Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sheo Shankar Pandey | -- | 4962 | 2023-09-13 11:35:01 | | | |
| 2 | Camila Xu | + 155 word(s) | 5117 | 2023-09-14 02:49:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pandey, S.S. The Role of Iron in Phytopathogenic Microbe–Plant Interactions. Encyclopedia. Available online: https://encyclopedia.pub/entry/49103 (accessed on 07 February 2026).
Pandey SS. The Role of Iron in Phytopathogenic Microbe–Plant Interactions. Encyclopedia. Available at: https://encyclopedia.pub/entry/49103. Accessed February 07, 2026.
Pandey, Sheo Shankar. "The Role of Iron in Phytopathogenic Microbe–Plant Interactions" Encyclopedia, https://encyclopedia.pub/entry/49103 (accessed February 07, 2026).
Pandey, S.S. (2023, September 13). The Role of Iron in Phytopathogenic Microbe–Plant Interactions. In Encyclopedia. https://encyclopedia.pub/entry/49103
Pandey, Sheo Shankar. "The Role of Iron in Phytopathogenic Microbe–Plant Interactions." Encyclopedia. Web. 13 September, 2023.
Copy Citation
Iron is an essential element required for the growth and survival of nearly all forms of life. It serves as a catalytic component in multiple enzymatic reactions, such as photosynthesis, respiration, and DNA replication.
iron
iron homeostasis
iron uptake and metabolism
pathogens
Host-Microbe Interactions
Plant-microbe
1. Introduction
Iron is accredited as the most abundant element on earth and ranks as the fourth most abundant element in the earth’s crust. It can exist in various oxidation states, ranging from −2 to +6. The primordial ocean had an abundance of ferrous iron, but oxygenation of the earth’s environment led to its oxidation into the ferric form of iron. Iron commonly occurs in biological systems in either the +3 (ferric) or +2 (ferrous) oxidation states [1]. Ferric iron is abundant, relatively unreactive, and insoluble, while ferrous iron is scarce, reactive, and soluble. The stable nucleus, ligand binding property, and ability to catalyze redox reactions are some of the reasons why iron is an indispensable element in the genesis and evolution of life [1][2].
Iron is an essential cofactor in cellular redox reactions due to its ability to transition between ferrous and ferric oxidation states with moderate oxidation potential and its broad range of ligand-binding capabilities [1][3]. The iron-bound proteins constitute around 50% of all metalloproteins in living beings. The abundance of iron sulfur proteins with [2Fe-2S] and [4Fe-4S] clusters in biological redox reactions is considered extremely ancient that incorporated in life at the early stage of evolution (Figure 1) [4][5][6][7]. Iron is abundantly distributed in various subcellular compartments, such as mitochondria, chloroplasts, lysosomes, Golgi complexes, nuclei, and nucleoli [8][9][10][11]. Iron metalloproteins play critical roles in a broad range of cellular and physiological reactions, including erythropoiesis, respiration, chloroplast/mitochondrial metabolism, host immunity, cell proliferation, and amino acid and nucleic acid metabolism. Additionally, iron plays a crucial role in regulating gene expression through various iron-associated transcription factors [12][13][14][15][16].
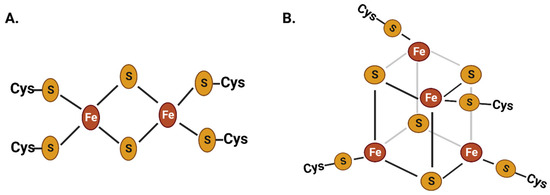
Figure 1. Chemical structure of two of the most common iron–sulfur clusters: the 2Fe-2S (A) and the 4Fe-4S (B) clusters. These iron–sulfur clusters have been present in life since the ancient stages of evolution. Iron is abundantly present in metalloproteins as part of these iron–sulfur clusters, which play a crucial role in cellular redox reactions.
The acquisition of iron is imperative for essential subcellular redox reactions in nearly all forms of life, making the struggle for control over this resource a critical aspect of the evolutionary battle between microbial pathogens and their hosts. Most research investigating the role of iron in host–microbe interactions has focused on mammalian–pathogen associations. Hosts have evolved sophisticated strategies for withholding iron to restrict free-iron availability to colonizing pathogens. The ability to extract iron and adapt to low-iron environments inside hosts is crucial in determining the survival and virulence of several bacterial, protozoan, viral, and fungal pathogens on mammals [17][18][19][20][21][22][23][24].
2. Mechanistic Insights into Phytopathogenic Bacterial Iron Homeostasis and Virulence
Host plants and phytopathogenic microbes have developed sophisticated strategies to regulate their own iron homeostasis, restricting the availability of this essential element to other organisms.
2.1. Microbial Iron Acquisition and Virulence
Bacteria encountering low iron levels either inside the host or under laboratory conditions typically produce and secrete siderophores, which scavenge ferric iron. Siderophore-mediated iron uptake is highly efficient, but requires the consumption of energy via ATP hydrolysis (Figure 2). Siderophores, which means “iron carriers” in Greek, are low-molecular-weight compounds secreted through exporters that usually belong to the major facilitator superfamily (MFS), ATP-binding cassette (ABC) superfamily, and resistance–nodulation–cell division (RND) superfamily transporters. In Escherichia coli, the enterobactin siderophore is secreted from the cytoplasm to the periplasm by the major facilitator EntS and then from the periplasm to the extracellular milieu via TolC, an outer membrane exporter of multidrug efflux pumps [25][26][27]. Siderophores exhibit high affinity to ferric iron and efficiently chelate iron in iron-depleted host environments or laboratory conditions. TonB-dependent outer membrane receptors, specific to ferric iron–siderophore complexes, recognize and internalize them into the periplasmic space with the involvement of the TonB-ExbBD complex. Further, periplasmic binding proteins (PBPs) deliver the ferric iron–siderophore complexes to the cognate ABC transporter, which transports them into the cytoplasm while ATP hydrolysis occurs (Figure 2) [28][29]. Specific porins transport ferrous iron from the extracellular milieu to the periplasm. The bacterial periplasmic and secreted β-cyclic glucan has the ability to sequester ferrous iron, which supports growth under low-iron conditions and protects cells against iron-induced toxicity under iron-replete conditions [30]. Several bacteria encode the FeoABC transporter for the import of ferrous iron from the periplasm to the cytoplasm (Figure 2) [31][32][33][34][35]. However, SitABCD in Salmonella typhimurium, YfeABCD in Yersinia pestis, SfuABC in Serratia marcescens, SitABCD in Salmonella enterica, and FbpABC in Neisseria gonorrhoeae are involved in ferrous iron import [36][37][38][39][40]. The FeoABC system is widely distributed among Gram-negative bacteria. Conversely, the SitABCD system is unique to certain pathogens such as Salmonella typhimurium, and it contributes to their virulence by effectively scavenging iron during infection. YfeABCD in Yersinia pestis acquires iron from host tissues, vital for pathogenicity. SfuABC, unique to Serratia marcescens, supports iron uptake from the environment, and the FbpABC transporter identified in Neisseria gonorrhoeae contributes to the bacterium’s growth and survival [36][37][38][39][40]. The outer membrane and cytoplasmic ferric reductases are responsible for reducing ferric iron to ferrous iron and maintaining the equilibrium between these two common oxidation states of iron (Figure 2) [41][42][43]. A more detailed understanding of bacterial iron uptake mechanisms can be found in reviews by Andrews et al. (2003), Krewulak and Vogel (2008), Chu et al. (2010), and Cornelis and Andrews (2010) [44][45][46][47].
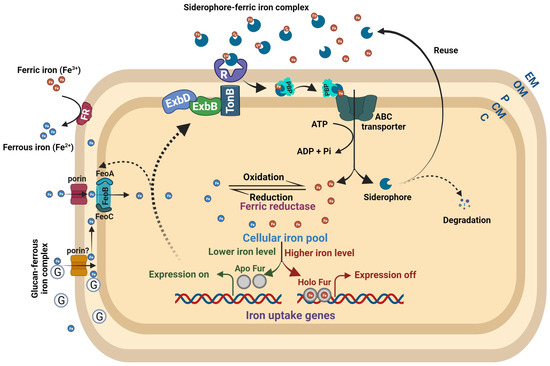
Figure 2. Bacterial iron homeostasis. The ferric and ferrous iron uptake pathways are independent in nature but markedly interdependent in regulation. Under high intracellular iron, the Holo Fur (Fur–Fe2+ complex) binds to the regulatory sites of iron uptake genes and turns off their expression. When intracellular iron levels are low, the Holo Fur releases the Fe2+ iron, and it turns into Apo Fur (Fur alone). The Apo Fur loses the ability to bind to regulatory sites, which makes the regulatory sites free from Fur and enables the expression of iron uptake genes. Bacteria synthesize ferric iron-chelating compounds, siderophores, and release them into the extracellular milieu to sequester ferric iron. The TonB-dependent outer membrane receptors recognize the Fe3+–siderophore complex, causing a conformational change in the plug domain of the receptor’s channel to internalize it. ExbB and ExbD energize TonB using an electrochemical charge gradient along the cytoplasmic membrane to release the Fe3+–siderophore complex into the periplasmic space. Further, periplasmic-binding proteins deliver the complex to the cognate ABC transporter to transport it into the cytoplasm. The ferrous iron is transported to the periplasm by Fe2+-specific porins. The glucan–Fe2+ complex can also bring ferrous iron to the periplasmic space. Further, the FeoB complex (FeoABC) transporter transports the ferrous iron to the cytoplasm. The outer membrane and cytoplasmic ferric reductases reduce ferric iron to ferrous iron at their respective places. Abbreviations: R = receptor; G = glucan; PBP = periplasmic-binding protein; EM = extracellular moiety; OM = outer membrane; P = periplasm; CM = cytoplasmic membrane; C = cytoplasm.
The enterobacterium Dickeya dadantii (formerly known as Erwinia chrysanthemi) causes soft rot disease on a broad host range of plants. Dickeya dadantii produces chrysobactin and achromobactin, two different types of siderophores that play an essential role in its full virulence on plants [48][49]. The enterobacterium Dickeya dadantii employs the type 2 secretion system (T2SS) to secrete the metal-binding protein IbpS, which exhibits high affinity for ferric and cupric ions. This substrate is essential for the virulence of Dickeya dadantii on plants and is conserved among numerous phytopathogenic microorganisms and fungi [50]. Erwinia amylovora, the pathogen responsible for the severe fire blight disease in apple and pear, produces the siderophore desferrioxamine (DFO) to sequester iron during the infection process. In addition to its role in supporting virulence by sequestering iron, DFO also plays a critical role in protecting the bacterium against the oxidative burst induced by the host plant’s defense response [51]. A recent study has reported that desferrioxamine (DFO) appears to be a significant virulence factor of Erwinia amylovora CFBP1430 in the low-iron state of apple flowers that is created after iron consumption by precolonized microorganisms [52]. Erwinia carotovora subsp. carotovora also produces the aerobactin and chrysobactin siderophores, but knocking out the genes encoding either of these siderophores does not affect the bacterium’s ability to macerate the potato tuber or develop aerial stem rot on potato [53][54]. Pseudomonas syringae pv. tabaci 6605, a foliar bacterial pathogen that causes wildfire disease in tobacco, produces the siderophore pyoverdine, which serves as a key virulence factor by facilitating bacterial growth on the host plants [55]. Taguchi and colleagues demonstrated that the mutants in pyoverdine biosynthesis enzyme–encoding genes exhibit low production of tabtoxin, extracellular polysaccharide, and quorum-sensing molecule acyl homoserine lactones (AHLs) but display accelerated swarming ability and increased biosurfactant production [55]. These studies have indicated that siderophores have a multifaceted role in the biological functions of phytopathogenic bacteria, extending beyond iron sequestration. However, in contrast, Pseudomonas syringae pv. phaseolicola 1448a, the causal agent of bean halo blight, produces two different types of siderophores, pyoverdine and achromobactin, yet neither of them appears to contribute to the virulence on bean plants [56]. The xanthomonads possess the Xanthomonas siderophore synthesis (xss) gene cluster, which is conserved in almost all members of the Xanthomonas genus and displays homology with the pvs gene cluster that encodes vibrioferrin in the Vibrio group of human pathogenic bacteria [57][58][59]. The Xanthomonas group of phytopathogens cause disease to approximately 400 plants, including several economically important crops, such as rice, pepper, cabbage, and tomato [60]. In the members of xanthomonads, the xss gene cluster encodes xanthoferrin, an α-hydroxycarboxylate-type siderophore, synthesis enzymes under low-iron conditions [57][58][61]. Xanthoferrin-mediated iron uptake promotes in planta growth of X. oryzae pv. oryzicola (causal agent bacterial leaf streak on rice) and X. campestris pv. campestris (causal agent black rot of crucifers) and is required for optimum virulence on their respective hosts [58][62]. Conversely, the xss gene cluster of X. oryzae pv. oryzae, a causal agent of bacterial blight of rice, does not express inside the plant host, and xanthoferrin-mediated iron uptake is not needed for virulence [57]. However, the feoB gene of X. oryzae pv. oryzae expresses inside the plant, and FeoB-mediated ferrous iron uptake critically contributes to the bacterial in planta growth and virulence on rice [57].
The above research works manifest the importance of siderophore-mediated iron uptake in the virulence of several phytopathogenic bacteria. Some phytopathogenic bacteria exhibit no impact on virulence even if the siderophore-encoding genes were knocked out (Table 1). The mutants in genes associated with siderophore biosynthesis in Agrobacterium tumefaciens C58, Pseudomonas syringae pv. tomato DC3000, and Ralstonia solanacearum AW1 displayed virulence proficiency on par with their wild-type strains [63][64][65]. The siderophore-mediated iron uptake is an expensive process but highly efficient in nature that secures the bacterial iron requirements by scavenging iron from iron-depleted environment. For instance, the chrysobactin produced by Dickeya dadantii outcompetes the plant ferritins for iron binding in Saintpaulia leaves [66]. The phytopathogenic bacteria have evolved with multiple iron uptake systems that confer an adaptive advantage to the pathogenic lifestyle in various host conditions. Apparently, the availability of iron inside the host and the tissue habitat of phytopathogens determine the requirement of siderophores during in planta bacterial growth and virulence. The two closely related xanthomonads, Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola, share the common host rice but colonize the xylem vessel and mesophyll apoplast, respectively. The lifestyle in different habitats and the iron constituents could be attributed to the requirement of ferrous uptake through the FeoB transport system and siderophore-mediated ferric-iron uptake for the optimum virulence of Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola, respectively. Pseudomonas syringae pv. tomato DC3000 has shown the ability to access iron from ferric citrate, but it does not contribute to pathogenicity [64].
Table 1. Iron-chelating compounds produced by different phytopathogenic bacteria, along with their respective roles in promoting virulence.
| S. No. | Phytopathogenic Bacteria | Iron Chelator | Role in Virulence | Reference |
|---|---|---|---|---|
| 01. | Dickeya dadantii (syn. Erwinia chrysanthemi) | Chrysobactin, achromobactin | Required for optimum virulence | [48][49] |
| 02. | Dickeya dadantii | IbpS | Required for optimum virulence | [50] |
| 03. | Erwinia amylovora | Desferrioxamine (DFO) | Required for optimum virulence | [51] |
| 04. | Erwinia carotovora subsp. carotovora | Aerobactin, Chrysobactin | Not required for virulence | [53][54][67] |
| 05. | Pseudomonas syringae pv. tabaci 6605 | Pyoverdine | Required for optimum virulence | [55] |
| 06. | Pseudomonas syringae pv. phaseolicola 1448a | Pyoverdine, achromobactin | Not required for virulence | [56] |
| 07. | Pseudomonas syringae pv. syringae B301D |
Pyoverdine | Not required for virulence | [68] |
| 08. | Pseudomonas syringae pv. tomato DC3000 |
Pyoverdine, yersiniabactin | Not required for virulence | [64] |
| 09. | Xanthomonas campestris pv. campestris 8004 | Xanthoferrin | Required for optimum virulence | [58] |
| 10. | Xanthomonas oryzae pv. oryzae BXO1 | Xanthoferrin | Not required for virulence | [57][61] |
| 11. | Xanthomonas oryzae pv. oryzicola BXOR1 | Xanthoferrin | Required for optimum virulence | [61][62] |
| 12. | Xanthomonas campestris pv. campestris 8004 | Cyclic β-(1,2)-glucans | Required for optimum virulence | [30] |
| 13. | Agrobacterium tumefaciens C58 | Unknown iron chelator | Not required for virulence | [63] |
| 14. | Agrobacterium tumefaciens strain B6 | Agrobactin | Not required for virulence | [69] |
| 15. | Ralstonia solanacearum AW1 |
Staphyloferrin B | Not required for virulence | [65] |
2.2. Phytopathogenic Microbial Iron Storage and Virulence
As described in the introduction section, iron constitutes an essential part of several metalloproteins, including proteins containing iron–sulfur (Fe-S) clusters (Figure 1). Phytopathogenic bacteria, such as Dickeya dadantii, encode SufABCDSE, which is involved in the biosynthesis of Fe–S clusters under oxidative stress and contributes to virulence [70][71]. However, bacteria also widely encode specific iron storage proteins, bacterioferritin (Bfr), and bacterial ferritin (Ftn), to maintain cellular iron homeostasis. Both Bfrs and Ftns assemble from 24 identical or similar subunits of ~19 kDa into spherical structures (~120 Å diameter) of ~450 kDa with a large hollow center (~80 Å inner diameter) [72][73]. The hollow center stores around 2000 iron atoms in the form of ferric-hydroxyphosphate core [72]. Despite being similar in fold and quaternary structure, bacterial Ftns and Bfrs vary in heme content (only Bfr contains heme), composition, electrostatic properties of the pores, and binding affinity of a few crucial residues [72][73]. In Dickeya dadantii, bfr and ftnA encode the iron storage proteins bacterioferritin and bacterial ferritins, respectively [74]. Interestingly, the bfr mutant did not show a difference in growth under low iron or intracellular iron content but exhibited delayed appearance maceration symptoms on the host plant [74]. However, knocking out ftnA in Dickeya dadantii resulted in impaired growth under low iron, more sensitivity to oxidative stress, and reduced virulence on African violets [74]. Bacterioferritin-mediated iron storage in the phytopathogen Agrobacterium tumefaciens is required for tolerance against H2O2 exposure, maintaining intracellular iron at an optimum level, growth under low iron, and full virulence on the host plant [75]. The triple deletion of bacterioferritin-like protein encoding genes (ΔyciE ΔyciF ΔXC_3754) in an operon of Xanthomonas campestris pv. campestris 8004 resulted in lower intracellular iron content than the wild-type strain [14].
2.3. Transcription Regulation of Phytopathogenic Microbial Iron Homeostasis and Virulence
Iron homeostasis in numerous bacterial species is tightly regulated through the iron-responsive transcriptional regulator, ferric uptake regulator (Fur), whose function is dependent on the availability of Fe2+. The Fur dimer and its corepressor Fe2+ form the holo–Fur complex in high iron conditions, which binds to the conserved fur-box located upstream of target genes, including those encoding siderophore biosynthesis and iron uptake proteins, to actively suppress gene expression. In contrast, during low-iron conditions, Fe2+ dissociates from Fur, resulting in the formation of apo-Fur, which in turn dissociates from the promoter regions of target genes. This dissociation of apo-Fur enables RNA polymerase to interact with the promoter and initiate gene expression (Figure 2). The mutant strain of Dickeya dadantii lacking functional Fur exhibited growth deficiencies in both minimal and rich media, yet did not exhibit a discernible difference in growth under low-iron conditions [76]. Furthermore, Franza et al. demonstrated that the fur mutant of Dickeya dadantii displays the constitutive expression of high-affinity iron transport systems and reduced virulence on African violet [76]. The fur mutant strain of Xanthomonas oryzae pv. oryzae demonstrated a heightened sensitivity to the metalloantibiotic streptonigrin, implying an elevated intracellular iron concentration. Additionally, this strain exhibited suboptimal growth on nutrient-rich media, reduced catalase activity, hypersensitivity to hydrogen peroxide, and decreased virulence on rice [77]. The fur mutant strain of Xanthomonas campestris pv. campestris similarly displays an increased intracellular iron content, constitutive overproduction of siderophores, upregulated expression of iron transport genes, heightened tolerance to peroxide toxicity, and decreased virulence on the host plant [78]. In conjunction with reduced swarming motility, the fur mutant strain of Pseudomonas syringae pv. tabaci 11,528 displays constitutive production of siderophores, virulence deficiency, diminished production of tabtoxin, and reduced quantities of the quorum-sensing molecule N-acyl homoserine lactones, in addition to its already-characterized slow-growth phenotype [79]. The regulatory interdependence between iron and quorum sensing is governed in an atypical manner among the group of phytopathogenic bacteria known as xanthomonads, as elucidated in a recent review by Pandey and Chatterjee [80].
The Xanthomonas iron-binding regulator (XibR) binds to Fe3+ iron and regulates the production of siderophores, motility, and chemotaxis in Xanthomonas campestris pv. campestris [14]. The XibR encoding gene is conserved across xanthomonads, and homologs are also present in Pseudoxanthomonas dokdonensis, Bordetella bronchiseptica, and Lysobacter sp. URHA0019. Under iron-replete conditions, XibR binds to the promoter region of genes involved in siderophore synthesis, thereby repressing their expression. XibR plays a positive regulatory role in iron storage and uptake, as well as in chemotaxis and motility, while negatively regulating siderophore production. It is required for optimal virulence on the host plant [14]. The two-component system VgrS/VgrR in Xanthomonas campestris pv. campestris senses iron and facilitates bacterial adaptation under low-iron conditions [81]. The membrane-bound histidine kinase receptor VgrS and its cognate sensor VgrR respond to periplasmic and intracellular iron levels, respectively. Wang et al. further reported that lowering the iron level in the periplasmic regions leads to VgrS autophosphorylation. Subsequently, phosphotransfer occurs to VgrR, which directly or indirectly affects several factors involved in iron uptake, signal transduction, detoxification, cell division, and cellular metabolism [81].
2.4. sRNA-Mediated Regulation of Microbial Iron Homeostasis
Small RNAs (sRNAs), which are 50–400 nucleotides in length, play a crucial role as post-transcriptional regulators in prokaryotes. They are required for the fine regulation of various cellular processes, such as carbon metabolism, iron homeostasis, stress responses, motility, chemotaxis, biofilm formation, quorum sensing, and virulence. Through short base pairings, sRNAs fine-tune the stability and translation efficiency of target mRNAs. One extensively studied sRNA is RyhB in E. coli, which contributes to maintaining cellular iron homeostasis by regulating multiple iron utilization and transport genes [82][83]. The sRNAs regulate virulence-associated functions and stress response in phytopathogenic bacteria, such as Pseudomonas, Xanthomonas, Agrobacterium, and Pectobacterium [84][85][86][87][88]. The sRNA DsRNA-Xoo4 of Xanthomonas oryzae pv. oryzae [89] and sX13 of Xanthomonas campestris pv. vesicatoria [90][91] regulate the expression of TonB. However, further extensive investigations are required to fully understand the regulation of iron homeostasis by sRNAs in phytopathogenic microbes.
3. Interplay between Iron Homeostasis and Plant Immune Response
Iron is an essentially required element for several vital cellular processes of plants. Plants employ two different strategies to uptake the iron through the roots. One in nongrass plants (e.g., Arabidopsis thaliana), iron starvation activates a cascade of signaling reaction to release protons and phenolics in rhizosphere, which lowers the pH and solubilizes ferric iron. The ferric reductases, such as ferric reduction oxidase 2 (FRO2) and ferric chelate reductase (FCR), reduce ferric iron to ferrous iron, which is further transported inside the roots through a specific transporter (IRT1). Another strategy in grass plants (e.g., barley, rice), iron starvation induces the synthesis and release of the iron-chelating compounds phytosiderophores (e.g., mugineic acid) in rhizosphere. Phytosiderophore–Ferric iron complexes are transported inside the root cells by specific transporters, such as YS1 and YSL. Further, iron is distributed to the sink tissues and utilized in the formation of enzyme cofactors, etc., or stored in vacuoles or complexed with ferritins. The mechanisms and regulations of iron homeostasis in plants have been extensively reviewed in recent years [92][93][94][95][96][97].
3.1. Bacterial Effectors Influencing Plant Iron Homeostasis
Initially, host plants detect the microbial/pathogen-associated molecular patterns (MAMPs/PAMPs) common to many classes of microbes/pathogens to trigger PAMP-triggered immunity (PTI). Then the pathogens inject effectors in host plants to suppress the PTI that facilitates the successful disease development (Figure 3) [98]. The phytopathogenic bacterium Pseudomonas syringae pv. tomato DC3000 delivers an effector, AvrRps4, inside the plants and interacts with the plant iron sensor protein BRUTUS [99]. The E3 ligase BRUTUS (BTS) is an iron sensor of plants that suppresses iron deficiency response by the ubiquitin-mediated degradation of the PYE-like (PYEL) proteins IAA-LEUCINE RESISTANT3 (ILR3), bHLH105 (basic helix–loop–helix family protein), and bHLH115 through ubiquitin-mediated degradation [100][101]. The PYE-like (PYEL) proteins IAA-LEUCINE RESISTANT3 (ILR3), bHLH105, and bHLH115 are known to be induced under iron-deficient conditions and improve the iron level of plants [100][102]. The Pseudomonas syringae pv. tomato DC3000 effector AvrRps4 interacts with BRUTUS and induces iron accumulation in Arabidopsis thaliana, which potentially facilitates bacterial iron uptake and proliferation [99].
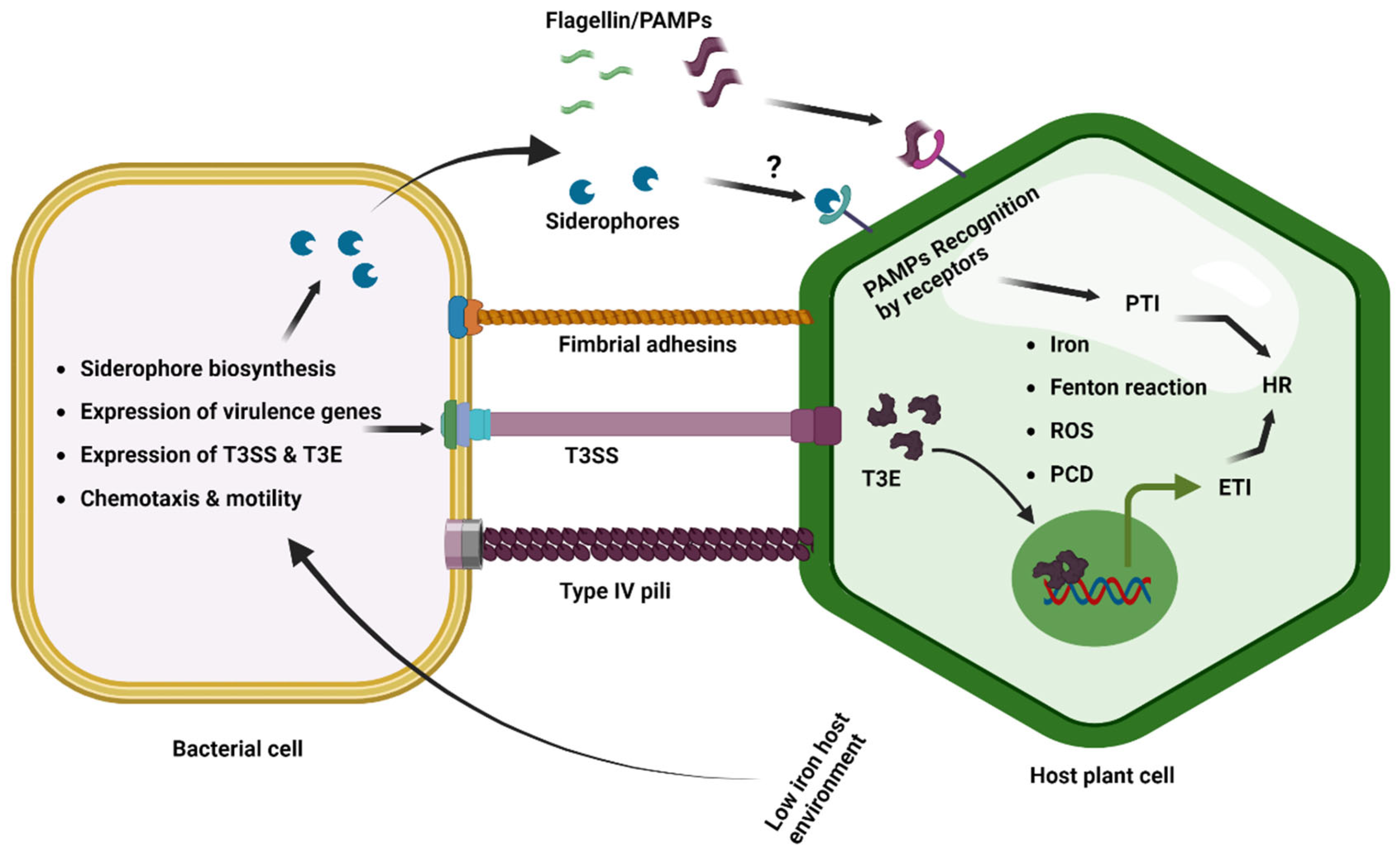
Figure 3. Iron in plant–phytopathogenic bacterial interactions. The host and phytopathogenic bacteria compete for iron resources during the in planta infection process and colonization. Plants limit the availability of iron for bacterial pathogens by transporting iron into the vacuole and sequestering it into ferritins. This results in low-iron conditions for bacteria, which trigger the induced expression of iron uptake genes and siderophore biosynthesis to obtain iron from the iron-depleted host environment. Low-iron conditions also induce bacterial motility and chemotaxis, as well as the expression of virulence genes, T3SS, and effectors. However, the response to low iron varies among bacterial pathogens. PAMP-induced PTI and effector-triggered ETI can cause the HR, which restricts bacterial growth. The siderophore pseudobactin has also been reported as a potential PAMP in Arabidopsis. Excess iron generates ROS via Fenton’s reaction, which triggers programmed cell death at low levels and necrosis at a threshold level.
The evidence suggests that the microbial siderophores act as effector and induce plant immunity along with manipulating the host iron homeostasis [103][104][105]. The application of iron-free achromobactin and chrysobactin activates the salicylic-acid-mediated signaling pathway in Arabidopsis thaliana [103]. Earlier, Dellagi and colleagues demonstrated that the siderophore null mutant strain of Dickeya dadantii displays a compromised expression of the ferritin-encoding AtFer1 gene in Arabidopsis thaliana [104]. Further, they showed that the infiltration of iron-free chrysobactin and desferrioxamine siderophores to Arabidopsis thaliana strongly increased AtFer1 expression. The ferritin accumulation during Dickeya dadantii infection appeared to be a siderophore-triggered basal defense mechanism in the host plant [104]. In another study, desferrioxamine siderophore infiltration in Arabidopsis thaliana resulted in the induced expression of the set of defense-related genes, accumulation of salicylic acid and jasmonic acid in leaves, and significantly higher callose deposition [105]. Several studies provided clear evidence of the modulation of host plant immune responses by siderophores. However, the potential external membrane receptors or internal receptors and the consequent cascade of reactions to induce host immune response needs more extensive investigations.
3.2. Plant Immune Response Influencing Microbial Iron Homeostasis
Microbial pathogens have evolved diverse strategies to manipulate host iron homeostasis for their own iron acquisition, which in turn has driven the evolution of intricate mechanisms in plants that target microbial iron homeostasis. The expression of the sigma factor, pvdS, responsible for regulating the biosynthesis of pyoverdine siderophores and supporting the in planta growth of Pseudomonas syringae pv. tomato DC3000, was found to be suppressed as a result of an AvrRpt2-triggered ETI response [106]. Among six clusters of host-genotype-dependent DEGs identified in the AvrRpt2 of Pseudomonas syringae pv. tomato DC3000, the siderophore biosynthesis genes were induced within Arabidopsis thaliana but suppressed by the activation of both PTI and ETI [106]. The bacterial genes that are implicated in the iron acquisition pathway demonstrate the highest levels of expression in planta at 6 h postinoculation, followed by a substantial reduction in expression at 48 h postinoculation. Despite this decrease, the expression levels at 48 h postinoculation remain higher than those observed during in vitro growth conditions [107]. The findings suggest that the PTI and ETI responses in Arabidopsis thaliana can suppress the expression of iron starvation genes in the phytopathogenic bacterium Pseudomonas syringae pv. tomato DC3000. However, despite this inhibition, the bacteria retain the capacity to express these genes to a certain extent in order to acquire the necessary iron for a successful disease development.
As discussed in the previous sections, microbial pathogens and host plants undergo a complex and dynamic competition for the acquisition of iron resources. The plant host actively seeks to sequester iron, limiting its availability to the invading microbial pathogens, with the ultimate goal of curbing their growth and survival. This intricate strategy constitutes a critical component of the host response, which helps to thwart the pathogenic challenge posed by phytopathogenic microorganisms. Plants have a natural mechanism to store iron, which involves encoding ferritin proteins. Ferritin serves as an iron storage protein in plants, and its expression is regulated by various internal and external factors. Multiple studies have demonstrated the upregulation of genes encoding ferritin proteins in various plant species as a result of pathogenic attacks, including but not limited to Arabidopsis thaliana in response to Dickeya dadantii [104] and potato against Streptomyces scabiei infestations [108]. The plants have developed an intricate system of regulation for the expression of ferritin, a protein that stores iron, which is precisely controlled during bacterial infections to potentially impact the bacterial iron homeostasis [109]. The ethylene response factor109 (erf109) mutations in Arabidopsis thaliana caused significant changes in gene expression, with an increased expression of genes related to iron homeostasis (bHLH38, bHLH39, bHLH101, NAS4, and FER1) and a decreased expression of defense-related genes (CML37, WRKY40, ERF13, and EXO70B2), while erf109 leaves showed elevated iron levels in both iron-sufficient and iron-deficient conditions, suggesting a potential involvement of ERF109 in regulating iron metabolism [110]. The natural resistance-associated macrophage protein (NRAMP) metal ion transporter plays a crucial role in the innate immunity of animal macrophages targeted by intracellular bacterial pathogens [111]. Segond et al. reported that AtNRAMP3, a homolog of NRAMP in Arabidopsis, is expressed at higher levels in leaves infected with the bacterial pathogens Pseudomonas syringae and Erwinia chrysanthemi [112]. They showed, using single and double mutants of nramp3 and nramp4 and ectopically expressing either of them, that AtNRAMP3 and, up to some extent, AtNRAMP4 contribute to the resistance against Erwinia chrysanthemi infection [112]. The susceptibility of the nramp3nramp4 double mutant is linked to reduced levels of ROS and the iron storage protein AtFER1 ferritin, which is known to provide resistance against microbial infections in Arabidopsis [112].
3.3. Iron Availability Influencing Microbial Pathogenicity and Plant Immune Response
The indispensable role of iron in plant–phytopathogenic microbe interactions is further supported by the visible expression of virulence functions by microbes and the resulting immune responses mounted by the host, both of which are closely linked to the levels of iron available in their respective environments. For instance, iron-deficient Arabidopsis thaliana exhibits reduced symptom severity, bacterial fitness, and the expression of bacterial pectate lyase-encoding genes when challenged with the bacterial phytopathogen Dickeya dadantii [113]. The reduced symptoms and bacterial fitness observed in iron-deficient plants also extended to the necrotrophic fungus Botrytis cinerea. The authors demonstrated that the plant’s iron status can influence the outcome of an infection by affecting both the pathogen’s virulence and the plant’s defense response [113]. The wild-type Arabidopsis Col-0 plants with iron deficiency exhibit heightened resistance to infections caused by pathogens with various lifestyles, including the necrotrophic fungus Botrytis cinerea, the hemibiotrophic bacterium Pseudomonas syringae pv. tomato DC3000, and the obligate biotrophic oomycete Hyaloperonospora arabidopsidis [114]. The iron-deficiency-induced systemic response provides defense against pathogenic infection by the involvement of ethylene and salicylic acid signaling pathways. Disturbance in iron homeostasis within the plant, through either inducing iron starvation stress or subjecting it to other nonhomeostatic conditions, is found to have a profound effect on the plant’s immune system. In fact, such disturbances act as a trigger, priming the plant’s immune system for an enhanced defense response [114].The deposition of ferritin, which is recognized for its ability to capture iron and minimize the iron availability, in the foliage of genetically modified tobacco displayed resistance to necrotic injury that resulted from viral (tobacco necrosis virus) and fungal (Alternaria alternata, Botrytis cinerea) infections [115].
On the contrary, certain phytopathogenic bacterial species possess the capacity to detect suboptimal concentrations of iron within host plants, which elicits the activation of virulence-associated mechanisms facilitating the efficacious establishment of the infection. In response to the limited iron availability, Xanthomonas campestris pv. campestris displays an induced expression of multiple virulence factors, including the type 3 secretion system (T3SS) and type 3 effectors (T3E), which could be repressed by exogenous iron supplementation [116]. AN exogenous supplementation of iron at a concentration of 100 µM FeS4 was observed to exert a significant suppressive effect on the disease development ability of Xanthomonas campestris pv. campestris on cabbage plants, while a complete abrogation of symptom development was achieved with a relatively high iron supplementation (250 µM FeS4). Interestingly, this modulation of pathogenicity was not accompanied by any significant impact on the in planta bacterial population dynamics, indicating that iron availability may specifically regulate virulence-associated functions rather than bacterial fitness in the plant host [116]. Interestingly, the Xanthomonas oryzae pv. oryzicola pathovars also exhibit an induced expression of hrp genes under in vitro low iron, albeit to a lesser extent in comparison with Xanthomonas campestris pv. campestris. However, Xanthomonas oryzae pv. oryzae fails to demonstrate any significant induction of hrp gene expression under in vitro low iron [116]. The results of the study indicate that different pathogenic bacteria belonging to the same group can adapt in various ways, depending on the host plant and tissue habitat they infect, as well as the extent of iron availability in their environment. This diversity in adaptation highlights the complexity of interactions between bacterial pathogens and their plant hosts, as well as the importance of available iron in shaping these interactions. A recent study demonstrated that the application of bioengineered chitosan–iron nanocomposites can effectively mitigate the severity of Xanthomonas oryzae pv. oryzae caused bacterial leaf blight disease in rice, primarily attributable to their capacity to modulate the expression of host defense genes and cellular physiology, as well as their inhibitory effects on bacterial proliferation [117].
3.4. Ferroptotic Cell Death (FCD)
Recent studies suggest that iron, acting as a catalyst, triggers the initiation of ferroptosis, a nonapoptotic programmed cell death pathway, through the generation of reactive oxygen species (ROS), particularly lipid peroxides. This phenomenon was initially identified in animals and has also been reported in plants as a response to heat stress and pathogenic interactions [118][119][120]. During an incompatible interaction between rice plants and the avirulent strain Magnaporthe oryzae INA 168, a notable elevation in intracellular ferric iron and ROS accumulation is observed. This surge in ferric iron and ROS levels subsequently triggers FCD, leading to the inhibition of pathogen growth. Conversely, in a compatible interaction with the virulent strain Magnaporthe oryzae PO6-6, there is no significant increase in iron accumulation within the rice plants, thereby facilitating the efficient growth and proliferation of the pathogen [119]. Remarkably, rice ferric iron storage protein ferritin 2 (OsFER2) plays a crucial role in iron–ROS–mediated FCD as a defense response against avirulent strain Magnaporthe oryzae INA 168 infection [121]. Furthermore, a recent study involving iTRAQ-based quantitative proteomics analysis and the use of iron chelators has indicated the occurrence of ferroptosis-like cell death in Nicotiana benthamiana plants infected with the highly virulent tobacco mosaic virus mutant 24A+UPD [122].
References
- Frey, P.A.; Reed, G.H. The Ubiquity of Iron. ACS Chem. Biol. 2012, 7, 1477–1481.
- Harel, A.; Bromberg, Y.; Falkowski, P.G.; Bhattacharya, D. Evolutionary History of Redox Metal-Binding Domains across the Tree of Life. Proc. Natl. Acad. Sci. USA 2014, 111, 7042–7047.
- Kosman, D.J. Redox Cycling in Iron Uptake, Efflux, and Trafficking. J. Biol. Chem. 2010, 285, 26729–26735.
- Fukuyama, K.; Hase, T.; Matsumoto, S.; Tsukihara, T.; Katsube, Y.; Tanaka, N.; Kakudo, M.; Wada, K.; Matsubara, H. Structure of S. Platensis Ferredoxin and Evolution of Chloroplast-Type Ferredoxins. Nature 1980, 286, 522–524.
- Cammack, R. Evolution and Diversity in the Iron-Sulphur Proteins. Chem. Scr. 1983, 22, 87–95.
- Meyer, J. Iron-Sulfur Protein Folds, Iron-Sulfur Chemistry, and Evolution. J. Biol. Inorg. Chem. 2008, 13, 157–170.
- Jordan, S.F.; Ioannou, I.; Rammu, H.; Halpern, A.; Bogart, L.K.; Ahn, M.; Vasiliadou, R.; Christodoulou, J.; Maréchal, A.; Lane, N. Spontaneous Assembly of Redox-Active Iron-Sulfur Clusters at Low Concentrations of Cysteine. Nat. Commun. 2021, 12, 5925.
- Wollman, F.A.; Minai, L.; Nechushtai, R. The Biogenesis and Assembly of Photosynthetic Proteins in Thylakoid Membranes. Biochim. Biophys. Acta-Bioenerg. 1999, 1411, 21–85.
- Roschzttardtz, H.; Grillet, L.; Isaure, M.P.; Conéjéro, G.; Ortega, R.; Curie, C.; Mari, S. Plant Cell Nucleolus as a Hot Spot for Iron. J. Biol. Chem. 2011, 286, 27863–27866.
- Hirayama, T.; Inden, M.; Tsuboi, H.; Niwa, M.; Uchida, Y.; Naka, Y.; Hozumi, I.; Nagasawa, H. A Golgi-Targeting Fluorescent Probe for Labile Fe(Ii) to Reveal an Abnormal Cellular Iron Distribution Induced by Dysfunction of VPS35. Chem. Sci. 2019, 10, 1514–1521.
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in Iron Metabolism, Ageing and Apoptosis. Histochem. Cell Biol. 2008, 129, 389–406.
- Kobayashi, T.; Itai, R.N.; Aung, M.S.; Senoura, T.; Nakanishi, H.; Nishizawa, N.K. The Rice Transcription Factor IDEF1 Directly Binds to Iron and Other Divalent Metals for Sensing Cellular Iron Status. Plant J. 2012, 69, 81–91.
- Schwartz, C.J.; Giel, J.L.; Patschkowski, T.; Luther, C.; Ruzicka, F.J.; Beinert, H.; Kiley, P.J. IscR, an Fe-S Cluster-Containing Transcription Factor, Represses Expression of Escherichia coli Genes Encoding Fe-S Cluster Assembly Proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 14895–14900.
- Pandey, S.S.; Patnana, P.K.; Lomada, S.K.; Tomar, A.; Chatterjee, S. Co-Regulation of Iron Metabolism and Virulence Associated Functions by Iron and XibR, a Novel Iron Binding Transcription Factor, in the Plant Pathogen Xanthomonas. PLoS Pathog. 2016, 12, e1006019.
- Deng, Z.; Wang, Q.; Liu, Z.; Zhang, M.; Machado, A.C.D.; Chiu, T.P.; Feng, C.; Zhang, Q.; Yu, L.; Qi, L.; et al. Mechanistic Insights into Metal Ion Activation and Operator Recognition by the Ferric Uptake Regulator. Nat. Commun. 2015, 6, 7642.
- Volbeda, A.; Dodd, E.L.; Darnault, C.; Crack, J.C.; Renoux, O.; Hutchings, M.I.; Le Bru, N.E.; Fontecilla-Camps, J.C. Crystal Structures of the NO Sensor NsrR Reveal How Its Iron-Sulfur Cluster Modulates DNA Binding. Nat. Commun. 2017, 8, 15052.
- Wilson, M.E.; Britigan, B.E. Iron Acquisition by Parasitic Protozoa. Parasitol. Today 1998, 14, 348–353.
- Drakesmith, H.; Prentice, A. Viral Infection and Iron Metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552.
- Bairwa, G.; Hee Jung, W.; Kronstad, J.W. Iron Acquisition in Fungal Pathogens of Humans. Metallomics 2017, 9, 215–227.
- Rodriguez, G.M.; Sharma, N.; Biswas, A.; Sharma, N. The Iron Response of Mycobacterium Tuberculosis and Its Implications for Tuberculosis Pathogenesis and Novel Therapeutics. Front. Cell. Infect. Microbiol. 2022, 12, 876667.
- Soares, M.P.; Weiss, G. The Iron Age of Host—Microbe Interactions. EMBO Rep. 2015, 16, 1482–1500.
- Aznar, A.; Chen, N.W.G.; Thomine, S.; Dellagi, A. Immunity to Plant Pathogens and Iron Homeostasis. Plant Sci. 2015, 240, 90–97.
- Verbon, E.H.; Trapet, P.L.; Stringlis, I.A.; Kruijs, S.; Bakker, P.A.H.M.; Pieterse, C.M.J. Iron and Immunity. Annu. Rev. Phytopathol. 2017, 55, 355–375.
- Herlihy, J.H.; Long, T.A.; McDowell, J.M. Iron Homeostasis and Plant Immune Responses: Recent Insights and Translational Implications. J. Biol. Chem. 2020, 295, 13444–13457.
- Bleuel, C.; Große, C.; Taudte, N.; Scherer, J.; Wesenberg, D.; Krauß, G.J.; Nies, D.H.; Grass, G. TolC Is Involved in Enterobactin Efflux across the Outer Membrane of Escherichia coli. J. Bacteriol. 2005, 187, 6701–6707.
- Furrer, J.L.; Sanders, D.N.; Hook-Barnard, I.G.; McIntosh, M.A. Export of the Siderophore Enterobactin in Escherichia coli: Involvement of a 43 KDa Membrane Exporter. Mol. Microbiol. 2002, 44, 1225–1234.
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451.
- Higgs, P.I.; Myers, P.S.; Postle, K. Interactions in the TonB-Dependent Energy Transduction Complex: ExbB and ExbD Form Homomultimers. J. Bacteriol. 1998, 180, 6031–6038.
- Braun, V. Energy-coupled Transport and Signal Transduction through the Gram-negative Outer Membrane via TonB-ExbB-ExbD-dependent Receptor Proteins. FEMS Microbiol. Rev. 1995, 16, 295–307.
- Javvadi, S.; Pandey, S.S.; Mishra, A.; Pradhan, B.B.; Chatterjee, S. Bacterial Cyclic Β-(1,2)-glucans Sequester Iron to Protect against Iron-induced Toxicity. EMBO Rep. 2018, 19, 172–186.
- Hantke, K. Is the Bacterial Ferrous Iron Transporter FeoB a Living Fossil? Trends Microbiol. 2003, 11, 192–195.
- Kammler, M.; Schön, C.; Hantke, K. Characterization of the Ferrous Iron Uptake System of Escherichia coli. J. Bacteriol. 1993, 175, 6212–6219.
- Marlovits, T.C.; Haase, W.; Herrmann, C.; Aller, S.G.; Unger, V.M. The Membrane Protein FeoB Contains an Intramolecular G Protein Essential for Fe(II) Uptake in Bacteria. Proc. Natl. Acad. Sci. USA 2002, 99, 16243–16248.
- Weaver, E.A.; Wyckoff, E.E.; Mey, A.R.; Morrison, R.; Payne, S.M. FeoA and FeoC Are Essential Components of the Vibrio Cholerae Ferrous Iron Uptake System, and FeoC Interacts with FeoB. J. Bacteriol. 2013, 195, 4826–4835.
- Stevenson, B.; Wyckoff, E.E.; Payne, S.M. Vibrio Cholerae FeoA, FeoB, and FeoC Interact to Form a Complex. J. Bacteriol. 2016, 198, 1160–1170.
- Janakiraman, A.; Slauch, J.M. The Putative Iron Transport System SitABCD Encoded on SPI1 Is Required for Full Virulence of Salmonella Typhimurium. Mol. Microbiol. 2000, 35, 1146–1155.
- Bearden, S.W.; Perry, R.D. The Yfe System of Yersinia Pestis Transports Iron and Manganese and Is Required for Full Virulence of Plague. Mol. Microbiol. 1999, 32, 403–414.
- Angerer, A.; Gaisser, S.; Braun, V. Nucleotide Sequences of the SfuA, SjiiB, and SfiC Genes of Serratia Marcestens Suggest a Periplasmic-Binding Protein-Dependent Iron Transport Mechanism. J. Bacteriol. 1990, 172, 572–578.
- Kehres, D.G.; Janakiraman, A.; Slauch, J.M.; Maguire, M.E. SitABCD Is the Alkaline Mn2+ Transporter of Salmonella Enterica Serovar Typhimurium. J. Bacteriol. 2002, 184, 3159–3166.
- Adhikari, P.; Berish, S.A.; Nowalk, A.J.; Veraldi, K.L.; Morse, S.A.; Mietzner, T.A. The FbpABC Locus of Neisseria Gonorrhoeae Functions in the Periplasm-to-Cytosol Transport of Iron. J. Bacteriol. 1996, 178, 2145–2149.
- Cowart, R.E. Reduction of Iron by Extracellular Iron Reductases: Implications for Microbial Iron Acquisition. Arch. Biochem. Biophys. 2002, 400, 273–281.
- Schröder, I.; Johnson, E.; De Vries, S. Microbial Ferric Iron Reductases. FEMS Microbiol. Rev. 2003, 27, 427–447.
- Sedláček, V.; van Spanning, R.J.M.; Kučera, I. Ferric Reductase A Is Essential for Effective Iron Acquisition in Paracoccus Denitrificans. Microbiology 2009, 155, 1294–1301.
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial Iron Homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237.
- Cornelis, P.; Andrews, S.C. Iron Uptake and Homeostasis in Microorganisms; Cornelis, P., Andrews, S.C., Eds.; Caister Academic Press: Norfolk, UK, 2010; ISBN 1904455654.
- Krewulak, K.D.; Vogel, H.J. Structural Biology of Bacterial Iron Uptake. Biochim. Biophys. Acta 2008, 1778, 1781–1804.
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore Uptake in Bacteria and the Battle for Iron with the Host; a Bird’s Eye View. BioMetals 2010, 23, 601–611.
- Enard, C.; Diolez, A.; Expert, D. Systemic Virulence of Erwinia Chrysanthemi 3937 Requires a Functional Iron Assimilation System. J. Bacteriol. 1988, 170, 2419–2426.
- Franza, T.; Mahé, B.; Expert, D. Erwinia Chrysanthemi Requires a Second Iron Transport Route Dependent of the Siderophore Achromobactin for Extracellular Growth and Plant Infection. Mol. Microbiol. 2005, 55, 261–275.
- Liu, L.; Gueguen-Chaignon, V.; Gonçalves, I.R.; Rascle, C.; Rigault, M.; Dellagi, A.; Loisel, E.; Poussereau, N.; Rodrigue, A.; Terradot, L.; et al. A Secreted Metal-Binding Protein Protects Necrotrophic Phytopathogens from Reactive Oxygen Species. Nat. Commun. 2019, 10, 4853.
- Dellagi, A.; Brisset, M.N.; Paulin, J.P.; Expert, D. Dual Role of Desferrioxamine in Erwinia Amylovora Pathogenicity. Mol. Plant. Microbe. Interact. 1998, 11, 734–742.
- Müller, L.; Müller, D.C.; Kammerecker, S.; Fluri, M.; Neutsch, L.; Emsermann, M.R.; Pelludat, C. Priority Effects in the Apple Flower Determine If the Siderophore Desferrioxamine Is a Virulence Factor for Erwinia Amylovora CFBP1430. Appl. Environ. Microbiol. 2022, 88, e02433-21.
- Bull, C.T.; Carnegie, S.R.; Loper, J.E. Pathogenicity of Mutants of Erwinia Carotovora Subsp. Carotovora Deficient in Aerobactin and Catecholate Siderophore Production. Mol. Plant Pathol. 1996, 86, 260–266.
- Barnes, H.H.; Ishimaru, C.A. Purification of Catechol Siderophores by Boronate Affinity Chromatography: Identification of Chrysobactin from Erwinia Carotovora Subsp. Carotovora. BioMetals 1999, 12, 83–87.
- Taguchi, F.; Suzuki, T.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. The Siderophore Pyoverdine of Pseudomonas Syringae Pv. Tabaci 6605 Is an Intrinsic Virulence Factor in Host Tobacco Infection. J. Bacteriol. 2010, 192, 117–126.
- Owen, J.G.; Ackerley, D.F. Characterization of Pyoverdine and Achromobactin in Pseudomonas Syringae Pv. Phaseolicola 1448a. BMC Microbiol. 2011, 11, 218.
- Pandey, A.; Sonti, R.V. Role of the FeoB Protein and Siderophore in Promoting Virulence of Xanthomonas Oryzae Pv. Oryzae on Rice. J. Bacteriol. 2010, 192, 3187–3203.
- Pandey, S.S.; Patnana, P.K.; Rai, R.; Chatterjee, S. Xanthoferrin, the α-Hydroxycarboxylate-Type Siderophore of Xanthomonas Campestris Pv. Campestris, Is Required for Optimum Virulence and Growth inside Cabbage. Mol. Plant Pathol. 2017, 18, 949–962.
- Tanabe, T.; Funahashi, T.; Nakao, H.; Miyoshi, S.; Shinoda, S.; Yamamoto, S. Identification and Characterization of Genes Required for Biosynthesis and Transport of the Siderophore Vibrioferrin in Vibrio Parahaemolyticus Identification and Characterization of Genes Required for Biosynthesis and Transport of the Siderophore Vibriof. J. Bacteriol. 2003, 185, 6938–6949.
- An, S.-Q.; Potnis, N.; Dow, M.; Vorhölter, F.-J.; He, Y.-Q.; Becker, A.; Teper, D.; Li, Y.; Wang, N.; Bleris, L.; et al. Mechanistic Insights into Host Adaptation, Virulence and Epidemiology of the Phytopathogen Xanthomonas. FEMS Microbiol. Rev. 2019, 44, 1–32.
- Pandey, S.S.; Singh, P.; Samal, B.; Verma, R.K.; Chatterjee, S. Xanthoferrin Siderophore Estimation from the Cell-Free Culture Supernatants of Different Xanthomonas Strains by HPLC. Bioprotocol 2017, 7, e2410.
- Rai, R.; Javvadi, S.; Chatterjee, S. Cell-Cell Signaling Promotes Ferric Iron Uptake in Xanthomonas Oryzae Pv. Oryzicola That Contribute to Its Virulence and Growth inside Rice. Mol. Microbiol. 2015, 96, 708–727.
- Rondon, M.R.; Ballering, K.S.; Thomas, M.G. Identification and Analysis of a Siderophore Biosynthetic Gene Cluster from Agrobacterium Tumefaciens C58. Microbiology 2004, 150, 3857–3866.
- Jones, A.M.; Wildermuth, M.C. The Phytopathogen Pseudomonas Syringae Pv. Tomato DC3000 Has Three High-Affinity Iron-Scavenging Systems Functional under Iron Limitation Conditions but Dispensable for Pathogenesis. J. Bacteriol. 2011, 193, 2767–2775.
- Bhatt, G.; Denny, T.P. Ralstonia Solanacearum Iron Scavenging by the Siderophore Staphyloferrin B Is Controlled by PhcA, the Global Virulence Regulator. J. Bacteriol. 2004, 186, 7896–7904.
- Neema, C.; Laulhère, J.P.; Expert, D. Iron Deficiency Induced by Chrysobactin in Saintpaulia Leaves Inoculated with Erwinia Chrysanthemi. Plant Physiol. 1993, 102, 967–973.
- Ishimaru, C.A.; Loper, J.E. High-Affinity Iron Uptake Systems Present in Erwinia Carotovora Subsp. Carotovora Include the Hydroxamate Siderophore Aerobactin. J. Bacteriol. 1992, 174, 2993–3003.
- Cody, Y.S.; Gross, D.C. Outer Membrane Protein Mediating Iron Uptake via Pyoverdin(Pss), the Fluorescent Siderophore Produced by Pseudomonas Syringae Pv. Syringae. J. Bacteriol. 1987, 169, 2207–2214.
- Leong, S.A.; Neilands, J.B. Relationship of Siderophore-Mediated Iron Assimilation to Virulence in Crown Gall Disease. J. Bacteriol. 1981, 147, 482–491.
- Nachin, L.; Loiseau, L.; Expert, D.; Barras, F. SufC: An Unorthodox Cytoplasmic ABC/ATPase Required for Biogenesis under Oxidative Stress. EMBO J. 2003, 22, 427–437.
- Nachin, L.; El Hassouni, M.; Loiseau, L.; Expert, D.; Barras, F. SoxR-Dependent Response to Oxidative Stress and Virulence of Erwinia Chrysanthemi: The Key Role of SufC, an Orphan ABC ATPase. Mol. Microbiol. 2001, 39, 960–972.
- Andrews, S.C. Iron Storage in Bacteria. Adv. Microb. Physiol. 1998, 40, 281–351.
- Rivera, M. Bacterioferritin: Structure, Dynamics, and Protein-Protein Interactions at Play in Iron Storage and Mobilization. Acc. Chem. Res. 2017, 50, 331–340.
- Boughammoura, A.; Matzanke, B.F.; Böttger, L.; Reverchon, S.; Lesuisse, E.; Expert, D.; Franza, T. Differential Role of Ferritins in Iron Metabolism and Virulence of the Plant-Pathogenic Bacterium Erwinia Chrysanthemi 3937. J. Bacteriol. 2008, 190, 1518–1530.
- Yang, J.; Pan, X.; Xu, Y.; Li, Y.; Xu, N.; Huang, Z.; Ye, J.; Gao, D.; Guo, M. Agrobacterium Tumefaciens Ferritins Play an Important Role in Full Virulence through Regulating Iron Homeostasis and Oxidative Stress Survival. Mol. Plant Pathol. 2020, 21, 1167–1178.
- Franza, T.; Sauvage, C.; Expert, D. Iron Regulation and Pathogenicity in Erwinia Chrysanthemi 3937: Role of the Fur Repressor Protein. Mol. Plant-Microbe Interact. 1999, 12, 119–128.
- Subramoni, S.; Sonti, R.V. Growth Deficiency of a Xanthomonas Oryzae Pv. Oryzae Fur Mutant in Rice Leaves Is Rescued by Ascorbic Acid Supplementation. Mol. Plant-Microbe Interact. MPMI 2005, 644, 644–651.
- Jittawuttipoka, T.; Sallabhan, R.; Vattanaviboon, P.; Fuangthong, M.; Mongkolsuk, S. Mutations of Ferric Uptake Regulator (Fur) Impair Iron Homeostasis, Growth, Oxidative Stress Survival, and Virulence of Xanthomonas Campestris Pv. Campestris. Arch. Microbiol. 2010, 192, 331–339.
- Cha, J.Y.; Lee, J.S.; Oh, J.I.; Choi, J.W.; Baik, H.S. Functional Analysis of the Role of Fur in the Virulence of Pseudomonas Syringae Pv. Tabaci 11528: Fur Controls Expression of Genes Involved in Quorum-Sensing. Biochem. Biophys. Res. Commun. 2008, 366, 281–287.
- Pandey, S.S.; Chatterjee, S. Insights into the Cell-Cell Signaling and Iron Homeostasis in Xanthomonas Virulence and Lifestyle. Phytopathology 2022, 112, 209–218.
- Wang, L.; Pan, Y.; Yuan, Z.H.; Zhang, H.; Peng, B.Y.; Wang, F.F.; Qian, W. Two-Component Signaling System VgrRS Directly Senses Extracytoplasmic and Intracellular Iron to Control Bacterial Adaptation under Iron Depleted Stress. PLoS Pathog. 2016, 12, e1006133.
- Massé, E.; Gottesman, S. A Small RNA Regulates the Expression of Genes Involved in Iron Metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 4620–4625.
- Chareyre, S.; Mandin, P. Bacterial Iron Homeostasis Regulation by SRNAs. Microbiol. Spectr. 2018, 6, RWR-0010-2017.
- Filiatrault, M.J.; Stodghill, P.V.; Bronstein, P.A.; Moll, S.; Lindeberg, M.; Grills, G.; Schweitzer, P.; Wang, W.; Schroth, G.P.; Luo, S.; et al. Transcriptome Analysis of Pseudomonas Syringae Identifies New Genes, Noncoding RNAs, and Antisense Activity. J. Bacteriol. 2010, 192, 2359–2372.
- Hu, Y.; Zhang, L.; Wang, X.; Sun, F.; Kong, X.; Dong, H.; Xu, H. Two Virulent SRNAs Identified by Genomic Sequencing Target the Type III Secretion System in Rice Bacterial Blight Pathogen. BMC Plant Biol. 2018, 18, 237.
- Wilms, I.; Overlöper, A.; Nowrousian, M.; Sharma, C.M.; Narberhaus, F. Deep Sequencing Uncovers Numerous Small RNAs on All Four Replicons of the Plant Pathogen Agrobacterium Tumefaciens. RNA Biol. 2012, 9, 446–457.
- Jiang, C.-H.; Li, Z.-J.; Zheng, L.-Y.; Yu, Y.-Y.; Niu, D.-D. Small RNAs: Efficient and Miraculous Effectors That Play Key Roles in Plant–Microbe Interactions. Mol. Plant Pathol. 2023, 24, 999–1013.
- Kwenda, S.; Gorshkov, V.; Ramesh, A.M.; Naidoo, S.; Rubagotti, E.; Birch, P.R.J.; Moleleki, L.N. Discovery and Profiling of Small RNAs Responsive to Stress Conditions in the Plant Pathogen Pectobacterium Atrosepticum. BMC Genom. 2016, 17, 47.
- Liang, H.; Zhao, Y.-T.; Zhang, J.-Q.; Wang, X.-J.; Fang, R.-X.; Jia, Y.-T. Identification and Functional Characterization of Small Non-Coding RNAs in Xanthomonas Oryzae Pathovar Oryzae. BMC Genom. 2011, 12, 87.
- Schmidtke, C.; Findeiss, S.; Sharma, C.M.; Kuhfuss, J.; Hoffmann, S.; Vogel, J.; Stadler, P.F.; Bonas, U. Genome-Wide Transcriptome Analysis of the Plant Pathogen Xanthomonas Identifies SRNAs with Putative Virulence Functions. Nucleic Acids Res. 2012, 40, 2020–2031.
- Schmidtke, C.; Abendroth, U.; Brock, J.; Serrania, J.; Becker, A.; Bonas, U. Small RNA SX13: A Multifaceted Regulator of Virulence in the Plant Pathogen Xanthomonas. PLoS Pathog. 2013, 9, e1003626.
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron Homeostasis in Plants-a Brief Overview. Metallomics 2017, 9, 813–823.
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395.
- Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The Transcriptional Control of Iron Homeostasis in Plants: A Tale of BHLH Transcription Factors? Front. Plant Sci. 2019, 10, 6.
- Vélez-Bermúdez, I.C.; Schmidt, W. How Plants Recalibrate Cellular Iron Homeostasis. Plant Cell Physiol. 2022, 63, 154–162.
- Li, M.; Watanabe, S.; Gao, F.; Dubos, C. Iron Nutrition in Plants: Towards a New Paradigm? Plants 2023, 12, 384.
- Vélez-Bermúdez, I.C.; Schmidt, W. Iron Sensing in Plants. Front. Plant Sci. 2023, 14, 1145510.
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329.
- Xing, Y.; Xu, N.; Bhandari, D.D.; Lapin, D.; Sun, X.; Luo, X.; Wang, Y.; Cao, J.; Wang, H.; Coaker, G.; et al. Bacterial Effector Targeting of a Plant Iron Sensor Facilitates Iron Acquisition and Pathogen Colonization. Plant Cell 2021, 33, 2015–2031.
- Long, T.A.; Tsukagoshi, H.; Busch, W.; Lahner, B.; Salt, D.E.; Benfey, P.N. The BHLH Transcription Factor POPEYE Regulates Response to Iron Deficiency in Arabidopsis Roots. Plant Cell 2010, 22, 2219–2236.
- Selote, D.; Samira, R.; Matthiadis, A.; Gillikin, J.W.; Long, T.A. Iron-Binding E3 Ligase Mediates Iron Response in Plants by Targeting Basic Helix-Loop-Helix Transcription Factors. Plant Physiol. 2015, 167, 273–286.
- Rampey, R.A.; Woodward, A.W.; Hobbs, B.N.; Tierney, M.P.; Lahner, B.; Salt, D.E.; Bartel, B. An Arabidopsis Basic Helix-Loop-Helix Leucine Zipper Protein Modulates Metal Homeostasis and Auxin Conjugate Responsiveness. Genetics 2006, 174, 1841–1857.
- Dellagi, A.; Segond, D.; Rigault, M.; Fagard, M.; Simon, C.; Saindrenan, P.; Expert, D. Microbial Siderophores Exert a Subtle Role in Arabidopsis during Infection by Manipulating the Immune Response and the Iron Status. Plant Physiol. 2009, 150, 1687–1696.
- Dellagi, A.; Rigault, M.; Segond, D.; Roux, C.; Kraepiel, Y.; Cellier, F.; Briat, J.F.; Gaymard, F.; Expert, D. Siderophore-Mediated Upregulation of Arabidopsis Ferritin Expression in Response to Erwinia Chrysanthemi Infection. Plant J. 2005, 43, 262–272.
- Aznar, A.; Chen, N.W.G.; Rigault, M.; Riache, N.; Joseph, D.; Desmaële, D.; Mouille, G.; Boutet, S.; Soubigou-Taconnat, L.; Renou, J.P.; et al. Scavenging Iron: A Novel Mechanism of Plant Immunity Activation by Microbial Siderophores. Plant Physiol. 2014, 164, 2167–2183.
- Nobori, T.; Velásquez, A.C.; Wu, J.; Kvitko, B.H.; Kremer, J.M.; Wang, Y.; He, S.Y.; Tsuda, K. Transcriptome Landscape of a Bacterial Pathogen under Plant Immunity. Proc. Natl. Acad. Sci. USA 2018, 115, E3055–E3064.
- Nobori, T.; Wang, Y.; Wu, J.; Stolze, S.C.; Tsuda, Y.; Finkemeier, I.; Nakagami, H.; Tsuda, K. Multidimensional Gene Regulatory Landscape of a Bacterial Pathogen in Plants. Nat. Plants 2020, 6, 883–896.
- Labidi, S.; Sandhu, R.K.; Beaulieu, C.; Beaudoin, N. Increased Ferritin and Iron Accumulation in Tubers of Thaxtomin A-Habituated Potato Var. Yukon Gold Somaclones with Enhanced Resistance to Common Scab. J. Plant Pathol. 2022, 105, 107–119.
- Briat, J.F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New Insights into Ferritin Synthesis and Function Highlight a Link between Iron Homeostasis and Oxidative Stress in Plants. Ann. Bot. 2010, 105, 811–822.
- Yang, C.L.; Huang, Y.T.; Schmidt, W.; Klein, P.; Chan, M.T.; Pan, I.C. Ethylene Response Factor109 Attunes Immunity, Photosynthesis, and Iron Homeostasis in Arabidopsis Leaves. Front. Plant Sci. 2022, 13, 841366.
- Govoni, G.; Gros, P. Macrophage NRAMP1 and Its Role in Resistance to Microbial Infections. Inflamm. Res. 1998, 47, 277–284.
- Segond, D.; Dellagi, A.; Lanquar, V.; Rigault, M.; Patrit, O.; Thomine, S.; Expert, D. NRAMP Genes Function in Arabidopsis Thaliana Resistance to Erwinia Chrysanthemi Infection. Plant J. 2009, 58, 195–207.
- Kieu, N.P.; Aznar, A.; Segond, D.; Rigault, M.; Simond-Côte, E.; Kunz, C.; Soulie, M.C.; Expert, D.; Dellagi, A. Iron Deficiency Affects Plant Defence Responses and Confers Resistance to Dickeya Dadantii and Botrytis Cinerea. Mol. Plant Pathol. 2012, 13, 816–827.
- Trapet, P.L.; Verbon, E.H.; Bosma, R.R.; Voordendag, K.; Van Pelt, J.A.; Pieterse, C.M.J. Mechanisms Underlying Iron Deficiency-Induced Resistance against Pathogens with Different Lifestyles. J. Exp. Bot. 2021, 72, 2231–2241.
- Deák, M.; Horváth, G.V.; Davletova, S.; Török, K.; Sass, L.; Vass, I.; Barna, B.; Király, Z.; Dudits, D. Plants Ectopically Expressing the Iron-Binding Protein, Ferritin, Are Tolerant to Oxidative Damage and Pathogens. Nat. Biotechnol. 1999, 17, 192–196.
- Pandey, S.S.; Patnana, P.K.; Padhi, Y.; Chatterjee, S. Low-Iron Conditions Induces the Hypersensitive Reaction and Pathogenicity Hrp Genes Expression in Xanthomonas and Is Involved in Modulation of Hypersensitive Response and Virulence. Environ. Microbiol. Rep. 2018, 10, 522–531.
- Ahmed, T.; Noman, M.; Jiang, H.; Shahid, M.; Ma, C.; Wu, Z.; Nazir, M.M.; Ali, M.A.; White, J.C.; Chen, J.; et al. Bioengineered Chitosan-Iron Nanocomposite Controls Bacterial Leaf Blight Disease by Modulating Plant Defense Response and Nutritional Status of Rice (Oryza sativa L.). Nano Today 2022, 45, 101547.
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072.
- Dangol, S.; Chen, Y.; Hwang, B.K.; Jwa, N.S. Iron- and Reactive Oxygen Species-Dependent Ferroptotic Cell Death in Rice-Magnaporthe Oryzae Interactions. Plant Cell 2019, 31, 189–209.
- Distéfano, A.M.; Martin, M.V.; Córdoba, J.P.; Bellido, A.M.; D’Ippólito, S.; Colman, S.L.; Soto, D.; Roldán, J.A.; Bartoli, C.G.; Zabaleta, E.J.; et al. Heat Stress Induces Ferroptosis-like Cell Death in Plants. J. Cell Biol. 2017, 216, 463–476.
- Nguyen, N.K.; Wang, J.; Liu, D.; Hwang, B.K.; Jwa, N.S. Rice Iron Storage Protein Ferritin 2 (OsFER2) Positively Regulates Ferroptotic Cell Death and Defense Responses against Magnaporthe Oryzae. Front. Plant Sci. 2022, 13, 1019669.
- Macharia, M.; Das, P.P.; Naqvi, N.I.; Wong, S.-M. ITRAQ-Based Quantitative Proteomics Reveals a Ferroptosis-like Programmed Cell Death in Plants Infected by a Highly Virulent Tobacco Mosaic Virus Mutant 24A+UPD. Phytopathol. Res. 2020, 2, 1–13.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
510
Revisions:
2 times
(View History)
Update Date:
14 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




