Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Bimetallic nanocomposites and nanoparticles have received tremendous interest recently because they often exhibit better properties than single-component materials. Improved electron transfer rates and the synergistic interactions between individual metals are two of the most beneficial attributes of these materials.
- nanoporous gold
- bimetallic
- electrochemistry
- nanomaterials
- nanoparticles
- sensors
1. Introduction
Nanoporous materials have had an enormous impact in many scientific and technological applications, including the development of adsorbents for cleaning up toxic waste, filtration and separation media for separating complex samples, catalytic materials for speeding up slow reactions, and chemical sensors for detecting trace constituents in complex samples, among others. Important properties include high surface area, a high surface area-to-volume ratio, high number of catalytically active sites, confinement effects, and unique and tailorable pore morphology. Examples of commonly used nanoporous materials include zeolites and clays, sol–gel-derived materials (xerogels and aerogels), the M41S family of materials, inverse opals, covalent organic frameworks (COFs), metal–organic frameworks (MOF), and nanoporous metals and foams [1,2,3,4,5,6,7,8,9,10]. The pore size, geometry, and interconnectivity strongly depend on the method of fabrication. Nanoporous metals are particularly valuable, because they have high electron conductivity, which allows them to serve as scaffolds for electrochemical devices such as electrochemical sensors, electrocatalysts, batteries, super capacitors, and fuel cells [11,12,13,14,15,16]. More importantly, they can have unique catalytic and electrochemical properties [13] due to their nanosized pores, high surface area-to-volume ratio, and large number of functional sites. The chemical and physical properties of nanoporous metals are often much better than those of nonporous metals, and thus they present exciting opportunities in the fields of chemical sensing, electrocatalysis, and energy storage and delivery.
One popular nanoporous material is nanoporous gold (NPG) [16,17,18,19,20,21,22,23]. NPG has been widely used in the field of analytical chemistry because of its unique pore structure and the ease with which it can be fabricated [18]. When prepared via dealloying methods, NPG has a characteristic three-dimensional bicontinuous nanopore framework, which has proven to be very valuable in electrochemical sensing [18] and electrocatalysis [19]. Compared to planar gold, NPG has many unique features [19]: (1) a high surface area that provides more places for electron transfer; (2) tunable pore size, ranging from a few nanometers to tens of nanometers, which allows pore-size-dependent effects to be evaluated [24,25]; (3) when prepared by dealloying in concentrated HNO3 [26], NPG has very clean surfaces, thus eliminating the need for harsh cleaning methods before use; (4) improved accessibility to internal sites due to the continuity and interconnectivity of pores [27]; and (5) high density of low-coordination surface gold atoms, which can improve electrocatalytic properties [28,29].
The unique properties of NPG have been exploited in several different ways. For example, NPG is a significantly better electrocatalyst for the oxidation of methanol than planar gold due to the presence of low coordination sites [30,31]. NPG is also effective for enzyme immobilization due to its small pores, which help stabilize the macromolecule under otherwise denaturing environments [32]. The high surface area of NPG means a greater amount of an adsorbate (e.g., enzyme) can be immobilized on the gold surface, allowing for greater signals relative to planar gold [24,33]. The bicontinuous, porous framework provides an avenue for small substrate molecules to reach inner surfaces and at the same time can reduce the effect of biofouling on an electrochemical signal due to a unique biosieving mechanism [34]. Because gold is easily modified with thiol groups, NPG can be modified with thiolated probe DNA molecules (or aptamers), enabling their use as DNA/RNA/nucleic acid sensors [35]. Electron transfer rates are also improved at nanoporous electrodes because of nanoconfinement effects [13].
While gold is an important element and has many useful attributes, it also has limitations. One important example is that gold is not as catalytically active as other metals. However, by either alloying gold or decorating gold with more catalytically active elements, improvements in the physicochemical properties of the nanoporous material can be obtained. Bimetallic nanocomposites and nanoparticles, in particular, have shown better physicochemical properties compared to their single-metal counterpart, and thus have shown their usefulness in chemical sensing and electrocatalysis [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Studies have shown that nanoporous alloys exhibit better electrocatalytic properties and have improved electrochemical sensing compared to single-component metals [37,52]. In some cases, these enhancements have been attributed to the synergistic interactions between the individual metals [37]. In chemical sensing, improved selectivity and sensitivity can also result from improved electron transfer rates when a second metal is added to a nanoporous structure [53,54]. Decorating a NPG framework with a precious metal can also reduce the consumption of that precious metal and provide high accessibility to it as needed.
2. Fabrication Methods for Porous Gold Electrodes
NPG can be made in many different ways. How NPG is made ultimately determines the pore structure, roughness factor, surface area, pore size, pore-size distribution, level of interconnectivity of the pores, and accessibility to the inner surfaces. All these properties are important, as they collectively determine the overall performance of the device or application. For electrochemical sensing applications, accessibility, surface area, pore size, and connectivity are especially critical, as they influence analytical figures-of-merit such as sensitivity, detection limit, response time, and selectivity. This section will briefly describe the most common ways of fabricating the NPG scaffold.
2.1. Templating
Templating methods provide a simple approach for preparing porous gold electrodes with a wide range of pore sizes and/or unique nanostructures [3,4,8,55,56]. This approach involves the selection or fabrication of the template(s) and the formation of the metal (e.g., gold) framework around the template, which is then followed by template removal. For materials that require large pores in the 100+ nm range, hard templating methods are valuable. In this case, ‘hard’ sacrificial templates such as anodic aluminum oxide (AAO), large silica particles, colloidal crystals, or copolymer particles are used [8]. Gold (or another suitable metal) is electrochemically (or chemically) deposited in and around an assembly of hard templates, which is then subsequently removed by chemical means or via heat treatment [57,58]. Polystyrene templates can be easily removed in chloroform or toluene. An example of this approach is shown in Figure 1. The pores and the arrangement of the pores are dictated by the size of the template and how it is assembled on the surface. Because of the size of the templates (and ultimately the pores), the overall surface area of the electrodes is not as high as it is when smaller templates are used.
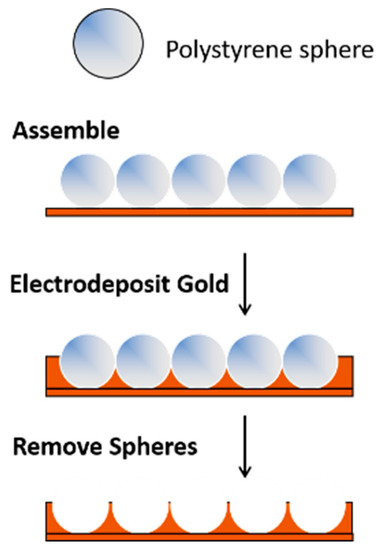
Figure 1. A templating approach used for the formation of porous gold electrodes.
Soft templates can be used to produce NPG with significantly smaller pore sizes, often in the sub-10 nm range, thus increasing the surface area of the electrode [4]. Examples of such ‘soft’ templates include surfactant micelles, amphiphilic block copolymers, and lyotropic liquid crystals [55]. Bimodal porosity can be achieved by combining templates with different sizes and types [7] or by using hierarchical templates [59]. Hierarchical porosity can also be achieved by combining different soft templates or hard templates with soft templates (e.g., large polystyrene or silica particles with lyotropic liquid crystals) [7,60].
Many templates and approaches have been developed to create nanoporous (meso- and microporous) gold electrodes with different pore arrangements. For instance, a one-step liquid-phase method using AgCl as a sacrificial template was developed for preparing zero-dimensional hollow NPG with adjustable particle size (150–1000 nm) and ligament thickness (21–54 nm) [61]. Strawberry- and raspberry-like hierarchical templates [62] were fabricated by linking different-sized polystyrene latex spheres together, and they were used to prepare hierarchical nanostructured gold electrodes with large outer pores (~μm in size) and ~10 s of nm sized inner pores [59]. In another example, Hsueh et al. prepared NPG with a bicontinuous morphology by using different types of block copolymer (BCP) [63]. Using electrodeposited silica as a template, NPG with a coral-like framework and high surface area was fabricated [64]. The one drawback of template-based methods for NPG formation is that the overall structure of the nanoporous matrix is dictated by the shape of the template and template assembly. Different structures typically require the design of corresponding templates [34,59].
2.2. Chemical Dealloying of a Pre-Formed Alloy
One common method for preparing NPG with a bicontinuous network of nanosized pores is via chemical or electrochemical dealloying of a pre-formed alloy [27,65,66]. In this approach, the least noble metal in a gold alloy is selectively removed, ultimately producing a nanopore structure containing a bicontinuous, well-connected network of ligands and pores. Examples of Au alloys that have been used include Au-Sn [67,68], Au-Zn [69,70], Au-Cu-Pd-Ag-Si [71,72], Au-Ni [73], Au-Cu [74], Au-Fe [75], Au-Al [76], and Au-Ag [65,77], with the latter alloy being the most common. White gold (~12K; Au-Ag alloy) has been widely used to form NPG [77], in part because it can be purchased commercially in the form of thin white gold leaves (~100 nm thick). Such materials are commonly used by artists, thus making this material popular and readily available. An Au-Ag alloy of the appropriate composition could also be electrochemically deposited from appropriate gold and silver salts on a conducting support [78]. It can also be sputtered or physically deposited under vacuum using appropriate targets [79].
A simple method for chemically dealloying white gold is to place it in nitric acid for a selected period of time [34,80]. This time can range from ~10 min to hours, depending on the thickness of the starting alloy [18]. During this corrosion process, the least noble metal (silver) is depleted, and the gold atoms diffuse at the electrolyte–metal interface and restructure on the surface to make gold-rich clusters surrounded by nanosized pores [81]. The pore size and shape strongly depend on the concentration of the acid, the time in the acid, the composition of the alloy, etc. [18]. Chemical dealloying in acid is simple and quick, but the corrosion process, and thus the pore formation, can be difficult to control [37]. Additionally, it is impossible to remove all the Ag from the alloy, and care needs to be taken to fully understand what role residual Ag has on the observed response [82,83,84]. A recent approach has been described in which NPG is formed that is free from residual Ag [85].
2.3. Electrochemical Dealloying of a Pre-Formed Alloy
Another approach to dealloying that can provide more control is electrochemical dealloying [37]. In this approach, the material to be dealloyed serves as the working electrode and is immersed in a suitable electrolyte solution (e.g., perchloric acid) along with a reference and counter electrode. A potential slightly higher than the ‘critical’ potential is applied to the electrode surface with respect to a reference for a set period of time. The critical potential is very important, and its value depends on the alloy, its composition, and the electrolyte [86]. Linear-sweep voltammetry can help determine the critical potential [86]. For both chemical and electrochemical dealloying, post-processing procedures such as heat treatment and/or electrochemical treatment can be used to further tune the ligament and pore size.
2.4. Electrochemical Formation of the Alloy Followed by Electrochemical Dealloying
A ‘combined’ electrochemical alloying–dealloying process can also be used to prepare NPG [69,70]. In this method, instead of starting with a previously fabricated alloy, the alloy is made on the spot electrochemically and then immediately dealloyed in the same electrochemical cell. The process is a multicyclic process [69,70]. The method utilizes a ZnCl2/benzyl alcohol electrolyte solution held at an elevated temperature and a gold electrode. First, a cathodic sweep reduces zinc ions, forming an Au-Zn alloy, and second, the anodic sweep then subsequently removes Zn (i.e., causes dealloying). After several cycles, a 3D nanoporous structure is obtained [69]. This method is relatively simple, and can be used to form NPG layers on a gold wire or gold needles, which are very useful when small electrodes are needed for monitoring redox events in confined environments [87,88,89].
2.5. Anodization and Surface Roughening
Another common and simple approach to prepare a high-surface-area NPG surface is anodization. This method is also conducive to the formation of NPG microelectrodes [90], and has commonly been performed using gold recordable compact disks cut into small pieces [91,92,93]. Different variants of this approach have been described in the literature. They typically involve, as a first step, the polishing or precleaning of the electrode surface. Next, the electrode is anodized via the application of a large positive potential in an electrolyte solution. In one example, 1.217 V (vs. Ag/AgCl) was applied to a precleaned gold microelectrode in HCl [90]. In another example, 0.9 V (vs. SMSE) was applied for 50 s [94]. An alternative procedure involves the application of 5 V (vs. Ag/AgCl) to a gold CD in phosphate buffer for three minutes [91,92,93,95]. Gas bubbles are produced, and an oxide is formed on the surface. The roughened electrode is then placed in a solution containing a chemical reducing agent (e.g., ascorbic acid) for a short period of time to reduce the gold oxide. Variations of this general approach include the application of square-wave potential pulses in sulfuric acid [96,97], the use of different electrolyte solutions such as phosphate buffer—KCl [98] or NaOH—with the use of a potential-pulse waveform from 1.2 to −4.6 V (vs. SCE) [99]. The mechanism for the formation of the roughened NPG surface depends on the reaction medium. In the case of KCl or HCl, the mechanism proceeds through electrodissolution, disproportion, and deposition [94,100]. In NaOH, upon application of a square-wave potential pulse between 0.8 V to −5 V (vs. SMSE), NPG is formed via the weak release of oxygen gas followed by the intense release of hydrogen [99,101].
The advantages, disadvantages (concerns), and applicability of these methods are compared in Table 1.
Table 1. Summary of Common Fabrication Methods for Porous Gold Electrodes.
| Method | Requirements | Advantages | Concerns |
|---|---|---|---|
Templating
|
|
|
|
| Chemical dealloying a pre-formed alloy |
|
|
|
| Electrochemical dealloying of a pre-formed alloy |
|
|
|
| Electrochemical alloying–dealloying |
|
|
|
| Anodization–Roughening |
|
|
|
This entry is adapted from the peer-reviewed paper 10.3390/nano13182515
This entry is offline, you can click here to edit this entry!
