Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lesego Tabea Maubane | -- | 7321 | 2023-09-12 12:11:20 | | | |
| 2 | Rita Xu | Meta information modification | 7321 | 2023-09-13 03:34:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Temane, L.T.; Orasugh, J.T.; Ray, S.S. 2D Nanomaterial-Based Flame-Retardant PLA Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/49066 (accessed on 08 March 2026).
Temane LT, Orasugh JT, Ray SS. 2D Nanomaterial-Based Flame-Retardant PLA Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/49066. Accessed March 08, 2026.
Temane, Lesego Tabea, Jonathan Tersur Orasugh, Suprakas Sinha Ray. "2D Nanomaterial-Based Flame-Retardant PLA Materials" Encyclopedia, https://encyclopedia.pub/entry/49066 (accessed March 08, 2026).
Temane, L.T., Orasugh, J.T., & Ray, S.S. (2023, September 12). 2D Nanomaterial-Based Flame-Retardant PLA Materials. In Encyclopedia. https://encyclopedia.pub/entry/49066
Temane, Lesego Tabea, et al. "2D Nanomaterial-Based Flame-Retardant PLA Materials." Encyclopedia. Web. 12 September, 2023.
Copy Citation
Poly (lactic acid) or polylactide (PLA) has gained widespread use in many industries and has become a commodity polymer. Its potential as a perfect replacement for petrochemically made plastics has been constrained by its extreme flammability and propensity to flow in a fire. Traditional flame-retardants (FRs), such as organo-halogen chemicals, can be added to PLA without significantly affecting the material’s mechanical properties.
2D nanomaterials
flame-retardants
polymers
1. Introduction
The extraordinary blend of qualities found in polymer materials, including their lightweight, high specific strength, simplicity of processing, adaptability, cost-effectiveness, and moldability, is driving their rising use in daily life [1]. The search for alternative bio-based/biodegradable polymeric materials has become increasingly important due to the current environmental awareness of the impact of synthetic polymers and the strict environmental laws adopted by governments worldwide [1]. By 2027, the global production of bio-based polymers is expected to expand by an impressive 14% [2]. For instance, the production of bio-based epoxy resin is on the rise, polytrimethylene terephthalate (PTT) has recovered its appeal after several years of stable capacities, and polyethylene (PE) and polypropylene (PP) made from bio-based naphtha are further establishing themselves with rising volumes [2]. The new market and trend report “Bio-based Building Blocks and Polymers—Global Capacities, Production, and Trends 2022–2027” by the International Nova Biopolymer expert group presented capacities and production data for 17 commercially available bio-based polymers in the year 2022 and a forecast to 2027. Polyhydroxyalkanoates (PHAs) and bio-based polyamides (PA) are both expected to see current and future growth. Also returning to the fray is the bio-based PET [2]. The new market and trend report “Bio-based Building Blocks and Polymers—Global Capacities, Production, and Trends 2022–2027” by the International Nova Biopolymer expert group presents capacities and production data for 17 commercially available bio-based polymers in the year 2022 and a forecast to 2027. In 2022, the production was 4.5 million tons, with a total installed capacity of 4.9 million tons, or 1% of the volume of all fossil-based polymers produced. A rise in capacity to 9.3 million tons in 2027 is anticipated, showing an average compound annual growth rate (CAGR) of roughly 14%, much higher than the growth of polymers (3–4%) previously. PHA is predicted to rise by 45%, PLA by 39%, PA by 37%, and PP by 34% until 2027. These polymers will all grow well above the average growth rate. Until 2027, PE use in Europe will rise by 18%, and casein polymers will rise by 15% [2]. After Asia, the leading region with the highest percentage of bio-based polymer(s) production capacity installed globally in 2022 (41%), with the highest capabilities for PLA and PA, Europe follows with 27%, primarily based on starch-containing polymer compounds, PE and PP. South America accounts for 13%, primarily based on PE, and North America accounts for 19%, with significant installed PLA and PTT capabilities. The Australia/Oceania market share of less than 1% is based on starch polymer compounds [2].
The focus has been on biodegradable qualities among the countless bio-based polymers, such as starch, methylcellulose, PLA, polycaprolactone, and PHA, as well as their mixtures [1]. Meanwhile, PLA has garnered much interest because of its distinctive qualities, including its attractive look, high mechanical strength, biodegradability, cytocompatibility, transparency, and more [3]. With a projected annual growth of 39% due to these qualities, its applications have expanded [2]. Recent applications of PLA in the automotive sector, industrial carpets, building and construction, furniture, high-end fashion electrical and electronics, items, foams, and fiberfill, among other industries, have sparked its interest in advanced materials [1]. However, PLA is renowned for having a low melting point and a high flammability level, which can occasionally release harmful gases during combustion in contaminated environments. Due to this, expanding the uses for PLA now requires enhancing its FR qualities, even though significant work has already been done in this area [4][5][6][7].
Like any other polymer, the flammability of PLA polymer is determined by well-known factors like solid degradation rate and/or peak heat release rate “pHRR” (burning rate), delay time, ignition temperature, critical heat flux for ignition (ignition characteristics), in particular toxic species emissions (product distribution), production of smoke [6], and others. Previously, the only FRs for PLA that were conventionally used were those with halogen moieties because they have been largely successful in most polymers [1]. However, due to their adverse health effects on both humans and animals, these FRs are now recognized as major potential global contaminants [1]. As the dust from indoor exposure, mechanical recycling of plastics and metals, and open burning and cremation of household items, halogenated FR leaks into the environment throughout their valuable lives. Throughout the lifespan of treated objects, halogenated FR leaches to the environment as dust through indoor exposure, mechanical recycling of plastics and metals, and cremation and open burning of household trash, such as electronic components, paints, solvents, and textiles [1]. They are also mainly linked to endocrine and thyroid disturbance, immunological toxicity, reproductive problems, cancer, neonatal and fetal defects, abnormal child development, and neurologic functions [1]. Due to rigorous government regulations and increased consumer awareness of the environment, halogenated FRs are consequently being phased out gradually [1]. The research community has produced many intriguing findings in response to the search for substitute eco-friendly FRs. However, no commercially available two-dimensional (2D)-based FR has been specifically developed to manage the flammability of PLA to retain its biological integrity while enhancing or maintaining its delicate crystalline and mechanical qualities [1].
1.1. PLA Feedstock
Lactic acid (LA) molecules create biodegradable and biobased PLA. Technically, PLA is suitable for various applications, from single-use packaging to lasting consumer items, because of its flexibility and other technical characteristics. The feedstocks used are glucose and sucrose. The primary biological source of glucose is still corn grains. The grains of maize are processed to remove the starch after harvest. Then, a process known as hydrolysis converts starch molecules into molecules of glucose. After being dried, glucose goes through an industrial fermentation process using bacteria. The resultant LA solutions are transformed into lactide and then crystallized to remove impurities before polymerizing into PLA. Similar general steps apply when using sugar cane, except the fermentation process uses sucrose extracted from the stalks [8]. Because scientific expertise and production capabilities have risen relatively high, PLA manufacture is currently more economical than other bioplastics. PLA has the greatest biopolymer production capacity installed as of 2022 in the global production capacities of all biopolymers, with a reported 39% growth rate in market shares [2]. Again, the European Bioplastics report on bioplastic production shows that PLA production stands out at 20.7% higher than all others and is predicted to rise to 37.9% by 2027 [9]. The three main application areas for PLA-based finished goods are rigid packaging, flexible packaging, & textiles.
The main goal of PLA feedstocks/synthesis processes has been to address the environmental concerns related to land usage and competition with food products tied to the current feedstock creation of food crops. Among these, the use of leftover plant material from their cultivation (stover) and processing (e.g., sugar cane bagasse) as cellulose-based feedstocks has also been studied for a while. These materials are obtained as a byproduct, eliminating or at least lessening first-generation feedstocks’ drawbacks. Several recommendations have been made to entirely uncouple PLA manufacturing from agricultural land usage in recent years. Waste and byproducts from the food business that would otherwise have little or no economic value are also used nowadays. Harbec [10] and Broeren et al. [11] have reportedly examined water waste generated during the industrial preparation of potatoes as feedstock for PLA synthesis. Using lactose and proteins as feedstocks, Liu et al. [10] look into manufacturing LA from cheese whey by contrasting various bacteria species and fermentation enzymes. Juodeikiene et al. [11] investigated how to increase the yield from cheese whey. Nguyen et al. [12] investigated an instance in which waste from the industrial extraction of curcuminoid from the Curcuma longa root utilized in medical applications is fermented to LA through simultaneous saccharification and fermentation. According to De la Torre et al. [13], orange peel waste and corn steep liquor have also been used as substrates. Coffee pulp, a byproduct of coffee processing, is the subject of Pleissner et al. [14] investigation. Alves de Oliveira et al. [15] suggested using sugar beet pulp as a byproduct of sugar extraction from sugar beets for animal feed. Another area looks at the possibility of switching from land-based to sea-based resources. It is thought that the cultivation and fermentation of carbohydrate-rich marine plants will provide a chance to create whole new production pathways, protecting current food supply chains from disruption by the creation of plastic. In this vein, Helmes and his group [16] investigate the utilization of the seaweed Ulva spp. in the LA synthesis process. Brown algae species Laminaria sp. have been reportedly grown as a feedstock source in an experiment by Ögmundarson et al. [17].
The number of novel feedstocks for PLA for which thorough studies on environmental performance are available is still relatively small. Lignocellulose, a second-generation feedstock produced as a byproduct of plant cultivation, has drawn particular interest in the already available data. Its primary benefits are clear: no environmental concerns are connected to land transformation when gathered only as a byproduct within the framework of currently used production processes for corn, sugar, or cereal. Additionally, the allocation principle’s emission reduction also applies to the cultivation of the feedstock since fertilizer input, water consumption, and machinery used are effectively shared between the agricultural outputs. By doing this, it would be possible to lessen the effects of climate change on both local and global environmental categories, including those linked to soil, water, and human health. Although these agricultural by-products are frequently not put to significant commercial use, they are not useless from an ecological standpoint, as shown by Ögmundarson et al. [17], and this should be taken into account in an environmental study. The plant material left on the field after harvesting offers ecological benefits, including erosion control and a boost to the soil’s carbon content, or it can be burned to provide electricity instead of using fossil fuels. The potential costs of these alternate uses should be considered in LCAs, examining the advantages of cellulose feedstocks, ideally through system expansion. Such a consideration can significantly degrade the overall environmental performance, according to the analysis by Ögmundarson et al. [17].
1.2. PLA Synthesis in Brief
The category of polymeric biomaterials referred to as poly-α-esters, poly-α-hydroxy acids, and/or aliphatic polyesters includes PLA, a hydrophobic polymer. It is made from LA, also known as 2-hydroxy propanoic acid, which has two enantiomeric forms, L-(+)-LA and D-(-)-LA and is a water-soluble monomer. LA is the starting material for this process, as indicated in Figure 1 [8].
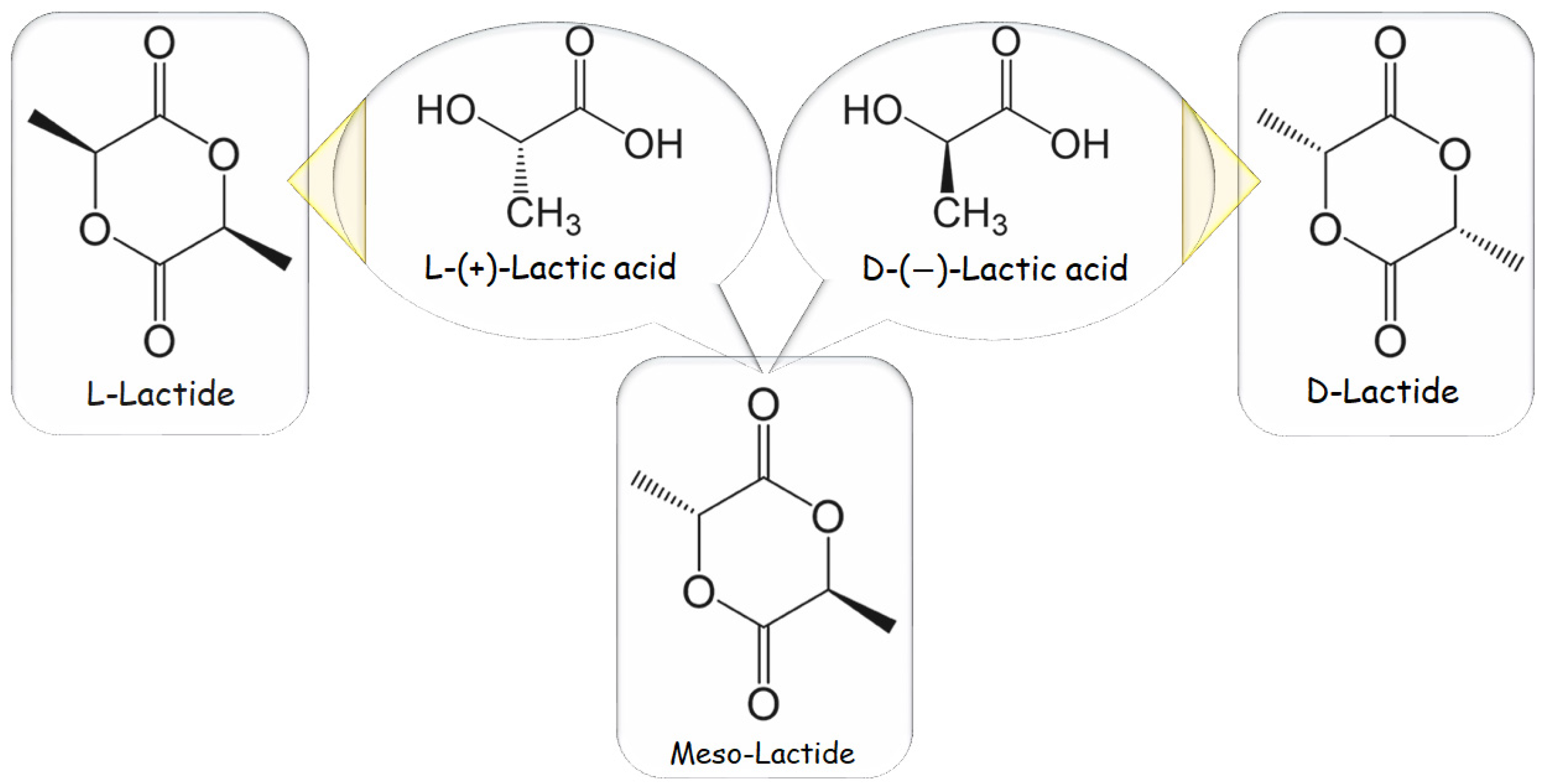
Figure 1. Lactic acid and lactide are initial stereoisomers.
Even though both enantiomers are often used in industrialized synthesis, L-(+)-LA is its isomer of interest for biotechnology, bioengineering, and biomechanics applications, seeing it participates in the metabolism at a cellular level within the body system and lowers the likelihood of adverse reactions. L-(+)-LA could be excluded from the body in the form of H2O and/or CO2 from the lungs in an in vivo setting by either incorporating it into the Krebs’ cycle or converting it into its stored form in the liver “glycogen” [18]. If L- and D-monomers racemic blend is utilized/adopted, poly-D,L-lactic acid (PDLLA) copolymer is generated. PLA could then be made by commencing with virgin L- and D-lactic isomers, corresponding to poly-L-lactic acid (PLLA) and poly-D-lactic acid (PDLA) homopolymers. Stereochemistry significantly impacts material properties: PLLA is a semi-crystalline polymer, whereas PDLLA is an amorphous polymeric material with no melting point. Additionally, because PLLA has crystalline areas, its degradation rate is considerably slower than PDLLA’s.
When lactonitrile is produced from hydrogen cyanide and acetaldehyde (1), it is hydrolyzed at a low pH to produce LA (2), which is then transformed to methyl lactate (3) via esterification and finally reclaimed, followed by its purification via distillation. After hydrolyzing, methanol is recycled in step two to create LA and methanol from lactate (3). In any case, a racemic mixture results from this kinetic process [19].
The most popular method for processing sugar solutions at the moment is bacterial fermentation; this method produces high yields and, depending on the bacteria used, permits the production of either a specific stereoisomer or a racemic mixture. According to estimates, this method yields around 90% of global LA today. The following LA purification procedure, which is costly and affects process profitability, is the crucial stage in this system. Reactive distillation, membrane separation, ion exchange, and liquid extraction are frequently used.
Step growth polymerization or ring-opening polymerization (ROP) are viable methods for creating PLA polymers. The reactivity of the two LA functional groups is exploited in step growth polymerization; in fact, the polycondensation of the hydroxyl and carboxyl moieties results in the production of the ester linkages that make up the polymer backbone. This method of synthesis has several drawbacks, including the need for prolonged residence times for longer chains (which can cause undesirable side reactions, such as transesterification), difficult reaction parameters with temperatures reaching 250 °C, and a vacuum pressure of ~100 mbar), along with the continuous exclusion of water (a polycondensation byproduct). It is theoretically possible to use chain extenders (such as isocyanates or epoxides), but doing so inevitably affects the quality and purity of the material.
ROP is the most used method at the industrial scale because of its benefits, mild process conditions, brief residence durations, the absence of side products, and high molecular weights. The most extensively used catalyst in PLA synthesis is 2-ethylhexanoic tin(II) salt, likewise known as stannous octoate “Sn(Oct)2”, which has been given U.S. Food and Drug Administration approval and is typically used in conjunction with alcohol as a co-catalyst. The availability of cyclic monomers and their optical and chemical purity are the primary bottlenecks of ROP because impurities have a negative impact on material quality due to the reaction’s sensitivity to remaining non-cyclic monomers. The cyclic dimer lactide, having trio-stereoisomeric forms and serves as PLA raw material, is represented in Figure 2 as the following: D, L-, LL-, and DD- (also referred to as a meso-lactide) [8][19].
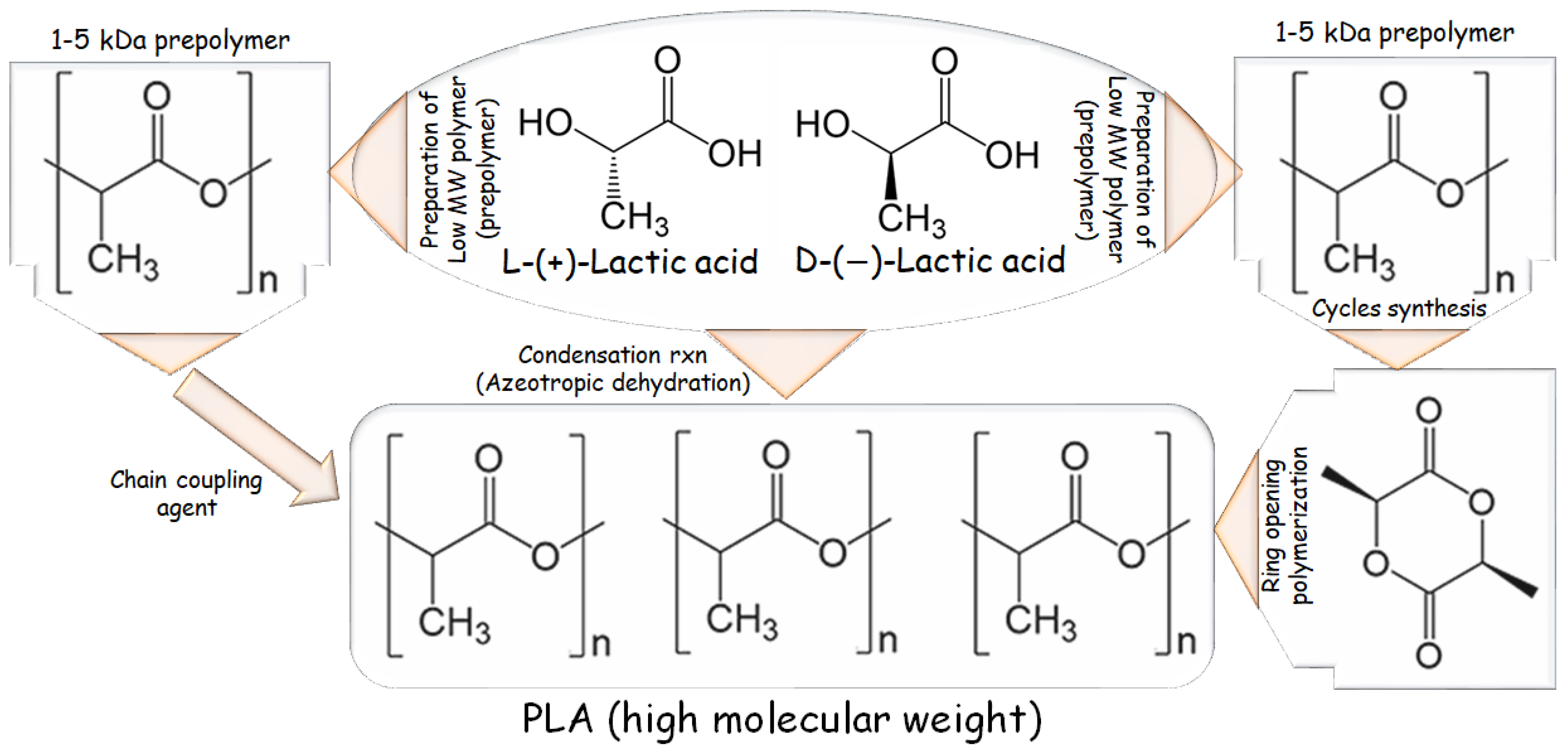
Figure 2. Major production routes for PLA.
A backbiting kinetic mechanism Is typically used to create lactide, starting with a low MW pre-polymer. After distillation, the cycles are eventually collected. Enzymatic polymerization and azeotropic dehydration are further methods of synthesis. The routes for synthesizing PLA-based polymers are listed in Figure 2 [20].
Due to its eco-friendliness, biocompatibility, and processability, PLA is now extensively used in materials science, even in applications where retarded flammability is required. PLA is processable using a variety of methods, including melt compounding and extrusion, injection stretch blow molding, injection molding, solution intercalation plus casting, blow film extrusion, foaming, melt spinning of fibers, electrospinning, 3D printing, etc. [3][21][22][23].
It is also important to note the advantages of PLA as a polymer: its eco-friendliness, biocompatibility, ease of processing, and less energy consumption (25–55% less energy) during production compared to petroleum-based polymers. Though several advantages have been stated, PLA has its disadvantages, which are poor toughness (highly brittle: <10 elongation at break), slow crystallization and degradation rate, relatively high hydrophobicity (contact angle of 80°), and a lack of reactive side chains or surface functional groups making it very difficult to almost impossible to modify PLA.
1.3. Polymeric Materials Behavior in the Fire: PLA Exacts
Before tackling the issue of the fire threat provided by polymeric materials, it is critical to gain a complete understanding of the combustion process of a polymer. Polymer combustion is a multi-step process involving several related chemical and physical steps. Three crucial components, heat, fuel, and oxygen, sometimes shown as a fire triangle, must all come together simultaneously for polymer combustion to begin and continue [24]. Due to their chemical composition, which is mainly composed of carbon and hydrogen, polymers are highly combustible [25].
A combustible substance (reducing agent) and a combustive substance are required for the combustion reaction (oxidizing agent). Combustion is usually brought on by oxygen in the air. When a heat source raises the temperature of the polymeric material to a threshold where polymer bonds split, the entire process frequently starts there. A combustible gaseous mixture is created when the polymer fragments are created due to the volatile portion diffusing into there (also called fuel). This gaseous mixture ignites and releases heat when the auto-ignition temperature, which is the temperature at which the activation energy of the combustion reaction is achieved, is reached. The fuel can ignite when exposed to a potent energy source outside of it at a lower temperature (referred to as the flash point) (spark, flame, etc.). How long the combustion cycle lasts depends on how much heat is produced during fuel combustion. When the amount of heat released exceeds a certain limit, new breakdown reactions in the solid phase start, forming more combustibles. Consequently, the combustion cycle is preserved, and the phrase “fire triangle” is utilized (Figure 3) [25].
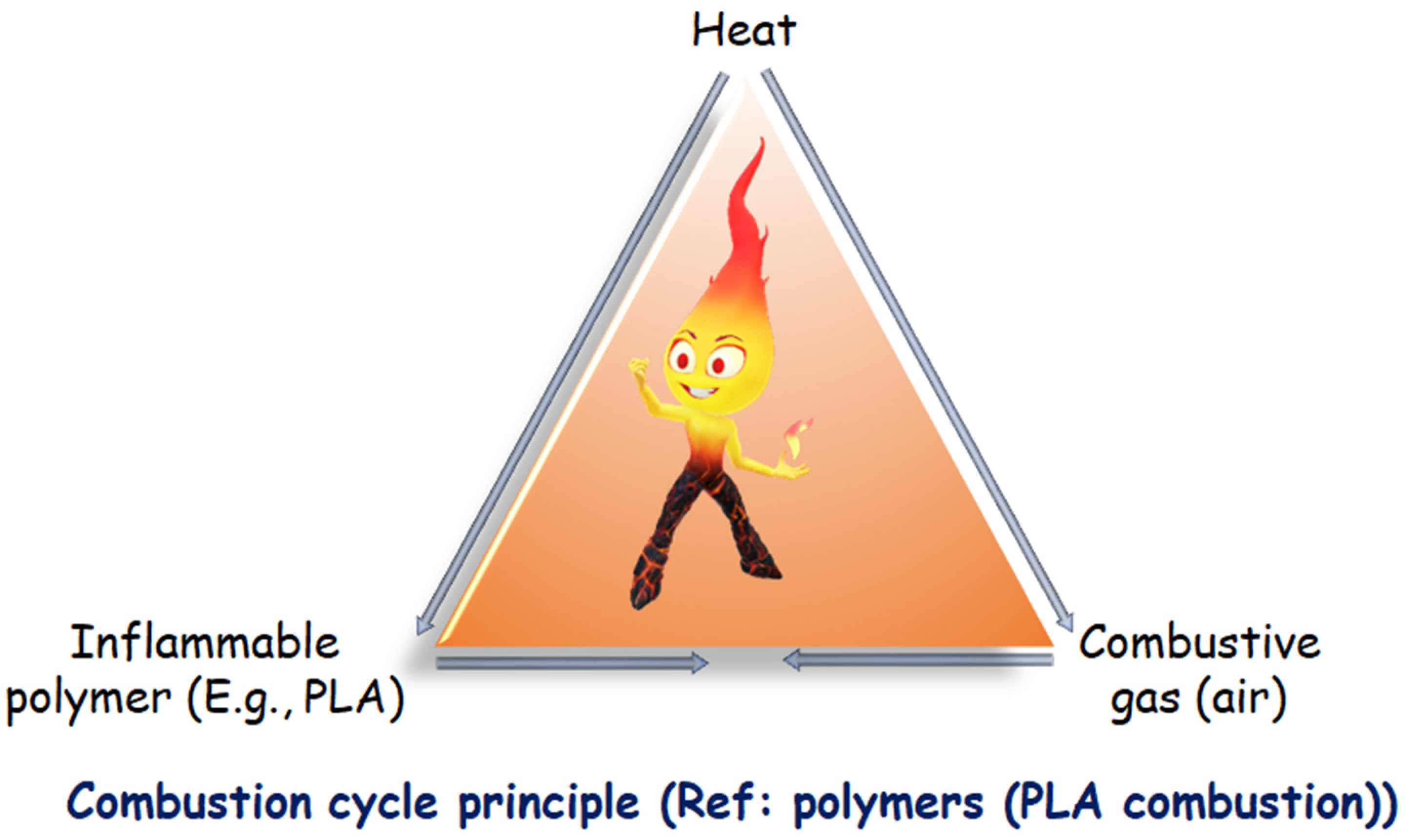
Figure 3. Combustion cycle principle with reference to PLA.
The physical characteristics of the material determine how much energy is required to initiate combustion in polymers. Semi-crystalline thermoplastics, for instance, cause the polymer to soften, melt, and drip when heated. The polymer’s capacity for heat storage, as well as its enthalpy of fusion and crystallinity level, all play a role in determining how much energy it can hold throughout these processes. The exothermicity of the processes, the specific heat, and the thermal conductivity of the semi-crystalline thermoplastic all contribute significantly to the temperature increase of the polymer and the accompanying rate. However, most thermosets and amorphous thermoplastics experience polymer breakage during heating because they lack a melting point [25].
The endothermic process of covalent bond dissolution, which results in the thermal breakdown of a polymer, requires energy input. The energy introduced into the system must be greater than necessary to bind the covalently linked atoms together (200–400 kJ/mol for the bulk of C–C polymers). The decomposition mechanism’s major factors include the weakest bonds and whether oxygen is present in the solid or gas phases. The combined effects of heat and oxygen typically bring on thermal breakdown. As a result, oxidizing and non-oxidizing heat degradation can be separated [26].
1.3.1. Principle of Polymeric Materials That Resist Fire
The pyrolysis and combustion of polymers occur in several phases, as shown in Figure 3. The polymeric substrate, which an outside heat source has heated, is converted into combustible fuel by pyrolysis. Only a part of this fuel is normally completely consumed when mixed with the stoichiometric amount of oxygen in the flame. Strong catalysts and abundant oxygen, among other severe techniques, can burn up the residual fraction. The substrate keeps pyrolyzing as the combustion cycle is maintained by returning some of the heated energy. Additional heat is removed by the environment [27]. Depending on their makeup, FR systems can function chemically or physically (by chilling, forming a protection barrier, or diluting fuel) (reaction in the condensed or gas phase). They might prevent different polymer combustion processes from happening (heating, pyrolysis, ignition, propagation of thermal degradation) [25].
Physical Action
Some FR additives experience an endothermic breakdown, which causes heat to be consumed and the temperature to drop. This necessitates a small amount of reaction medium cooling to below the temperature of polymer combustion. Magnesium hydroxide and hydrated trialumina also belong to this category and start to produce water vapor at temperatures of about 200 and 300 °C, respectively. This kind of strong endothermic reaction is said to act as a “heat sink”. The breakdown of the FRs dilutes the combination of flammable gases. It produces inert gases like water, carbon dioxide, etc., which lowers the concentration of reagents and the risk of ignition. Various FR additions may also form a protective solid or gaseous layer between the solid phase, where thermal degradation occurs, and the gaseous phase, where combustion occurs. The passage of such a barrier prevents gases like oxygen and flammable, volatile vapors. As a result, significantly less decomposition gas is produced. Additionally, the fuel gases and oxygen can be physically separated, halting the combustion process [25].
Chemical Action
The amount and composition of the gaseous products and the energy needed to heat the polymer to the pyrolysis temperature and break down, gasify, or volatilize the combustibles all affect how flammable the substrate is. An FR with a condensed phase chemical mechanism can alter the pyrolytic route of the substrate and considerably reduce the number of gaseous combustibles by encouraging the formation of carbonaceous char and water. In this case, as the flame-retarding chemical concentration is increased, the heat released during combustion is decreased [27].
The heat released during combustion decreases typically as the flame-retarding chemical grows, although the amount of combustible material in the gas-phase mechanism doesn’t change. The pyrolysis decreases or stops when the surface temperature decreases because less heat is returned to the polymer surface. When it comes into contact with the flame, the flame-retarding substance must be gaseous and volatile. Instead, it must disassemble and deliver its molecule’s active component to the gaseous phase. The less active agent will be present in the char that remains in the substrate. In the worst situation, the polymer pyrolysis should proceed as if no FR had been added. As they approach the flame, the composition of the volatiles should also not be affected by the presence of the gas-phase active agent [27].
1.3.2. The Condensed-Phase Mechanism
The condensed-phase method relies on the idea that the polymer and the FR component, frequently provided in large amounts, would interact chemically. The pyrolytic breakdown occurs at higher temperatures than this contact. It was suggested that the two main types of this interaction were cross-linking and dehydration. They are well-established for various polymers, including synthetic and cellulosic ones [27].
1.3.3. FR Mechanism of PLA-2D NPs NCP in Brief
There is no one method for determining flame retardancy because the FR chemistry of composites is typically overly complex. For this reason, scientists frequently select FRs based on models for the chemistry of heat degradation of polymers and fire hazards. In addition, various commercial specifications, including those relating to the way a product is processed, its cost, environmental stability, color, and, more recently, its sustainability and recyclable nature, are imposed on commercial goods.
It is commonly acknowledged that the basic FR mechanism of polymeric NCPs, such as PLA systems made with 2D layered nanomaterials, is based on those materials’ barrier characteristics. Furthermore, the addition of layered nanomaterials can operate as a carbon donor (charring agent) to increase the char residue, forming an insulating char layer between the burned and unburnt structures during the combustion process [28]. For instance, it has been shown that layered double hydroxides (LDH) increase the PLA’s flame retardancy by leaving behind refractory oxide residues on the surface of the material and releasing water vapor and carbon dioxide during the polymer’s breakdown [29].
Through a 2D layered structure with a lamella blocking effect, graphene can have numerous special properties that limit oxygen access and delay the heat transfer between interfaces, thwart the escape of pyrolysis products, and mix the oxygen [30]. Again, it has been hypothesized that MMT creates a carbonaceous silicate during combustion on the surface of the polymer material. Heat and mass transport are inhibited by the carbonaceous silicate [31]. The intercalated particles under the heat source can expand perpendicular to the carbon layers in the crystal structure because expanded graphite (EG), one type of graphite intercalation compound, retards flame in its PLA systems. These structures are intumescent and can rely on thermally induced disintegration to create a char layer that separates the substrate from the heat source [32]. It has also been claimed that the dispersion and exfoliation of molybdenum disulfide (MoS2) in PLA provide a physical barrier effect that can impede the transfer of heat and the products of polymer decomposition. To limit the transmission of heat and mass during combustion, the transition metal element Mo encourages the formation of a physical barrier known as the char layer [33]. Layered materials slow down hazardous gas escape from matrices into the surface of PLA-2D NPs NCPs, reducing the amount of flammable gases in combustion zones. The “labyrinth effect” is another name for this.
Furthermore, incorporating layered nanomaterials increases the viscosity of polymer matrices, which may help slow down oxygen diffusion rates inside polymer melt and the generation of breakdown products from below. As the temperature rises during pyrolysis and combustion, the viscosity and density of the polymer melt also rise. As a result, the mass loss rate (MLR) gradually decreases, and the movement of FR particles to the flame slows down. In addition, the higher the viscosity of the polymer melts, the lower the likelihood of dripping and the greater the likelihood of forming an expanding layer of char [34].
2. 2D Flame Retarding NPs as FRs in PLA Composite Systems
Several layered NPs (2D nanomaterials), such as expandable graphite (EG) [35], MXenes [36], MMT [37], LDH [38], MoS2 [33], and others, have so far demonstrated outstanding flame-retarding properties in PLA-based polymer composite systems.
It has been suggested that the geometry of NPs, such as zero-dimensional (0D), one-dimensional (1D), 2D, and/or three-dimensional 3D, greatly affects the FR properties of polymeric matrices and their ultimate NCP(s) [39]. It has been demonstrated that fire performance increases with the order of rod-like, spherical, and plate-like geometries that qualitatively match the effective surface area of NPs in the PLA-based NCP(s). This phenomenon was claimed to be dependent on the intercalation/exfoliation (nanodispersion) of the clay (MMT), which resulted in a significant increase in the surface area that aided in the rapid migration and accumulation of platelets on the exposed sample surface before the formation of intumescent char. A thicker aluminum phosphate/MMT NCP char formed as a result, acting as a potent transport barrier and preventing intumescence [39]. Additionally, investigations into the microstructural development of the leftovers revealed remarkably uniform, hollow-fibrillar formations after PLA/aluminum diethylphosphinate (AlPi) combustion.
2.1. Nanoclays (NCs)
The most widely used and significant class of two-dimensional NPs are often NCs (amongst which a notable exception of increasing importance is one-dimensional (1D) sepiolite). Most of them are phyllosilicate minerals that are found in nature. Although boehmite (an aluminosilicate) and other clays have also been utilized in polymeric (nano)composites, MMT is by far the least expensive and most frequently employed [40]. Even compared to other nanomaterials, they compare favorably in cost-benefit ratio [41].
2D Layered mineral silicate NPs called NCs have been developed for usage in various niches. Due to NC's potential advantages, including enhanced mechanical strength, lower gas permeability, and superior flame-resistance [6], polymer-clay NCPs are a class of nanomaterials (NMTs) that researchers have widely explored. Based on their chemical makeup and NP form, NCs are classified into various classes, including MMT, halloysites, bentonite, kaolinite, and hectorite [3][42][43]. The alluring class of hybrid organic-inorganic NPs known as “organoclays” can be used in polymer NCPs as FRs, gas absorbents, drug delivery vehicles, and rheological modifiers [6]. Along with the well-known FR chemistries, it has been extensively reported that the application of NC enhances the qualities of PLA and its good FR capabilities [1][6][21][42][43][44]. The addition of organo-modified NC improves the storage modulus of PLA in both the solid and molten stages and the polymer’s biodegradability and flexural strength [6][42][43]. NCs also can reduce the cone calorimeter test’s pHRR [6]. In a typical experiment, 3% organo-modified MMT (O-MMT) and sodium MMT (Na+ MMT) loaded PLA/clay NCPs were created, and their FR properties, molecular and supramolecular characteristics were investigated using a thermogravimetric analyzer (TGA), differential scanning calorimetry (DSC), and light microscopy (LM) [45]. It was determined that the filler changed the PLA matrix’s ordering at the molecular and supramolecular levels but that it encouraged char formation, decreased flammability, and increased thermal stability instead. A related study examined the melt stability and FR characteristics of O-MMT at 5 wt.% and intumescent FR (IFR) at 15 wt.% [46]. The two systems produced outstanding flame retardancy, resulting in a UL-94 V-0 rating, an LOI of 27.5%, and improved melt dripping suppression. Similar results have been published [22] for knitted fabrics finished with PLA/clay NCP in the presence of a plasticizer, in which a significant reduction in pHRR (38%) was reached. EG and O-MMT were used in synergy to create PLA-based NCPs, and their thermal and mechanical properties were assessed [32].
The synergistic effect of SiO2, Zinc Borate (ZnB), and O-MMT NPs in PLA aimed at retarding the flammability of PLA matrix containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) has been investigated by Long et al. [47]. These researchers adopted MCD and injection molding to prepare their NCPs. The virgin PLA displayed no retardancy against fire, though upon including 10 wt.% DOPO, an improvement in the LOI from 19.1% to 27.1% was observed, along with a UL-94 rating of V-0. Additionally, with the inclusion of the O-MMT, the UL-94 rating dropped from V-0 to V-1, and the system showed melt drops during the fire test. The same was true with the NCP containing DOP, O-MMT, and ZnB. However, upon the inclusion of SiO2 in the composite system, the LOI value was 27% compared to the LOI value of 25.8% and 26.5% for the composite systems containing O-MMT and ZnB at 2 wt.% [47].
There is an interesting report on the utilization of C30B in synergy with EG to fabricate a PLA NCP with not just flame retardancy but enhanced thermal stability, mechanical performance and also enhanced co-dispersion of 2D NPs with PLA [32]. The improvement in the FR properties of the virgin polymer upon the inclusion of the NPs was attributed partly to accelerated PLA crystallization primarily governed by the added EG. Additionally, the enhanced thermal stability of the NCP system was attributed to the addition of organoclay. The presence of both 2D NPs was said to improve the mechanical performance and partially enhance thermal properties simultaneously. In this way, the authors prepared an NCP having either of the fillers and a ternary system having the synergized 2D NPs.
When C30B and EG are added to PLA, better property performance is achieved during combustion, resulting in significant reductions in sample BR compared to neat PLA. This phenomenon was postulated to be related to forming a carbonized surface layer rich in silicate or graphite, which can shield the bulk of the PLA from the heat source. These researchers also noted that PLA-EG NCPs are entirely shattered by burning and dripping, in contrast to PLA-C30B NCPs, which retain the original shape of specimens with only partial fragmentation (Figure 4a,b) [32].
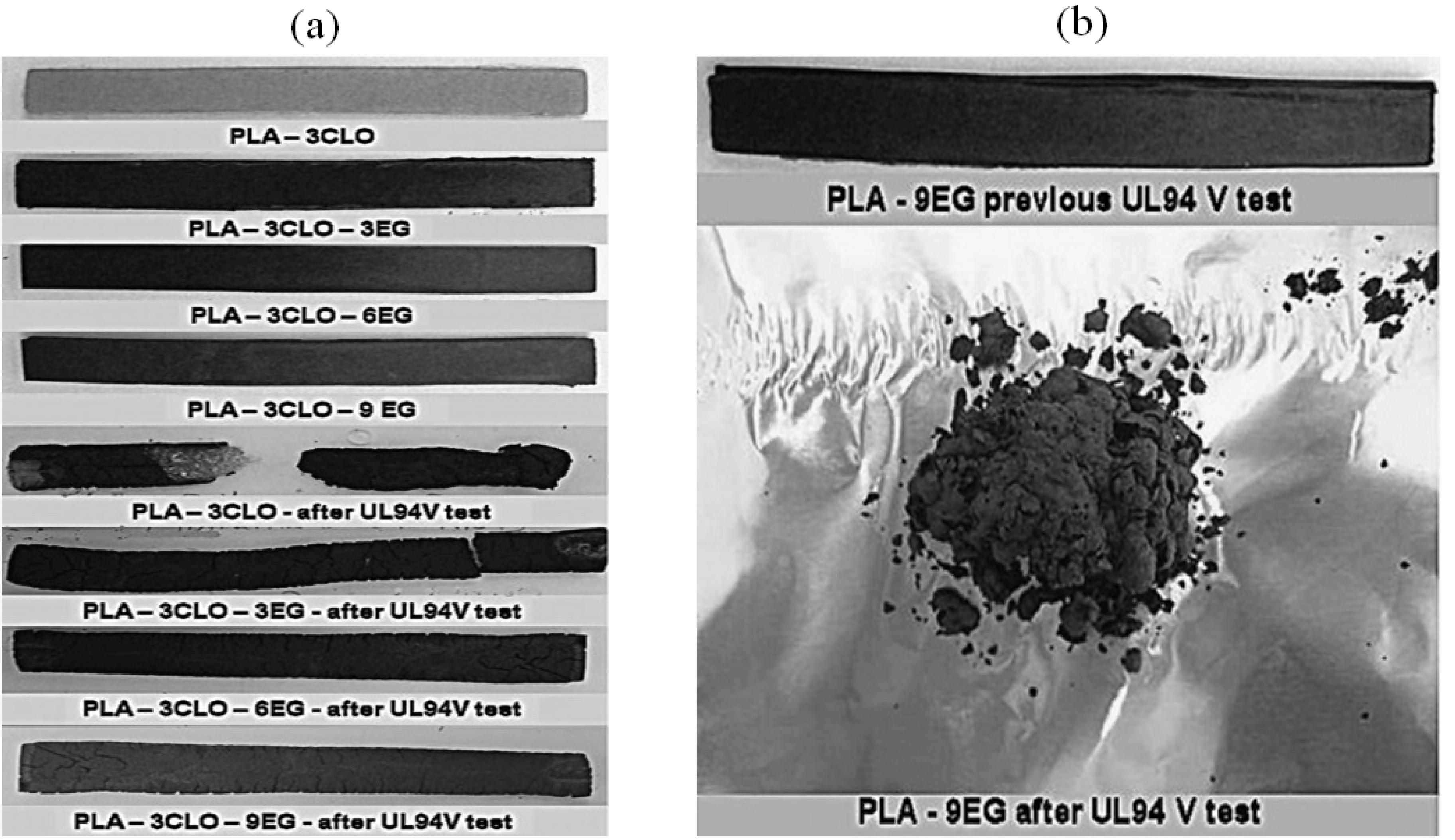
Figure 4. (a) Images of neat PLA and PLA specimens containing CLO, previous and after UL94V test and (b) Images of PLA-9EG specimen before UL94V testing and residue obtained by burning. Similar observations were obtained for PLA-3EG and PLA-6EG NCP.
Another experimental investigation conducted by Solarski and his group aimed at preparing PLA-clay NCPs for creating FR textile fibers has been explored [22]. Melt mixing was adopted in their work to process PLA and 1–10 wt.% of a particular organomodified bentonite clay (Bentone1 104-B104) in order to investigate the impact of processing variables, such as residence time, temperature, and shear on the morphology of PLA/clay NCPs. It was revealed that the dispersion of B104 occurred under various conditions without difficulty due to strong compatibility with the PLA matrix, and a comparable morphology was formed [22]. Their outcomes demonstrated that the extent of intercalation and delamination at low mixing temperatures is significantly influenced by the shear stress applied to the polymer. By adding 4 wt.% B104 to the PLA matrix during melt blending, upscale tests were carried out under improved circumstances to create NCP for the spinning process. It was then shown that, surprisingly, it is not necessary to utilize a plasticizer to spin a mixture with 4 wt.% B104 by melt spinning to obtain NCP-based multifilament yarns [22]. The yarns’ thermal, mechanical, and shrinkage properties and clay dispersion were all investigated. Even at high draw ratios, B104 may be added to PLA in amounts up to 4 wt.% without negatively affecting the tensile strength of melt-spun filaments. Interestingly, the flammability of the NCP-based multifilaments was investigated using a cone calorimeter at 35 kW/m2. The HRR experienced a significant decline of up to 46% [22].
Another study examined how O-MMT, an IFR, affected PLA’s melt stability and flame retardancy. A twin-screw extruder and a two-roll mill created the FR PLA. Then, adopting LOI, vertical burning test, TGA, scanning electronmicroscopy (SEM), melt flow index (MFI), and parallel plate rheological studies, the impact of IFR and MMT on flame retardancy and melt stability was carefully examined. The testing findings demonstrate that the IFR system, in conjunction with MMT, has good fire retardancy, as evidenced by the sample’s ability to receive a UL94 V-0 rating and an increase in the LOI value from 20.1 for unaltered PLA to 27.5 for the flame-retarded PLA. O-MMT greatly improves melt stability and reduces melt leaking, according to MFI and rheological measurements [46].
In another instance, clay particles were added to polylactide (PLA) together with a plasticizer, inorganic additives, plus O-MMT or unmodified O-MMT NPs (ethylene glycol) [45] to fabricate FR PLA NCP. Melt blending PLA with other components produced the PLA-based systems. While adding microparticles produced a microcomposite, combining PLA with O-MMT particles produced an NCP with an intercalated nanostructure. In both systems, the proportion of O-MMT was kept at 3 wt.%. The same blending conditions were also used to create unfilled PLA, plasticized PLA, and plasticized NCP. Poly(ethylene glycol) in the amount of 10% was utilized for plasticization. The melt filling of PLA with organomodified NPs results in an NCP with an intercalated nanostructure, as was demonstrated. In plasticized NCPs, the intercalated nanostructure was also generated. PEG molecules take part in or promote the intercalation process. Unaltered clay particles create a traditional microcomposite with a PLA matrix.
Contrary to neat PLA, it was discovered that thermo-mechanical processing of PLA improves its capacity to crystallize by heating up from the glassy amorphous form [45]. Because of the intercalated nanostructure in NCPs, including inorganic clay particles, it has a stronger inhibitory effect on PLA crystallization from the glassy amorphous state. Plasticized PLA and polymer matrices crystallize more easily in NCPs thanks to plasticizers. Independent of the sample composition, the heating technique used to induce crystallization from the glassy amorphous stage resulted in the same crystalline alteration of the PLA matrix. The sample composition impacted the spherulites’ sizes and perfection as they formed along with the crystallization. Spherulites are typically generated with smaller filler inclusions and with worse order. The plasticizer’s presence, particularly, is reported as a factor in developing delicate spherulitic morphology. In this case, the amorphous phase’s organization is said to change little with age; it is more plasticized in PLA, more constrained in NCPs, and stabilized by intercalated nanostructures [45]. It was demonstrated that intercalation is not destroyed by crystallization in NCP materials or plasticized NCP materials. Additionally, depending on the overall morphology and responsiveness to the sample mix were mechanical properties.
Among the most popular organic biodegradable polymers, PLA is utilized extensively. Its FR properties, however, are poor, as earlier stated: In order to overcome this drawback, a group of authors performed melt-blending of PLA with IFRs (melamine phosphate and pentaerythritol) in the presence of organically modified MMT (O-MMT) [6]. The authors carefully analyzed these produced nano-biocomposites with excellent intumescent char formation and improved FR properties. The MMTs utilized in this work were modified with tributyl hexadecyl phosphonium (O-MMT-2) and triphenyl benzyl phosphonium (O-MMT-1). According to a thermogravimetric investigation and Fourier Transform Infrared spectroscopy, these NCPs emit fewer hazardous gases during thermal decomposition than unmodified PLA. Concluding statements based on cone calorimeter data and char structure of different nanobiocomposites were supported by melt-rheological behaviors.
Additionally, the surfactant characteristics utilized to modify MMT were very important in regulating the fire properties of the composites. Compared to unmodified PLA, the fire characteristics of the NCP containing 5 wt.% O-MMT-1 were dramatically enhanced, with peak heat and total heat release rates (THRR) declining by 47% and 68%, respectively. In conclusion, developing high-performance PLA-based sustainable materials may benefit from the melt-blending of PLA, IFR, and O-MMT [6].
An innovative IFR PLA system has reportedly been used to study the effects of various organically modified MMTs (labeled DK1, DK2, and DK4) [4]. Lignin and microencapsulated ammonium polyphosphate made up of the IFR system were adopted, while appropriate characterization techniques were used to study the morphology of PLA/O-MMT NCPs. The composites FR and their thermal properties were assessed using the UL-94, LOI, and the cone calorimeter (CC). According to the findings, the sample containing DK2 had superior flame retardance supported by its lower pHRR and more excellent LOI value [4]. Sample PLA8 (including DK2) demonstrates a more outstanding increase in the flame retardancy of IFR-PLA when compared to DK1 and DK4, having the best LOI value of 35.3 and UL-94 V-0 at the loading of 2 wt.% of DK2. According to the cone calorimeter’s findings, the addition of IFR greatly reduces the HRR and THR of the IFR-PLA, and the addition of DK2 to IFR-PLA can further reduce the corresponding values of IFR-PLA above; however, the addition of DK2 and DK4 cannot have the same impact. According to the TGA data, the sample containing DK2, which has a higher char yield at high temperatures, has superior thermal stability than the samples containing DK1 and DK4. The TG-FTIR data demonstrate that all O-MMTs stimulated the breakdown of PLA and that the sample containing DK2 released fewer combustible gas products than the samples containing DK1 and DK4. The integration of DK2 could enhance the char quality and result in a considerably more compact and continuous morphology, as the study of the char residues demonstrates [4].
As per considered literature, it is well established that the phenomenon responsible for impacting flame retardancy to PLA-CL systems may be related to the formation of a carbonized surface layer rich in silicate, which can shield the bulk of the PLA from the heat source and the barricading of the volatiles within the composite thereby retarding degradation caused by oxidation. Additionally, considering the inexpensive nature of CLs NPs, their commercialization is achievable.
2.2. MXene
Through an etching process of titanium aluminum carbon (Ti3AlC2) followed by liquid exfoliation, a novel 2D nanomaterial known as titanium carbide (Ti3C2Tx) was identified for the first time in 2011 [48][49][50]. Ti3C2Tx nanosheets have a wide range of potential uses in the fields of sensors, water treatment, catalysis, energy storage, electromagnetic interference shielding, and so on because of their inherently high metal conductivity, adjustable surface active sites, and notable mechanical stability [50]. Additionally, because of its low thermal conductivity and exceptional lamella thermal stability, Ti3C2Tx can have a lamella barrier effect during the pyrolysis and combustion of polymeric materials [48]. As a result, Ti3C2Tx has been considered a viable polymer FR additive for polymer matrices, such as PLA [48].
As a potential nontoxic functional ingredient for creating FR polymer composites, a novel 2D transition metal carbide called MXene (Ti3C2) has recently received much attention [36]. Mxene limits the emission of flammable volatiles while preventing further burning of the underlying polymer composite thanks to its layered flake shape, which creates a physical barrier effect. The Ti component of Mxene has been discovered to exert a catalytic attenuation effect on most polymers, unlike most traditional FRs and other 2D nanomaterials, leading to a considerable decrease in composites’ heat and smoke release [36].
Zhou et al. [36] reportedly synthesized a PLA/DOPO-Ti3C2 NCP system having excellent property performance as a FR material. The fabricated FR presented a pHRR reduction of 33.7%, a V-0 UL-94 rating owing to the DOPO- Ti 3 C 2 ability to interplay the catalytic, barrier, and condensed effects within the PLA system.
Cone calorimetry, the UL-94 test, and the LOI were used to demonstrate their findings on PLA composites’ thermal and burning capabilities. The authors also looked at the tensile and UV-shielding capabilities. According to the findings in the UL-94 test, PLA/ Ti 3 C 2 /DOPO (3 wt.%) demonstrated a V-0 rating. The large decrease in pHRR (33.7%), total heat release (47%) and peak CO output (58.8%), as well as the improvement in fire safety, were all indicators of this (41.7%). The interaction of catalytic, barrier, and condensed effects of the Ti3C2/DOPO nanosheets in the PLA matrix, as seen in Figure 5, was the cause of the composites’ increased fire-safety performance [36].
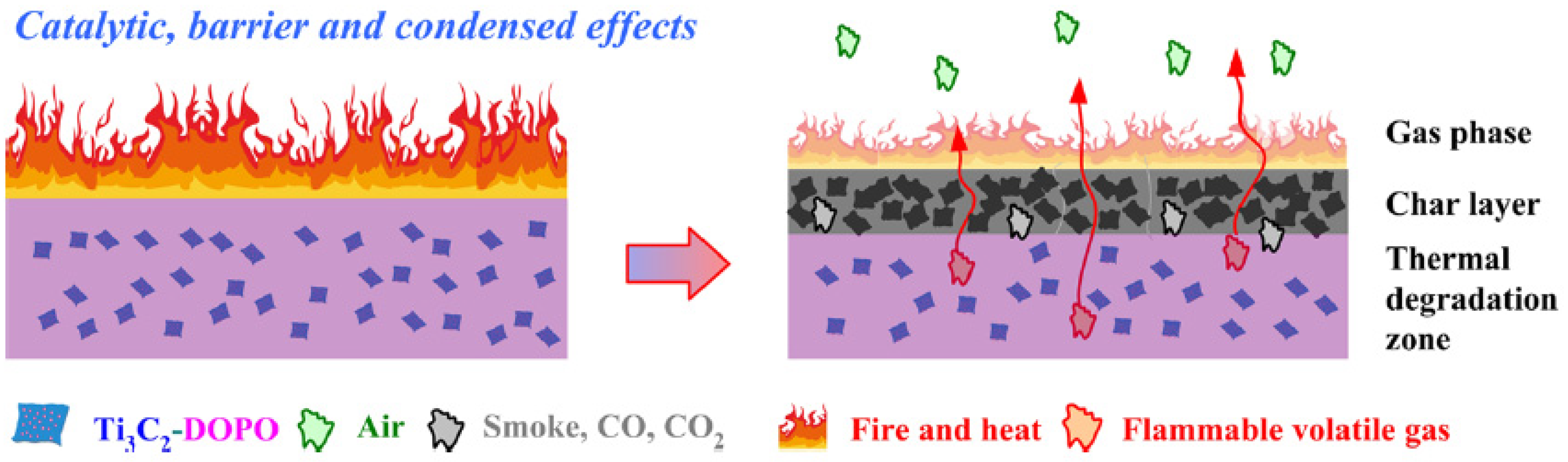
Figure 5. Proposed mechanism of FR.
In this interesting example where Mxene was adopted as reinforcing 2D NPs in the PLA system, the tensile strength of PLA/Ti3C2-DOPO increased by about 9%, and it scored “Excellent” (UPF 50+) for UV protection [36]. This study presents a novel chemical modification technique for 2D Mxene flakes to create multifunctional PLA composites, which show promise as candidates for the next wave of sustainable and protective plastic products [36].
There is an existing instance where Shi and his group [48] pointed out that Mxene (Ti3C2Tx) can be effectively used as FR in its pristine state, and it modified form Ti3C2Tx (benzyldimethylhexadecylammonium chloride (HDBAC)-Ti3C2Tx. They prepared Mxene (Ti3C2Tx) via the etching of Ti3AlC2 using LiF/HCl at a temperature of 35 °C for 48 h, followed by washing with deionized H2O to neutral via centrifugation. The prepared Mxene was then dried and ready for modification with HDBAC through a facile solution mixing under a nitrogen environment for 2h, washed and dried for inclusion in the PLA matrix, as depicted in Figure 6a.
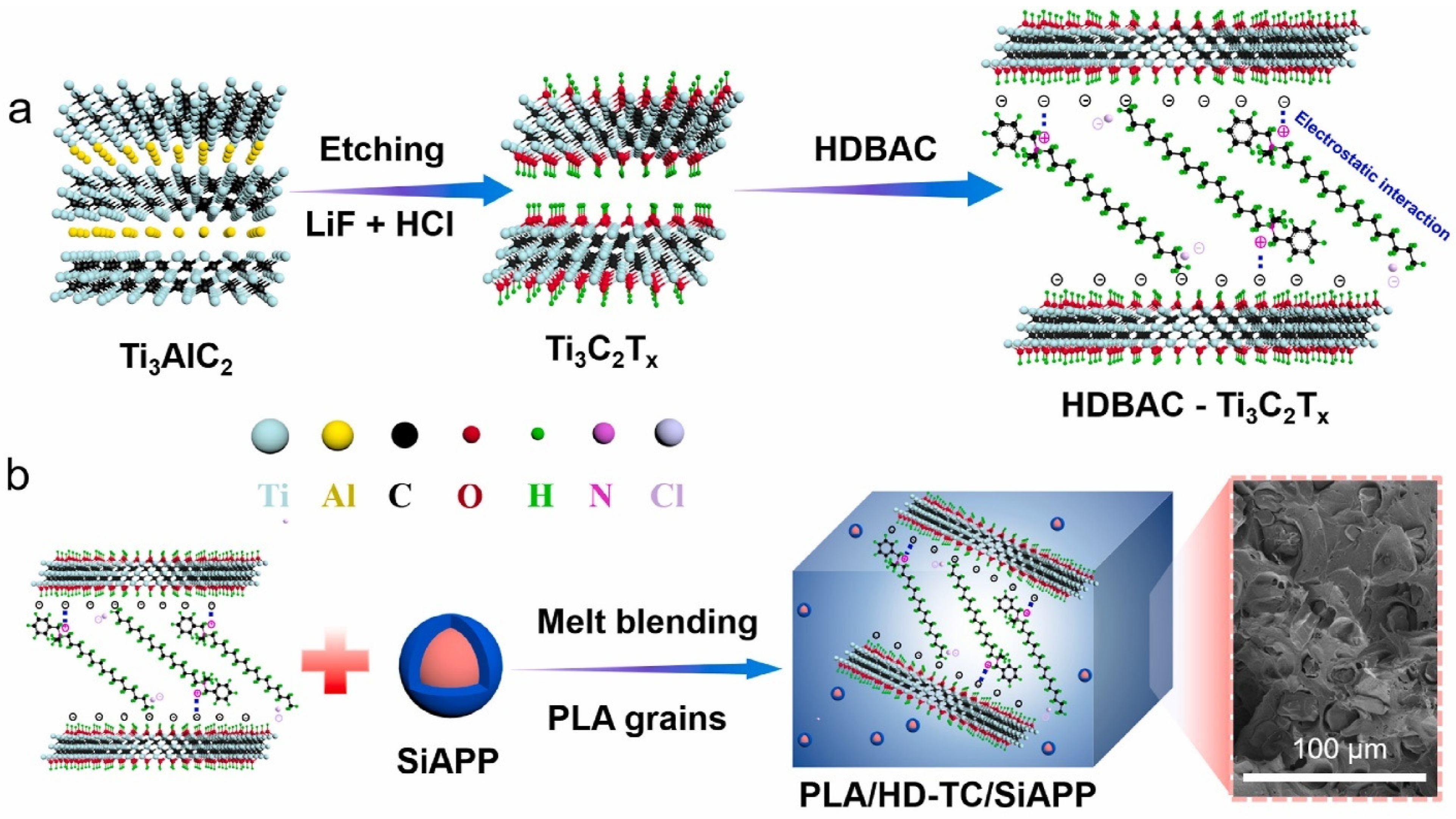
Figure 6. Schematic diagrams for HDBAC-Ti3C2Tx (a) and PLA/HD-TC/SiAPP composites (b).
According to Figure 6b, melt blending was used to process PLA composites. The formulations for the PLA/HDBAC-Ti3C2Tx(HD-TC)/SiAPP composites were made by adding 0.2, 0.5, 1.0, and 2.0 wt.% of HDBAC-Ti3C2Tx and 15.0, 14.8, 14.5, 14.0, and 13.0 wt.% of SiAPP, respectively. Raw PLA was first vacuum-dried at 80 °C for 12 h before processing. The samples were then created using an internal mixer at 180 °C with a 50 r/min rotation speed. First, pure PLA was poured into the internal mixer. The modified Ti3C2Tx and SiAPP were introduced into the mixer for 20 min to obtain an excellent visible dispersion after melting the PLA. The sample was heated under 10 Mpa at 180 °C for additional analysis [48]. Their prepared composite/hybrid system presented the best results for their formulation: PLA/2.0HD-TC/13.0SiAPP, which contained 2 wt.% Mxene and 13 wt.% SiPPA: the LOI was enhanced from 24.4% to 33.3% with a UL-94 rating of V-0, a pHRR reduction of 49.8% along with T5%(°C) of 327 (residual wt.% of 10.33 at 700 °C) [48]. They investigated the residual char from the NCP in order to ascertain the FR mechanism of the PLA/Mxene/SiAPP: the formation of dense continuous intumescent C-layer was said to separate the thermal decomposition zone and hinder the transfer of heat into the underlying NCP. Additionally, the addition of HDBAC-Ti3C2Tx enhanced the char formation, thereby hindering the spread of the fire, as depicted in Figure 7 [48].
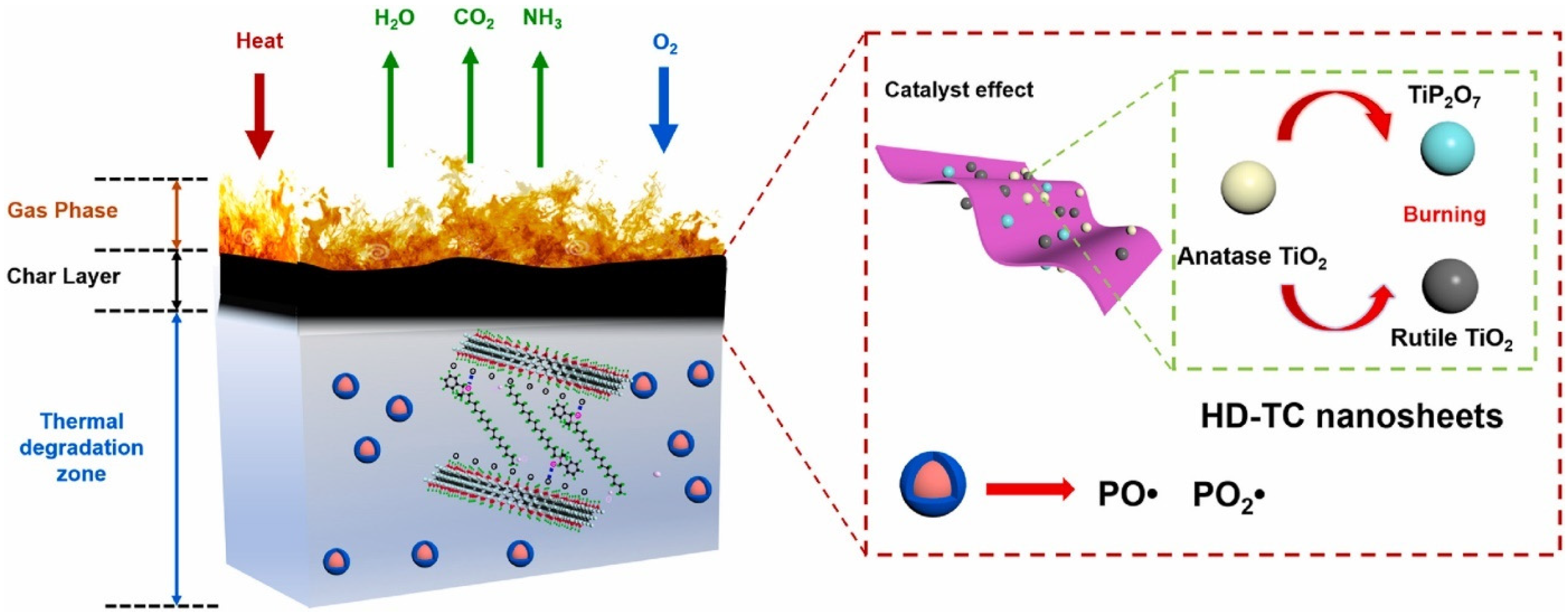
Figure 7. Schematic illustration for the proposed flame-retardant mechanism.
In their study, Huang et al. [51] prepared PLA/IFR composites with Ti3C2 Mxene nanosheets using melt blending, and they looked at the synergistic impacts of Mxene on the fire performance of PLA/IFR systems by replacing some of the IFR with Mxene. The incorporation of a small amount of Mxene might significantly improve the flame retardancy of PLA/IFR composites, according to their LOI, UL-94, and CCT results. The addition of 1.0 wt.% Mxene and 11.0 wt.% IFR resulted in a V-0 UL-94 rating, an apparent rise in LOI (160.4%), and a clear decrease in pHRR (64.6%). Intumescent char formation capability of PLA/IFR systems could be effectively improved by the presence of nano TiO2 catalyst and 2D nanosheet barrier effect of Mxene (as per Figure 8), thereby preventing the further flame spread and the spread of fire hazard, according to CCTs analysis, SEM, photographs, laser Raman spectroscopy (LRS), XRD, and X-ray photoelectron spectroscopy (XPS) of carbon residues of PLA/IFR/Mxene composites. It was claimed that the use of 2D Mxene in this research was successful as FR additive-based nanomaterials with self-charring and self-catalyzing functions as a high-performance synergist for various kinds of IFR polymeric matrix [51].
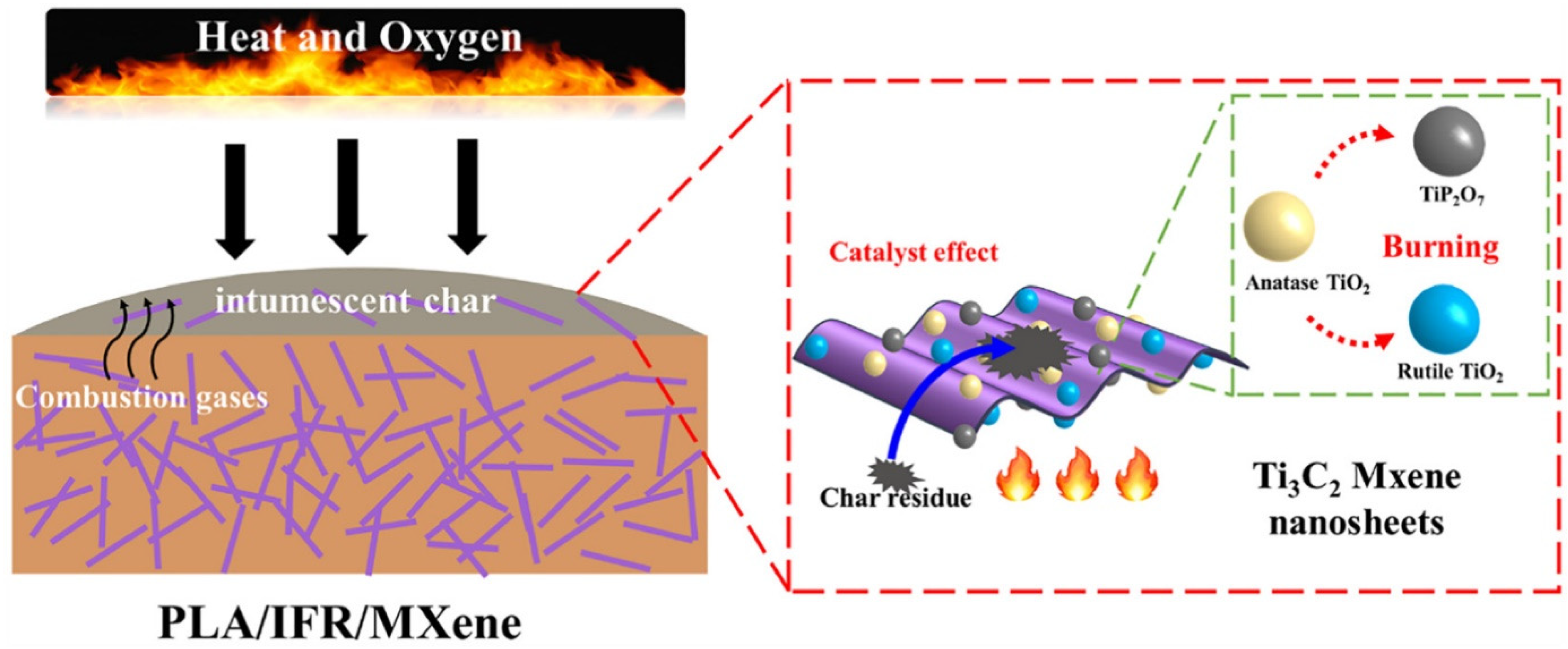
Figure 8. Schematic illustration for the synergistic flame retarded mechanism of PLA/IFR/Mxene NCPs.
In summary, the 2D nanosheet barrier effect of Mxene is hypothesized to significantly increase the ability of PLA/IFR systems to create intumescent char, hence halting further flame development and the spread of fire hazards. Mxene’s layered flake form, which produces a physical barrier effect, controls the emission of combustible volatiles while inhibiting additional burning of the underlying polymer mixture.
References
- Tawiah, B.; Yu, B.; Fei, B. Advances in Flame Retardant Poly(Lactic Acid). Polymers 2018, 10, 876.
- SpecialChem. Bio-Based Polymers Market to Grow at a CAGR of 14% by 2027. Available online: https://omnexus.specialchem.com/news/industry-news/bio-based-polymer-market-2027-000230145 (accessed on 19 June 2023).
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silicate nanocomposites. 2. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer 2003, 44, 857–866.
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. The effect of different organic modified montmorillonites (OMMTs) on the thermal properties and flammability of PLA/MCAPP/lignin systems. J. Appl. Polym. Sci. 2013, 127, 4967–4973.
- Tong, X.-Z.; Song, F.; Li, M.-Q.; Wang, X.-L.; Chin, I.-J.; Wang, Y.-Z. Fabrication of graphene/polylactide nanocomposites with improved properties. Compos. Sci. Technol. 2013, 88, 33–38.
- Malkappa, K.; Bandyopadhyay, J.; Ray, S.S. Thermal Degradation Characteristic and Flame Retardancy of Polylactide-Based Nanobiocomposites. Molecules 2018, 23, 2648.
- Cao, Y.; Feng, J.; Wu, P. Polypropylene-grafted graphene oxide sheets as multifunctional compatibilizers for polyolefin-based polymer blends. J. Mater. Chem. 2012, 22, 14997–15005.
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649.
- Bioplastics, E. Continued Growth: Global Production Capacities of Bioplastics 2022–2027. Available online: https://docs.european-bioplastics.org/publications/market_data/2022/Report_Bioplastics_Market_Data_2022_short_version.pdf (accessed on 19 June 2023).
- Liu, P.; Zheng, Z.; Xu, Q.; Qian, Z.; Liu, J.; Ouyang, J. Valorization of dairy waste for enhanced D-lactic acid production at low cost. Process Biochem. 2018, 71, 18–22.
- Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Klupsaite, D. Application of acid tolerant Pedioccocus strains for increasing the sustainability of lactic acid production from cheese whey. LWT-Food Sci. Technol. 2016, 72, 399–406.
- Nguyen, C.M.; Kim, J.-S.; Nguyen, T.N.; Kim, S.K.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.-C. Production of L-and D-lactic acid from waste Curcuma longa biomass through simultaneous saccharification and cofermentation. Bioresour. Technol. 2013, 146, 35–43.
- De la Torre, I.; Ladero, M.; Santos, V. Production of d-lactic acid by Lactobacillus delbrueckii ssp. delbrueckii from orange peel waste: Techno-economical assessment of nitrogen sources. Appl. Microbiol. Biotechnol. 2018, 102, 10511–10521.
- Pleissner, D.; Neu, A.-K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173.
- Alves de Oliveira, R.; Schneider, R.; Hoss Lunelli, B.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. A simple biorefinery concept to produce 2G-lactic acid from sugar beet pulp (SBP): A high-value target approach to valorize a waste stream. Molecules 2020, 25, 2113.
- Helmes, R.J.; López-Contreras, A.M.; Benoit, M.; Abreu, H.; Maguire, J.; Moejes, F.; van den Burg, S.W. Environmental impacts of experimental production of lactic acid for bioplastics from Ulva spp. Sustainability 2018, 10, 2462.
- Ögmundarson, Ó.; Sukumara, S.; Laurent, A.; Fantke, P. Environmental hotspots of lactic acid production systems. GCB Bioenergy 2020, 12, 19–38.
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794.
- Storti, G.; Lattuada, M. Synthesis of bioresorbable polymers for medical applications. In Bioresorbable Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 153–179.
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259.
- Selatile, M.K.; Ojijo, V.; Sadiku, R.; Ray, S.S. Development of bacterial-resistant electrospun polylactide membrane for air filtration application: Effects of reduction methods and their loadings. Polym. Degrad. Stab. 2020, 178, 109205.
- Solarski, S.; Ferreira, M.; Devaux, E.; Fontaine, G.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Da Silva Ferreira, A. Designing polylactide/clay nanocomposites for textile applications: Effect of processing conditions, spinning, and characterization. J. Appl. Polym. Sci. 2008, 109, 841–851.
- Ghosh, A.; Orasugh, J.T.; Ray, S.S.; Chattopadhyay, D. Integration of 3D Printing–Coelectrospinning: Concept Shifting in Biomedical Applications. ACS Omega 2023, 8, 28002–28025.
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater Sci. 2020, 114, 100687.
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125.
- Macskásy, H.; Palyi, G. Plastics: Their Behaviour in Fires; Elsevier: Amsterdam, The Netherlands, 2012.
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire Retard. Mater. 2001, 1, 31–68.
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105.
- Shan, X.; Hu, Y.; Chen, H.; Yuan, X. Study on thermal property, flame retardancy and mechanism of poly (lactic acid)/intumescent flame retardant/NiAl layer double hydroxide nanocomposites. Sci. Adv. Mater. 2015, 7, 1848–1857.
- Zhang, M.; Shi, X.; Dai, X.; Huo, C.; Xie, J.; Li, X.; Wang, X. Improving the crystallization and fire resistance of poly (lactic acid) with nano-ZIF-8@ GO. J. Mater. Sci. 2018, 53, 7083–7093.
- Chow, W.S.; Teoh, E.L. Flexible and flame resistant poly(lactic acid)/organomontmorillonite nanocomposites. J. Appl. Polym. Sci. 2015, 132, 41253.
- Fukushima, K.; Murariu, M.; Camino, G.; Dubois, P. Effect of expanded graphite/layered-silicate clay on thermal, mechanical and fire retardant properties of poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1063–1076.
- Homa, P.; Wenelska, K.; Mijowska, E. Enhanced thermal properties of poly (lactic acid)/MoS2/carbon nanotubes composites. Sci. Rep. 2020, 10, 740.
- Lewin, M.; Zhang, J.; Pearce, E.; Zammarano, M. Polyamide 6 treated with pentabromobenzyl acrylate and layered silicates. Polym. Adv. Technol. 2010, 21, 825–834.
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Lu, H.; Hu, Y. Cobalt oxide/graphene composite for highly efficient CO oxidation and its application in reducing the fire hazards of aliphatic polyesters. J. Mater. Chem. 2012, 22, 3426–3431.
- Zhou, Y.; Lin, Y.; Tawiah, B.; Sun, J.; Yuen, R.K.K.; Fei, B. DOPO-Decorated Two-Dimensional MXene Nanosheets for Flame-Retardant, Ultraviolet-Protective, and Reinforced Polylactide Composites. ACS Appl. Mater. Interfaces 2021, 13, 21876–21887.
- Ye, L.; Ren, J.; Cai, S.-y.; Wang, Z.-g.; Li, J.-b. Poly(lactic acid) nanocomposites with improved flame retardancy and impact strength by combining of phosphinates and organoclay. Chin. J. Polym. Sci. 2016, 34, 785–796.
- Wang, D.-Y.; Leuteritz, A.; Wang, Y.-Z.; Wagenknecht, U.; Heinrich, G. Preparation and burning behaviors of flame retarding biodegradable poly(lactic acid) nanocomposite based on zinc aluminum layered double hydroxide. Polym. Degrad. Stab. 2010, 95, 2474–2480.
- Isitman, N.A.; Dogan, M.; Bayramli, E.; Kaynak, C. The role of nanoparticle geometry in flame retardancy of polylactide nanocomposites containing aluminium phosphinate. Polym. Degrad. Stab. 2012, 97, 1285–1296.
- Fu, Z.; Wang, H.; Zhao, X.; Li, X.; Gu, X.; Li, Y. Flame-retarding nanoparticles as the compatibilizers for immiscible polymer blends: Simultaneously enhanced mechanical performance and flame retardancy. J. Mater. Chem. A 2019, 7, 4903–4912.
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958.
- Sinha, R.S. Clay-Containing Polymer Nanocomposites: From Fundamentals to Real Applications; Newnes: Newton, MA, USA, 2013.
- Solarski, S.; Mahjoubi, F.; Ferreira, M.; Devaux, E.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Da silva Ferreira, A.; et al. (Plasticized) Polylactide/clay nanocomposite textile: Thermal, mechanical, shrinkage and fire properties. J. Mater. Sci. 2007, 42, 5105–5117.
- Urbanczyk, L.; Ngoundjo, F.; Alexandre, M.; Jérôme, C.; Detrembleur, C.; Calberg, C. Synthesis of polylactide/clay nanocomposites by in situ intercalative polymerization in supercritical carbon dioxide. Eur. Polym. J. 2009, 45, 643–648.
- Pluta, M. Morphology and properties of polylactide modified by thermal treatment, filling with layered silicates and plasticization. Polymer 2004, 45, 8239–8251.
- Li, S.; Yuan, H.; Yu, T.; Yuan, W.; Ren, J. Flame-retardancy and anti-dripping effects of intumescent flame retardant incorporating montmorillonite on poly (lactic acid). Polym. Adv. Technol. 2009, 20, 1114–1120.
- Long, L.; Yin, J.; He, W.; Xiang, Y.; Qin, S.; Yu, J. Synergistic effect of different nanoparticles on flame retardant poly(lactic acid) with bridged DOPO derivative. Polym. Compos. 2019, 40, 1043–1052.
- Shi, Y.; Wang, Z.; Liu, C.; Wang, H.; Guo, J.; Fu, L.; Feng, Y.; Wang, L.; Yang, F.; Liu, M. Engineering titanium carbide ultra-thin nanosheets for enhanced fire safety of intumescent flame retardant polylactic acid. Compos. Part B Eng. 2022, 236, 109792.
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253.
- Orasugh, J.T.; Temane, L.T.; Ray, S.S. Application of MXenes in Water Purification, CO2 Capture and Conversion. In Two-Dimensional Materials for Environmental Applications; Kumar, N., Gusain, R., Sinha Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 17–74.
- Huang, H.; Dong, D.; Li, W.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Synergistic effect of MXene on the flame retardancy and thermal degradation of intumescent flame retardant biodegradable poly (lactic acid) composites. Chin. J. Chem. Eng. 2020, 28, 1981–1993.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
802
Revisions:
2 times
(View History)
Update Date:
13 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





