
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alice Vilela | -- | 2610 | 2023-09-12 12:03:59 | | | |
| 2 | Alice Vilela | -66 word(s) | 2544 | 2023-09-12 12:34:07 | | | | |
| 3 | Peter Tang | Meta information modification | 2544 | 2023-09-13 04:01:56 | | |
Video Upload Options
A relevant trend in winemaking is to reduce the use of chemical compounds in both the vineyard and winery. In organic productions, synthetic chemical fertilizers, pesticides, and genetically modified organisms must be avoided, aiming to achieve the production of a “safer wine.” Safety represents a significant threat all over the world, being one of the most important goals to be completed in both Western society and developing countries. An occurrence in wine safety results in the recovery of a broad variety of harmful compounds for human health such as amines, carbamate, and mycotoxins. The perceived increase in sensory complexity and superiority of successful uninoculated wine fermentations, as well as a thrust from consumers looking for a more “natural” or “organic” wine produced with fewer additives and perceived health attributes, has led to more investigations into the use of non-Saccharomyces yeasts in winemaking, namely in organic wines.
1. General Introduction
2. Wine Contamination by Ochratoxin A (OTA) and Other Mycotoxins
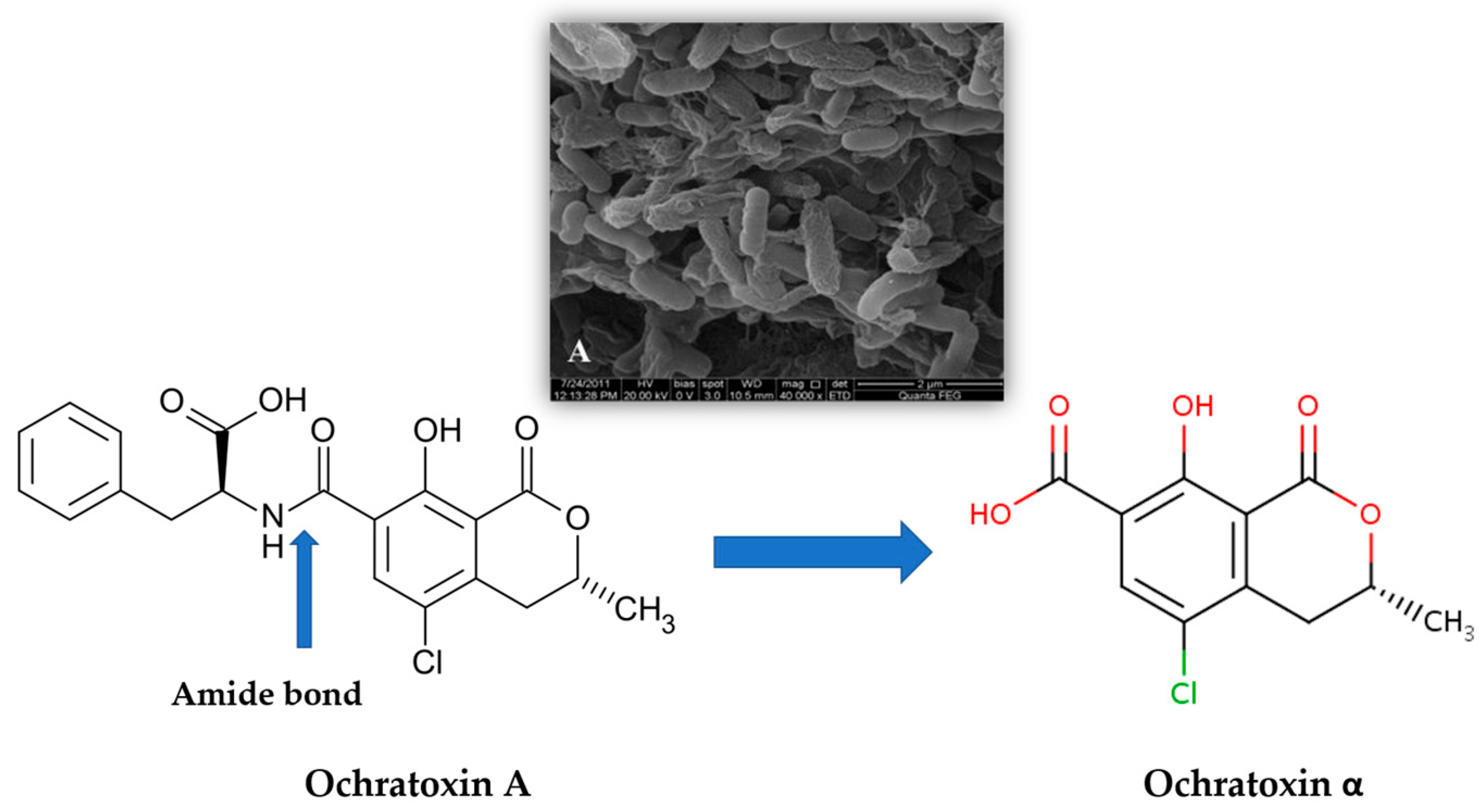
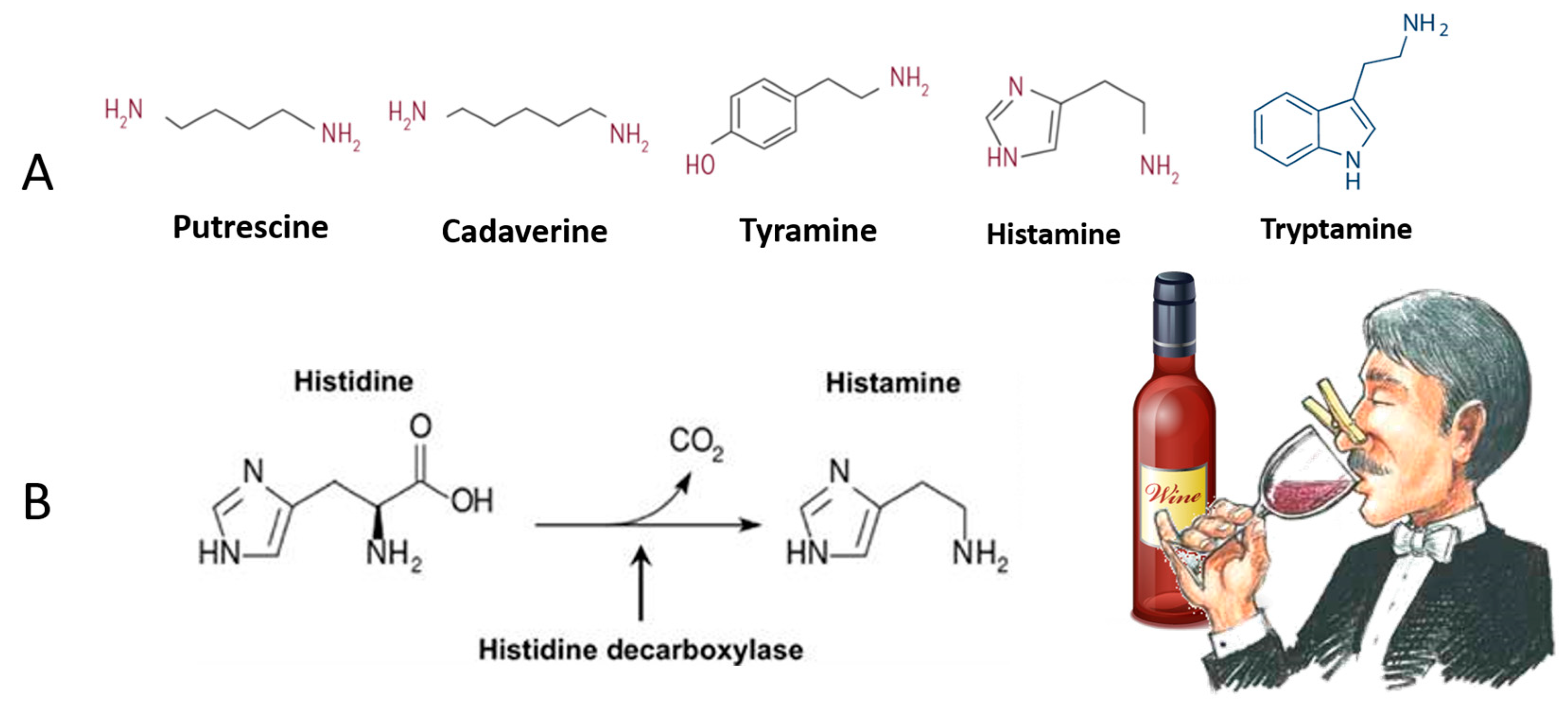
3. Organic Wines Contamination with Biogenic Amines
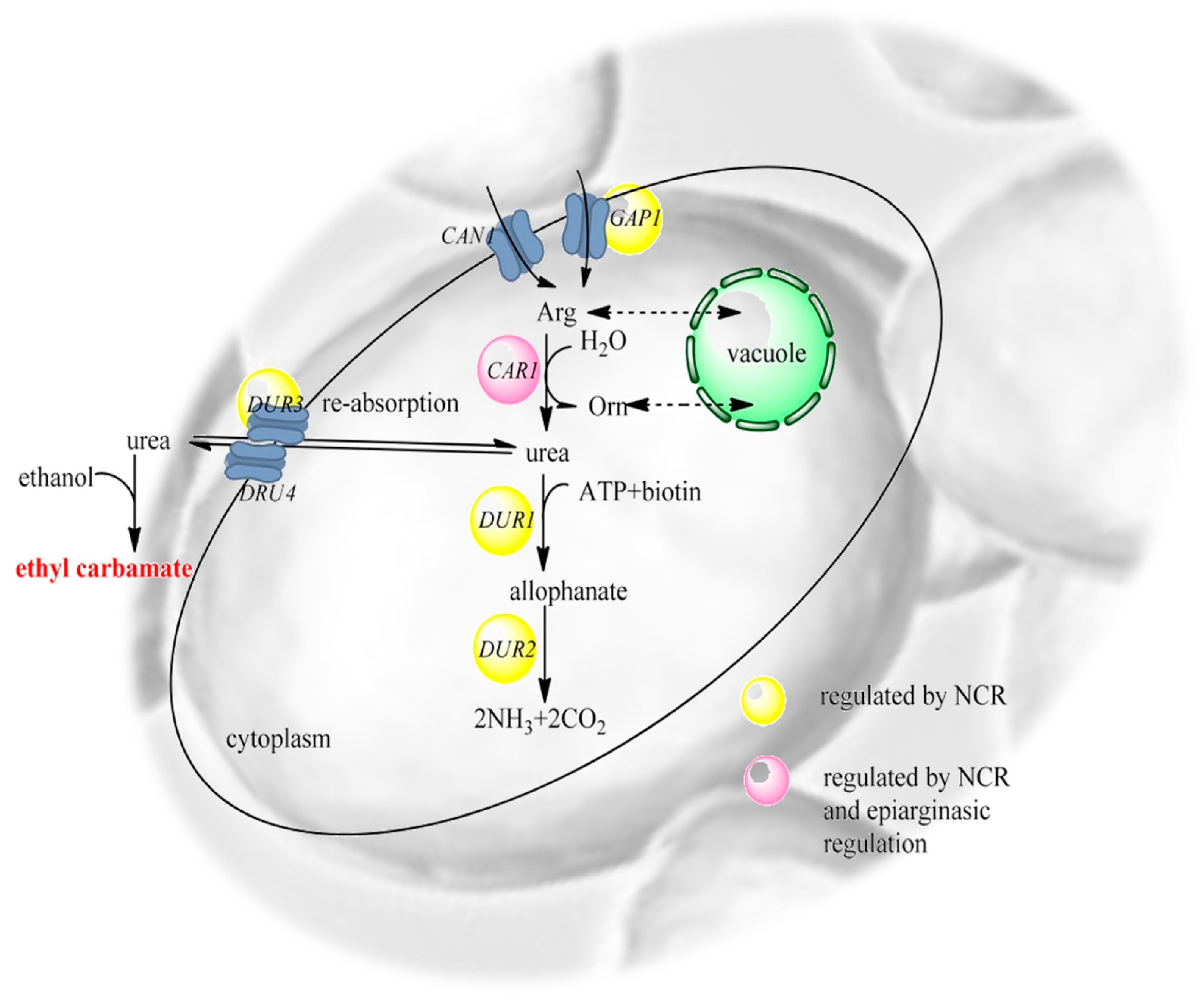
4. Wines and Ethyl Carbamate Contamination
References
- IFOAM. Basic Standards for Organic Production and Processing. Bonn-Germany. 2014. Available online: http://www.ifoam.orghttps://www.ifoam.bio/sites/default/files/ifoam_norms_july_2014_t.pdf (accessed on 15 April 2020).
- Trioli, G.; Hofmann, U. ORWINE: Code of good organic viticulture and winemaking. In ECOVIN-Federal Association of Organic Wine-Producer; Ecovin: Oppenheim, Germany, 2009.
- IFAOM. EU Rules for Organic Wine Production: Background, Evaluation, and Further Sector Development. 2013. Available online: https://orgprints.org/29867/1/ifoameu_reg_wine_dossier_201307.pdf (accessed on 17 April 2020).
- Commission Implementing Regulation (EU) No 203/2012 of 8 March 2012 Amending Regulation (EC) No 889/2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007, as Regards Detailed Rules on Organic Wine. OJ L 71. 9 March 2012, pp. 42–47. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012R0203 (accessed on 10 May 2020).
- Schäufele, I.; Hamm, U. Consumers’ perceptions, preferences, and willingness-to-pay for wine with sustainability characteristics: A review. J. Clean. Prod. 2017, 147, 379–394.
- Cravero, M.C. Organic, and biodynamic wines quality and characteristics: A review. Food Chem. 2019, 295, 334–340.
- Tofalo, R.; Schirone, M.; Telera, G.C.; Manetta, A.C.; Corsetti, A.; Suzzi, G. Influence of organic viticulture on non-Saccharomyces wine yeast populations. Ann. Microbiol. 2011, 61, 57–66.
- Russo, P.; Capozzi, V.; Spano, G.; Corbo, M.R.; Sinigaglia, M.; Antonio, B. Metabolites of Microbial Origin with an Impact on Health: Ochratoxin A and Biogenic Amines. Front. Microbiol. 2016, 7, 482.
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32.
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237.
- Maurizio, C.; Francesca, C.; Ilaria, M.; Paola, D. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133.
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46.
- Vilela, A. Modulating Wine Pleasantness Throughout Wine-Yeast Co-Inoculation or Sequential Inoculation. Fermentation 2020, 6, 22.
- Walker, R. Risk assessment of ochratoxin: Current views of the European Scientific Committee on Food, the JECFA and the Codex Committee on Food Additives and Contaminants. Adv. Exp. Med. Biol. 2002, 504, 249–255.
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133.
- Barreira, M.J.; Alvito, P.C.; Almeida, C.M. Occurrence of patulin in apple-based foods in Portugal. Food Chem. 2010, 121, 653–658.
- Peraica, M.; Radic, B.; Lucicć, P.; Pavlovic, M. Toxic effects of mycotoxins in humans. Int. J. Public Health 1999, 77, 754–766.
- Ferenczi, S.; Cserháti, M.; Krifaton, C.; Szoboszlay, S.; Kukolya, J.; Szőke, Z.; Kovács, K.J. A New Ochratoxin A Biodegradation Strategy Using Cupriavidus basilensis Őr16 Strain. PLoS ONE 2014, 9, e109817.
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Rattray, F. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, 95–100.
- Petruzzi, L.; Sinigaglia, M.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A. Decontamination of Ochratoxin A by yeasts: Possible approaches and factor leading to toxin removal in wine. Appl. Microbiol. Biotechnol. 2014, 98, 6555–6567.
- Battilani, P.; Giorni, P.; Bertuzzi, T.; Formenti, S.; Pietri, A. Black Aspergilli and Ochratoxin A in grapes in Italy. Int. J. Food Microbiol. 2006, 111, S53–S60.
- Bellver Soto, J.; Fernández-Franzón, M.; Ruiz, M.J.; García, A.J. Presence of Ochratoxin A (OTA) mycotoxin in alcoholic drinks from southern European countries: Wine and beer. J. Agric. Food Chem. 2014, 62, 7643–7651.
- IARC. Mycotoxins and Human Health (Chapter 6). 2020; pp. 87–104. Available online: file:///C:/Users/avimo/Downloads/IARC_SP158_Chapter%206.pdf (accessed on 17 April 2020).
- EFSA (European Food Safety Authority). Opinion of the scientific panel on contaminants in the food chain on a request. Commission related to Ochratoxin A in food. EFSA J. 2006, 365, 1–56.
- Quintela, S.; Villarán, M.C.; Armentia, I.L.; Elejalde, E. Ochratoxin A removal in wine: A review. Food Control 2013, 30, 439–445.
- Pitout, M.J. The hydrolysis of ochratoxin A by some proteolytic enzymes. Biochem. Pharmacol. 1969, 18, 485–491.
- Abrunhosa, L.; Inês, A.; Rodrigues, A.I.; Guimarães, A.; Pereira, V.L.; Paropt, P.; Venâncio, A. Biodegradation of Ochratoxin A by Pediococcus parvulus isolated from Douro wines. Int. J. Food Microbiol. 2014, 188, 45–52.
- De Bellis, P.; Tristezza, M.; Haidukowski, M.; Fanelli, F.; Sisto, A.; Mulè, G.; Grieco, F. Biodegradation of Ochratoxin A by Bacterial Strains Isolated from Vineyard Soils. Toxins (Basel) 2015, 7, 5079–5093.
- Friman, H.; Schechter, A.; Ioffe, Y.; Nitzan, Y.; Cahan, R. Electricity formation in a microbial fuel cell. Microb. Biotechnol. 2013, 6, 425–434.
- Caridi, A.; Cufari, A.; Lovino, R.; Palumbo, R.; Tedesco, I. Influence of yeast on polyphenol composition of wine. Food Technol. Biotechnol. 2004, 42, 37–40.
- Petruzzi, L.; Baiano, A.; De Gianni, A.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Differential Adsorption of Ochratoxin A and Anthocyanins by Inactivated Yeasts and Yeast Cell Walls during Simulation of Wine Aging. Toxins (Basel) 2015, 7, 4350–4365.
- Bevilacqua, A.; Petruzzi, L.; Corbo, M.R.; Baiano, A.; Garofalo, C.; Sinigaglia, M. Ochratoxin A released back into the medium by Saccharomyces cerevisiae as a function of the strain, washing medium, and fermentative conditions. J. Sci. Food Agric. 2014, 94, 3291–3295.
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881.
- Palacios, S.; Vasserot, Y.; Maujean, A. Evidence for sulfur volatile products adsorption by yeast lees. Am. J. Enol. Vitic. 1997, 48, 525–526.
- Alexandre, H.; Lubbers, S.; Charpentier, C. Interactions between toxic fatty acids for yeasts and colloids, cellulose and yeast ghost using the equilibrium dialysis method in a model wine system. Food Biotechnol. 1997, 11, 89–99.
- Navarro, S.; Barba, A.; Oliva, J.; Navarro, G.; Pardo, F. Evolution of residual levels of six pesticides during elaboration of red wines. Effect of wine-making procedures in their disappearance. J. Agric. Food Chem. 1999, 47, 264–270.
- Pradelles, R.; Chassagne, D.; Vichi, S.; Gougeon, R.; Alexandre, H. (−) Geosmin sorption by enological yeasts in model wine and FTIR spectroscopy characterization of the sorbent. Food Chem. 2010, 120, 531–538.
- Palomero, F.; Ntanos, K.; Morata, A.; Benito, S.; Suárez-Lepe, J.A. Reduction of wine 4-ethylphenol concentration using lyophilized yeast as a bioadsorbent: Influence on anthocyanin content and chromatic variables. Eur. Food Res. Technol. 2011, 232, 971–977.
- Anwar, M.I.; Muhammad, F.; Awais, M.M.; Akhtar, M. A review of β-glucans as a growth promoter and antibiotic alternative against enteric pathogens in poultry. World’s Poult. Sci. J. 2017, 73, 651–661.
- Wolken, W.A.M.; Lucas, P.M.; Lonvaud-Funel, A.; Lolkema, J.S. The mechanism of the tyrosine transporter TyrP supports a proton motive tyrosine decarboxylation pathway in Lactobacillus brevis. J. Bacteriol. 2006, 188, 2198–2206.
- Smit, A.Y.; du Toit, W.J.; du Toit, M. Biogenic amines in wine: Understanding the headache. South Afr. J. Enol. Vitic. 2008, 29, 109–127.
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Review article. Int. J. Food Microbiol. 2017, 243, 16–27.
- Guo, Y.-Y.; Yang, Y.-P.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532.
- Martuscelli, M.; Mastrocola, D. Biogenic Amines: A Claim for Wines, Biogenic Amines, Charalampos Proestos; IntechOpen: London, UK, 2018.
- Beneduce, L.; Romano, A.; Capozzi, V.; Lucas, P.; Barnavon, L.; Bach, B.; Spano, G. Biogenic amines in regional wines. Ann. Microbiol. 2010, 60, 573–578.
- Ancín-Azpilicueta, C.; González-Marco, A.; Jiménez-Moreno, N. Current knowledge about the presence of amines in wine. Crit. Rev. Food Sci. Nutr. 2008, 48, 257–275.
- Tassoni, A.; Tango, N.; Ferri, M. Comparison of biogenic amine and polyphenol profiles of grape berries and wines obtained following conventional, organic and biodynamic agricultural and oenological practices. Food Chem. 2013, 139, 405–413.
- Thibon, C.; Marullo, P.; Claisse, O.; Cullin, C.; Dubourdieu, D.; Tominaga, T. Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. Fems Yeast Res. 2008, 8, 1076–1086.
- Thorgeirsson, U.P.; Dalgard, D.W.; Reeves, J.; Adamson, R.H. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul. Toxicol. Pharm. 1994, 19, 130–151.
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl Carbamate in Fermented Beverages: Presence, Analytical Chemistry, Formation Mechanism, and Mitigation Proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626.
- Salmon, A.G.; Zeise, L. Risks of Carcinogenesis from Urethane Exposure; CRC Press: Boca Raton, FL, USA, 1991; p. 115.
- Beland, F.A.; Benson, R.W.; Mellick, P.W.; Kovatch, R.M.; Roberts, D.W.; Fang, J.-L.; Doerge, D.R. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 2005, 43, 1–19.
- Dahabieh, M.; Husnik, J.; Van Vuuren, H. Functional enhancement of sake yeast strains to minimize the production of ethyl carbamate in sake wine. J. Appl. Microbiol. 2010, 109, 963–973.
- Arena, M.; Saguir, F.; Manca de Nadra, M. Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int. J. Food Microbiol. 1999, 52, 155–161.
- Polychroniadou, E.; Kanellaki, M.; Iconomopoulou, M.; Koutinas, A.; Marchant, R.; Banat, I. Grape and apple wines volatile fermentation products and possible relation to spoilage. Bioresour. Technol. 2003, 87, 337–339.
- Zhao, X.; Zou, H.; Fu, J.; Chen, J.; Zhou, J.; Du, G. Nitrogen regulation involved in the accumulation of urea in Saccharomyces cerevisiae. Yeast 2013, 30, 437–447.
- Fidaleo, M.; Esti, M.; Moresi, M. Assessment of urea degradation rate in model wine solutions by acid urease from Lactobacillus fermentum. J. Agric. Food Chem. 2006, 54, 6226–6235.
- Benito, S. The Management of Compounds that Influence Human Health in Modern Winemaking from an HACCP Point of View. Fermentation 2019, 5, 33.
- Andrich, L.; Esti, M.; Moresi, M. Urea removal in model wine solutions by immobilized acid urease in a stirred bioreactor. Chem. Eng. Trans. 2009, 17, 915–920.
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367.




