrolyte–metal interface and restructure on the surface to make gold-rich clusters surrounded by nanosized pores [81]. The pore size and shape strongly depend on the concentration of the acid, the time in the acid, the composition of the alloy, etc. [18]. Chemical dealloying in acid is simple and quick, but the corrosion process, and thus the pore formation, can be difficult to control [37]. Additionally, it is impossible to remove all the Ag from the alloy, and care needs to be taken to fully understand what role residual Ag has on the observed response [82][83][84]. A recent approach has been described in which NPG is formed that is free from residual Ag [85].
2.3. Electrochemical Dealloying of a Pre-Formed Alloy
Another approach to dealloying that can provide more control is electrochemical dealloying [37]. In this approach, the material to be dealloyed serves as the working electrode and is immersed in a suitable electrolyte solution (e.g., perchloric acid) along with a reference and counter electrode. A potential slightly higher than the ‘critical’ potential is applied to the electrode surface with respect to a reference for a set period of time. The critical potential is very important, and its value depends on the alloy, its composition, and the electrolyte [86]. Linear-sweep voltammetry can help determine the critical potential [86]. For both chemical and electrochemical dealloying, post-processing procedures such as heat treatment and/or electrochemical treatment can be used to further tune the ligament and pore size.
2.4. Electrochemical Formation of the Alloy Followed by Electrochemical Dealloying
A ‘combined’ electrochemical alloying–dealloying process can also be used to prepare NPG [69][70]. In this method, instead of starting with a previously fabricated alloy, the alloy is made on the spot electrochemically and then immediately dealloyed in the same electrochemical cell. The process is a multicyclic process [69][70]. The method utilizes a ZnCl2/benzyl alcohol electrolyte solution held at an elevated temperature and a gold electrode. First, a cathodic sweep reduces zinc ions, forming an Au-Zn alloy, and second, the anodic sweep then subsequently removes Zn (i.e., causes dealloying). After several cycles, a 3D nanoporous structure is obtained [69]. This method is relatively simple, and can be used to form NPG layers on a gold wire or gold needles, which are very useful when small electrodes are needed for monitoring redox events in confined environments [87][88][89].
2.5. Anodization and Surface Roughening
Another common and simple approach to prepare a high-surface-area NPG surface is anodization. This method is also conducive to the formation of NPG microelectrodes [90], and has commonly been performed using gold recordable compact disks cut into small pieces [91][92][93]. Different variants of this approach have been described in the literature. They typically involve, as a first step, the polishing or precleaning of the electrode surface. Next, the electrode is anodized via the application of a large positive potential in an electrolyte solution. In one example, 1.217 V (vs. Ag/AgCl) was applied to a precleaned gold microelectrode in HCl [90]. In another example, 0.9 V (vs. SMSE) was applied for 50 s [94]. An alternative procedure involves the application of 5 V (vs. Ag/AgCl) to a gold CD in phosphate buffer for three minutes [91][92][93][95]. Gas bubbles are produced, and an oxide is formed on the surface. The roughened electrode is then placed in a solution containing a chemical reducing agent (e.g., ascorbic acid) for a short period of time to reduce the gold oxide. Variations of this general approach include the application of square-wave potential pulses in sulfuric acid [96][97], the use of different electrolyte solutions such as phosphate buffer—KCl [98] or NaOH—with the use of a potential-pulse waveform from 1.2 to −4.6 V (vs. SCE) [99]. The mechanism for the formation of the roughened NPG surface depends on the reaction medium. In the case of KCl or HCl, the mechanism proceeds through electrodissolution, disproportion, and deposition [94][100]. In NaOH, upon application of a square-wave potential pulse between 0.8 V to −5 V (vs. SMSE), NPG is formed via the weak release of oxygen gas followed by the intense release of hydrogen [99][101].
The advantages, disadvantages (concerns), and applicability of these methods are compared in Table 1.
Table 1. Summary of Common Fabrication Methods for Porous Gold Electrodes.
| Method | Requirements | Advantages | Concerns |
|---|---|---|---|
Templating
|
|
|
|
| Chemical dealloying a pre-formed alloy |
|
|
|
| Electrochemical dealloying of a pre-formed alloy |
|
|
|
| Electrochemical alloying–dealloying |
|
|
|
| Anodization–Roughening |
|
|
|
References
- Taguchi, A.; Schüth, F. Ordered Mesoporous Materials in Catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45.
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420.
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77.
- Yamauchi, Y.; Kuroda, K. Rational Design of Mesoporous Metals and Related Nanomaterials by a Soft-Template Approach. Chem. Asian J. 2008, 3, 664–676.
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.D.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322.
- Bryce, C.T.; Stephen, A.S.; Luther, E.P. Nanoporous Metal Foams. Angew. Chem. Int. Ed. 2010, 49, 4544–4565.
- Ying, J.; Lenaerts, S.; Symes, M.D.; Yang, X.Y. Hierarchical Design in Nanoporous Metals. Adv. Sci. 2022, 9, 2106117.
- Rebbecchi, T.A.; Chen, Y. Template-Based Fabrication of Nanoporous Metals. J. Mater. Res. 2018, 33, 2–15.
- Díaz, U.; Corma, A. Ordered Covalent Organic Frameworks, COFs and PAFs. From Preparation to Application. Coord. Chem. Rev. 2016, 311, 85–124.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933.
- Sang, Q.; Hao, S.; Han, J.; Ding, Y. Dealloyed Nanoporous Materials for Electrochemical Energy Conversion and Storage. EnergyChem 2022, 4, 100069.
- Zhang, J.; Li, C.M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016–7031.
- Park, S.; Kim, H.C.; Chung, T.D. Electrochemical Analysis Based on Nanoporous Structures. Analyst 2012, 137, 3891.
- Dai, Z.; Ju, H. Bioanalysis Based on Nanoporous Materials. TrAC Trends Anal. Chem. 2012, 39, 149–162.
- Şeker, E.; Shih, W.C.; Stine, K.J. Nanoporous Metals by Alloy Corrosion: Bioanalytical and Biomedical Applications. MRS Bull. 2018, 43, 49–56.
- Gonçalves, J.M.; Kumar, A.; da Silva, M.I.; Toma, H.E.; Martins, P.R.; Araki, K.; Bertotti, M.; Angnes, L. Nanoporous Gold-Based Materials for Electrochemical Energy Storage and Conversion. Energy Technol. 2021, 9, 2000927.
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215.
- Collinson, M.M. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 1–21.
- Wittstock, A.; Wichmann, A.; Bäumer, M. Nanoporous Gold as a Platform for a Building Block Catalyst. ACS Catal. 2012, 2, 2199–2215.
- Wittstock, A.; Biener, J.; Erlebacher, J.; Bäumer, M. Nanoporous Gold: From an Ancient Technology to a High-Tech Material; The Royal Society of Chemistry: London, UK, 2012.
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous Gold: A Review and Potentials in Biotechnological and Biomedical Applications. Nano Sel. 2021, 2, 1437–1458.
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899.
- Xiao, X.; Si, P.; Magner, E. An Overview of Dealloyed Nanoporous Gold in Bioelectrochemistry. Bioelectrochemistry 2016, 109, 117–126.
- Chen, L.Y.; Fujita, T.; Chen, M.W. Biofunctionalized Nanoporous Gold for Electrochemical Biosensors. Electrochim. Acta 2012, 67, 1–5.
- Qiu, H.; Xu, C.; Huang, X.; Ding, Y.; Qu, Y.; Gao, P. Immobilization of Laccase on Nanoporous Gold: Comparative Studies on the Immobilization Strategies and the Particle Size Effects. J. Phys. Chem. C 2009, 113, 2521–2525.
- Ding, Y.; Erlebacher, J. Nanoporous Metals with Controlled Multimodal Pore Size Distribution. J. Am. Chem. Soc. 2003, 125, 7772–7773.
- Chen, Q. Bicontinuous Nanoporous Metals with Self-Organized Functionalities. Chem. Mater. 2022, 34, 10237–10248.
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic Origins of the High Catalytic Activity of Nanoporous Gold. Nat. Mater. 2012, 11, 775–780.
- Ding, Y.; Chen, M. Nanoporous Metals for Catalytic and Optical Applications. MRS Bull. 2009, 34, 569–576.
- Ge, X.; Wang, R.; Liu, P.; Ding, Y. Platinum-Decorated Nanoporous Gold Leaf for Methanol Electrooxidation. Chem. Mater. 2007, 19, 5827–5829.
- Zhang, J.; Liu, P.; Ma, H.; Ding, Y. Nanostructured Porous Gold for Methanol Electro-Oxidation. J. Phys. Chem. C 2007, 111, 10382–10388.
- Qiu, H.; Xu, C.; Huang, X.; Ding, Y.; Qu, Y.; Gao, P. Adsorption of Lacease on the Surface of Nanoporous Gold and the Direct Electron Transfer between Them. J. Phys. Chem. C 2008, 112, 14781–14785.
- Stine, K.J. Enzyme Immobilization on Nanoporous Gold: A Review. Biochem. Insights 2017, 10, 117862641774860.
- Patel, J.; Radhakrishnan, L.; Zhao, B.; Uppalapati, B.; Daniels, R.C.; Ward, K.R.; Collinson, M.M. Electrochemical Properties of Nanostructured Porous Gold Electrodes in Biofouling Solutions. Anal. Chem. 2013, 85, 11610–11618.
- Daggumati, P.; Matharu, Z.; Wang, L.; Seker, E. Biofouling-Resilient Nanoporous Gold Electrodes for DNA Sensing. Anal. Chem. 2015, 87, 8618–8622.
- Stephanie, R.; Kim, M.W.; Kim, S.H.; Kim, J.K.; Park, C.Y.; Park, T.J. Recent Advances of Bimetallic Nanomaterials and Its Nanocomposites for Biosensing Applications. TrAC Trends Anal. Chem. 2021, 135, 116159.
- Lu, L. Nanoporous Noble Metal-Based Alloys: A Review on Synthesis and Applications to Electrocatalysis and Electrochemical Sensing. Microchim. Acta 2019, 186, 664.
- Rajeev, R.; Datta, R.; Varghese, A.; Sudhakar, Y.N.N.; George, L. Recent Advances in Bimetallic Based Nanostructures: Synthesis and Electrochemical Sensing Applications. Microchem. J. 2021, 163, 105910.
- Rick, J.; Tsai, M.C.; Hwang, B.J. Biosensors Incorporating Bimetallic Nanoparticles. Nanomaterials 2015, 6, 5.
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412.
- Kannan, P.; Maduraiveeran, G. Bimetallic Nanomaterials-Based Electrochemical Biosensor Platforms for Clinical Applications. Micromachines 2022, 13, 76.
- Wang, D.; Li, Y. Bimetallic Nanocrystals: Liquid-Phase Synthesis and Catalytic Applications. Adv. Mater. 2011, 23, 1044–1060.
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472.
- Mandal, R.; Baranwal, A.; Srivastava, A.; Chandra, P. Evolving Trends in Bio/Chemical Sensor Fabrication Incorporating Bimetallic Nanoparticles. Biosens. Bioelectron. 2018, 117, 546–561.
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260.
- Porter, N.S.; Wu, H.; Quan, Z.; Fang, J. Shape-Control and Electrocatalytic Activity-Enhancement of Pt-Based Bimetallic Nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877.
- Jiang, J.; Zhou, X.L.; Lv, H.G.; Yu, H.Q.; Yu, Y. Bimetallic-Based Electrocatalysts for Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2212160.
- Chavan, V.A.; Bhagat, D.S.; Gangawane, A.K.; Khawashi, H.P.; Thorat, B.R. Bimetallic Nanomaterials-Based Electroanalytical Methods for Detection of Pesticide Residues. Biointerface Res. Appl. Chem. 2023, 13, 468.
- Cui, C.; Hu, X.; Wen, L. Recent Progress on Nanostructured Bimetallic Electrocatalysts for Water Splitting and Electroreduction of Carbon Dioxide. J. Semicond. 2020, 41, 091705.
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble Metal-Based Bimetallic Nanoparticles: The Effect of the Structure on the Optical, Catalytic and Photocatalytic Properties. Adv. Colloid Interface Sci. 2016, 229, 80–107.
- Dindar, C.K.; Erkmen, C.; Uslu, B. Electroanalytical Methods Based on Bimetallic Nanomaterials for Determination of Pesticides: Past, Present, and Future. Trends Environ. Anal. Chem. 2021, 32, e00145.
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 846–910.
- Noyhouzer, T.; Valdinger, I.; Mandler, D. Enhanced Potentiometry by Metallic Nanoparticles. Anal. Chem. 2013, 85, 8347–8353.
- Islam, M.S.; Collinson, M.M. Improved Sensitivity and Selectivity for the Redox Potentiometric Measurement of Biological Redox Molecules Using Nafion-Coated Platinum Decorated Nanoporous Gold Electrodes. J. Electrochem. Soc. 2022, 169, 057503.
- Vukovic, I.; ten Brinke, G.; Loos, K. Block Copolymer Template-Directed Synthesis of Well-Ordered Metallic Nanostructures. Polymer 2013, 54, 2591–2605.
- Raman, N.K.; Anderson, M.T.; Brinker, C.J. Template-Based Approaches to the Preparation of Amorphous, Nanoporous Silicas. Chem. Mater. 1996, 8, 1682–1701.
- Bartlett, P.N.; Birkin, P.R.; Ghanem, M.A. Electrochemical Deposition of Macroporous Platinum, Palladium and Cobalt Films Using Polystyrene Latex Sphere Templates. Chem. Commun. 2000, 1671–1672.
- Bartlett, P.N.; Baumberg, J.J.; Birkin, P.R.; Ghanem, M.A.; Netti, M.C. Highly Ordered Macroporous Gold and Platinum Films Formed by Electrochemical Deposition through Templates Assembled from Submicron Diameter Monodisperse Polystyrene Spheres. Chem. Mater. 2002, 14, 2199–2208.
- Zhao, B.; Collinson, M.M. Hierarchical Porous Gold Electrodes: Preparation, Characterization, and Electrochemical Behavior. J. Electroanal. Chem. 2012, 684, 53–59.
- Yang, X.Y.; Chen, L.H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.L. Hierarchically Porous Materials: Synthesis Strategies and Structure Design. Chem. Soc. Rev. 2017, 46, 481–558.
- Pedireddy, S.; Lee, H.K.; Tjiu, W.W.; Phang, I.Y.; Tan, H.R.; Chua, S.Q.; Troadec, C.; Ling, X.Y. One-Step Synthesis of Zero-Dimensional Hollow Nanoporous Gold Nanoparticles with Enhanced Methanol Electrooxidation Performance. Nat. Commun. 2014, 5, 4947.
- Zhao, B.; Collinson, M.M. Well-Defined Hierarchical Templates for Multimodal Porous Material Fabrication. Chem. Mater. 2010, 22, 4312–4319.
- Hsueh, H.Y.; Chen, H.Y.; Hung, Y.C.; Ling, Y.C.; Gwo, S.; Ho, R.M. Well-Defined Multibranched Gold with Surface Plasmon Resonance in near-Infrared Region from Seeding Growth Approach Using Gyroid Block Copolymer Template. Adv. Mater. 2013, 25, 1780–1786.
- Farghaly, A.A.; Collinson, M.M. Electroassisted Codeposition of Sol-Gel Derived Silica Nanocomposite Directs the Fabrication of Coral-like Nanostructured Porous Gold. Langmuir 2014, 30, 5276–5286.
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of Nanoporosity in Dealloying. Nature 2001, 410, 450–453.
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286.
- Xu, Y.; Ke, X.; Yu, C.; Liu, S.; Zhao, J.; Cui, G.; Higgins, D.; Chen, Z.; Li, Q.; Wu, G. A Strategy for Fabricating Nanoporous Gold Films through Chemical Dealloying of Electrochemically Deposited Au-Sn Alloys. Nanotechnology 2014, 25, 445602.
- Chen, S.; Chu, Y.; Zheng, J.; Li, Z. Study on the Two Dealloying Modes in the Electrooxidation of Au-Sn Alloys by in Situ Raman Spectroscopy. Electrochim. Acta 2009, 54, 1102–1108.
- Jia, F.; Yu, C.; Ai, Z.; Zhang, L. Fabrication of Nanoporous Gold Film Electrodes with Ultrahigh Surface Area and Electrochemical Activity. Chem. Mater. 2007, 19, 3648–3653.
- Huang, J.F.; Sun, I.W. Fabrication and Surface Functionalization of Nanoporous Gold by Electrochemical Alloying/Dealloying of Au-Zn in an Ionic Liquid, and the Self-Assembly of L-Cysteine Monolayers. Adv. Funct. Mater. 2005, 15, 989–994.
- Xue, Y.; Scaglione, F.; Paschalidou, E.M.; Rizzi, P.; Battezzati, L. Excellent Surface Enhanced Raman Scattering Obtained with Nanoporous Gold Fabricated by Chemical De-Alloying. Chem. Phys. Lett. 2016, 665, 6–9.
- Yu, J.; Ding, Y.; Xu, C.; Inoue, A.; Sakurai, T.; Chen, M. Nanoporous Metals by Dealloying Multicomponent Metallic Glasses. Chem. Mater. 2008, 20, 4548–4550.
- Cheng, C.; Lührs, L. Robust Metallic Actuators Based on Nanoporous Gold Rapidly Dealloyed from Gold–Nickel Precursors. Adv. Funct. Mater. 2021, 31, 2107241.
- Zhong, Y.; Markmann, J.; Jin, H.J.; Ivanisenko, Y.; Kurmanaeva, L.; Weissmüller, J. Crack Mitigation during Dealloying of Au25Cu75. Adv. Eng. Mater. 2014, 16, 389–398.
- Raj, D.; Scaglione, F.; Fiore, G.; Rizzi, P. Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance. Coatings 2022, 12, 831.
- Zhonghua, Z.; Yan, W.; Zhen, Q.; Jikui, L.; Xiufang, B. Nanoporous Gold Ribbons with Bimodal Channel Size Distributions by Chemical Dealloying of Al-Au Alloys. J. Phys. Chem. C 2009, 113, 1308–1314.
- Ding, Y.; Kim, Y.-J.; Erlebacher, J. Nanoporous Gold Leaf: “Ancient Technology”. Adv. Mater. 2004, 16, 1897–1900.
- Bozzini, B.; De Gaudenzi, G.P.; Mele, C. A SERS Investigation of the Electrodeposition of Ag-Au Alloys from Free-Cyanide Solutions. J. Electroanal. Chem. 2004, 563, 133–143.
- Kim, M.; Ha, W.J.; Anh, J.W.; Kim, H.S.; Park, S.W.; Lee, D. Fabrication of Nanoporous Gold Thin Films on Silicon Substrate by Multilayer Deposition of Au and Ag. J. Alloys Compd. 2009, 484, 28–32.
- Islam, M.S.; Branigan, A.J.; Ullah, B.; Freeman, C.J.; Collinson, M.M. The Measurement of Mixed Potentials Using Platinum Decorated Nanoporous Gold Electrodes. J. Electrochem. Soc. 2022, 169, 016503.
- Erlebacher, J. An Atomistic Description of Dealloying Porosity Evolution, the Critical Potential, and Rate-Limiting Behavior. J. Electrochem. Soc. 2004, 151, C614–C626.
- Silva Olaya, A.R.; Kühling, F.; Mahr, C.; Zandersons, B.; Rosenauer, A.; Weissmüller, J.; Wittstock, G. Promoting Effect of the Residual Silver on the Electrocatalytic Oxidation of Methanol and Its Intermediates on Nanoporous Gold. ACS Catal. 2022, 12, 4415–4429.
- Wittstock, A.; Neumann, B.; Schaefer, A.; Dumbuya, K.; Kübel, C.; Biener, M.M.; Zielasek, V.; Steinrüek, H.P.; Gottfried, J.M.; Biener, J.; et al. Nanoporous Au: An Unsupported Pure Gold Catalyst? J. Phys. Chem. C 2009, 113, 5593–5600.
- Silva Olaya, A.R.; Zandersons, B.; Wittstock, G. Effect of the Residual Silver and Adsorbed Lead Anions towards the Electrocatalytic Methanol Oxidation on Nanoporous Gold in Alkaline Media. Electrochim. Acta 2021, 383, 138348.
- Kwon, H.; Barad, H.N.; Silva Olaya, A.R.; Alarcón-Correa, M.; Hahn, K.; Richter, G.; Wittstock, G.; Fischer, P. Dry Synthesis of Pure and Ultrathin Nanoporous Metallic Films. ACS Appl. Mater. Interfaces 2023, 15, 5620–5627.
- Sieradzki, K.; Dimitrov, N.; Movrin, D.; McCall, C.; Vasiljevic, N.; Erlebacher, J. The Dealloying Critical Potential. J. Electrochem. Soc. 2002, 149, B370.
- Xiao, C.; Liu, Y.L.; Xu, J.Q.; Lv, S.W.; Guo, S.; Huang, W.H. Real-Time Monitoring of H2O2 Release from Single Cells Using Nanoporous Gold Microelectrodes Decorated with Platinum Nanoparticles. Analyst 2015, 140, 3753–3758.
- Li, W.; Qi, H.; Wang, B.; Wang, Q.; Wei, S.; Zhang, X.; Wang, Y.; Zhang, L.; Cui, X. Ultrathin NiCo2O4 Nanowalls Supported on a 3D Nanoporous Gold Coated Needle for Non-Enzymatic Amperometric Sensing of Glucose. Microchim. Acta 2018, 185, 124.
- Liu, Z.; Puumala, E.; Chen, A. Sensitive Electrochemical Detection of Hg(II) via a FeOOH Modified Nanoporous Gold Microelectrode. Sens. Actuators B Chem. 2019, 287, 517–525.
- Ikegami, M.; Hirano, Y.; Mie, Y.; Komatsu, Y. Adsorptive Stripping Voltammetry for the Determination of Dissolved Oxygen Using a Mesoporous Pt Microelectrode. J. Electrochem. Soc. 2019, 166, B542–B546.
- Tavakkoli, N.; Soltani, N.; Sadeghi, M.; Salavati, H. Electrochemical Determination of Methimazole Using Nanoporous Gold Film Electrode Modified with MoO2 Thin Film. Microchem. J. 2019, 150, 104153.
- Tavakkoli, N.; Soltani, N.; Khorshidi, E. Fabrication of Ru-Pd Bimetallic Monolayer on Nanoporous Gold Film Electrode with Excellent Electrocatalytic Performance towards Captopril Oxidation. Electrochim. Acta 2015, 164, 1–11.
- Tavakkoli, N.; Nasrollahi, S. Non-Enzymatic Glucose Sensor Based on Palladium Coated Nanoporous Gold Film Electrode. Aust. J. Chem. 2013, 66, 1097–1104.
- Deng, Y.; Huang, W.; Chen, X.; Li, Z. Facile Fabrication of Nanoporous Gold Film Electrodes. Electrochem. Commun. 2008, 10, 810–813.
- Sadeghi, M.; Shabani-Nooshabadi, M.; Ansarinejad, H. A Nanoporous Gold Film Sensor Modified with Polypyrrole/CuO Nanocomposite for Electrochemical Determination of Piroxicam and Tramadole. Environ. Res. 2023, 216, 114633.
- Zhong, G.; Liu, A.; Chen, X.; Wang, K.; Lian, Z.; Liu, Q.; Chen, Y.; Du, M.; Lin, X. Electrochemical Biosensor Based on Nanoporous Gold Electrode for Detection of PML/RARα Fusion Gene. Biosens. Bioelectron. 2011, 26, 3812–3817.
- Rezaei, B.; Damiri, S. Fabrication of a Nanostructure Thin Film on the Gold Electrode Using Continuous Pulsed-Potential Technique and Its Application for the Electrocatalytic Determination of Metronidazole. Electrochim. Acta 2010, 55, 1801–1808.
- Lee, E.; Sung, M.; Wang, Y.; Kim, J. Atomic Layer Electrodeposition of Pt on Nanoporous Au and Its Application in PH Sensing. Electroanalysis 2018, 30, 2028–2034.
- Qiu, H.; Huang, X. Effects of Pt Decoration on the Electrocatalytic Activity of Nanoporous Gold Electrode toward Glucose and Its Potential Application for Constructing a Nonenzymatic Glucose Sensor. J. Electroanal. Chem. 2010, 643, 39–45.
- Xia, Y.; Huang, W.; Zheng, J.; Niu, Z.; Li, Z. Nonenzymatic Amperometric Response of Glucose on a Nanoporous Gold Film Electrode Fabricated by a Rapid and Simple Electrochemical Method. Biosens. Bioelectron. 2011, 26, 3555–3561.
- Huang, W.; Wang, M.; Zheng, J.; Li, Z. Facile Fabrication of Multifunctional Three-Dimensional Hierarchical Porous Gold Films via Surface Rebuilding. J. Phys. Chem. C 2009, 113, 1800–1805.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
741
Revisions:
2 times
(View History)
Update Date:
15 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




