Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jayakumar Rangasamy | -- | 1146 | 2023-09-08 15:33:38 | | | |
| 2 | Jessie Wu | Meta information modification | 1146 | 2023-09-11 04:35:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Murugaiyan, K.; Amirthalingam, S.; Hwang, N.S.; Jayakumar, R. FGF-18 in Bone Regeneration. Encyclopedia. Available online: https://encyclopedia.pub/entry/48970 (accessed on 04 March 2026).
Murugaiyan K, Amirthalingam S, Hwang NS, Jayakumar R. FGF-18 in Bone Regeneration. Encyclopedia. Available at: https://encyclopedia.pub/entry/48970. Accessed March 04, 2026.
Murugaiyan, Kavipriya, Sivashanmugam Amirthalingam, Nathaniel Suk-Yeon Hwang, Rangasamy Jayakumar. "FGF-18 in Bone Regeneration" Encyclopedia, https://encyclopedia.pub/entry/48970 (accessed March 04, 2026).
Murugaiyan, K., Amirthalingam, S., Hwang, N.S., & Jayakumar, R. (2023, September 08). FGF-18 in Bone Regeneration. In Encyclopedia. https://encyclopedia.pub/entry/48970
Murugaiyan, Kavipriya, et al. "FGF-18 in Bone Regeneration." Encyclopedia. Web. 08 September, 2023.
Copy Citation
The fibroblast growth factor family contains 22 members. Fibroblast growth factors such as 2, 9, and 18 are mainly associated with the differentiation of osteoblasts and in bone regeneration. FGF-18 stimulates the PI3K/ERK pathway and smad1/5/8 pathway mediated via BMP-2 by blocking its antagonist, which is essential for bone formation.
bone regeneration
fibroblasticgrowth factors
gene
1. Introduction
Bone is a metabolically active organ comprising both organic and inorganic components with a plethora of functions to maintain homeostasis [1]. Bone loss happens in hereditary, deficiency, and pathological conditions as well as in trauma [2]; in these conditions, replacement certainly becomes vital for the survival of the patient. Autografts are regarded as the gold standard technique in the case of bone regeneration due to their aspects such as no risk of immunogenicity and their immediate availability from the donor, but the replacement of the donor site affects its morbidity, and restricted amounts of available graft make us reconsider the option. Allograft is an alternative option and is also greatly considered due to its ready availability and lack of requirement for a donor site. However, again it requires appropriate storage and sterilization techniques and carries a risk of immunogenicity [3]. Keeping in mind the complexity of the grafts and the structure, function, and composition of the bone itself, innovations are being conducted with new materials and methods to bio-mimic natural bone tissue.
Tissue engineering is an integrative field in which biological tissues are engineered accordingly to repair, replace, or regenerate lost tissues. This field uses a variety of materials ranging from natural to synthetic ones, and methods use various technologies to achieve its purpose [4]. The three important triads of tissue engineering are scaffolds, biological/mechanical cues, and cells [5]. Scaffolds are being developed to mimic the natural environment by binding certain bioactive molecules, while drugs are also being used to achieve this purpose [6]. Growth factors act either in an autocrine or in a paracrine manner and influence the cells around the scaffolds [7]. However, the problem arises when researchers expect regeneration for larger defects. Hence, synthetic bone grafts have been widely used in clinical scenarios due to their favorable properties to augment such large defects and stimulate bone regeneration [8][9]. Synthetic grafts can also be made osteoconductive/osteoinductive by the appropriate selection of graft materials [10]. The incorporation of fibroblastic growth factors (FGFs) has enhanced the performance of these synthetic grafts [11][12]. Fibroblastic growth factors play an important role in many physiological processes (i.e., inflammation, angiogenesis, etc.); one among these is skeletal development [2][8][13][14][15], where continuous bone remodeling happens in the presence of osteoblasts and osteoclasts ultimately necessitating the inevitable involvement of fibroblastic growth factors [16]. In the FGF family (Figure 1), FGF-2, -9, and -18 plays a significant role in bone regeneration [17]. Though in previous studies, more emphasis was laid on FGF-2, researchers here highlight the potential of FGF-18 when incorporated with hydrogel and scaffolds showing implicit bone regeneration.
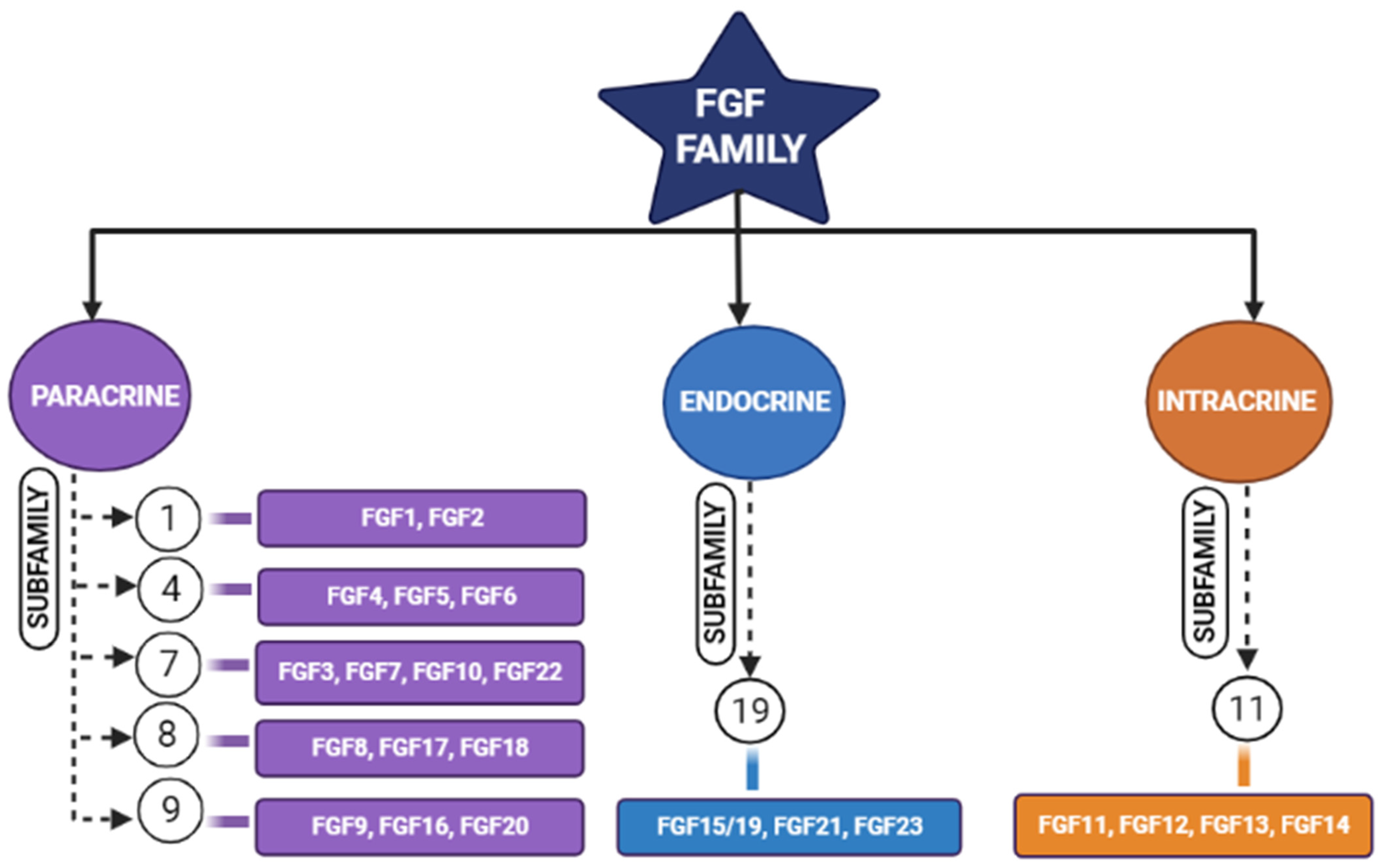
Figure 1. Schematic diagram representing different members of the fibroblastic growth factors (FGF) family and their subfamilies [17].
2. FGF-18 in Regeneration of Bone
FGF-18 was first reported in 1998 and was initially known for its activity in soft tissue proliferation, such as in the liver and intestine. The purified FGF-18 from mice contains 207 amino acids. FGF-18 is a glycoprotein with the first 26 amino acids forming a signal peptide sequence [18]. FGF-18 belongs to the paracrine FGF-8 subfamily. It exerts its action through FGFR-1, -2, and -3 (Figure 2) [19][20][21]. The gene which encodes for this protein is located in the fifth chromosome (5q35.1) [21][22]. The biological function of this growth factor is mainly through the proliferation of cells such as osteoblasts, chondrocytes, and osteoclasts [23].
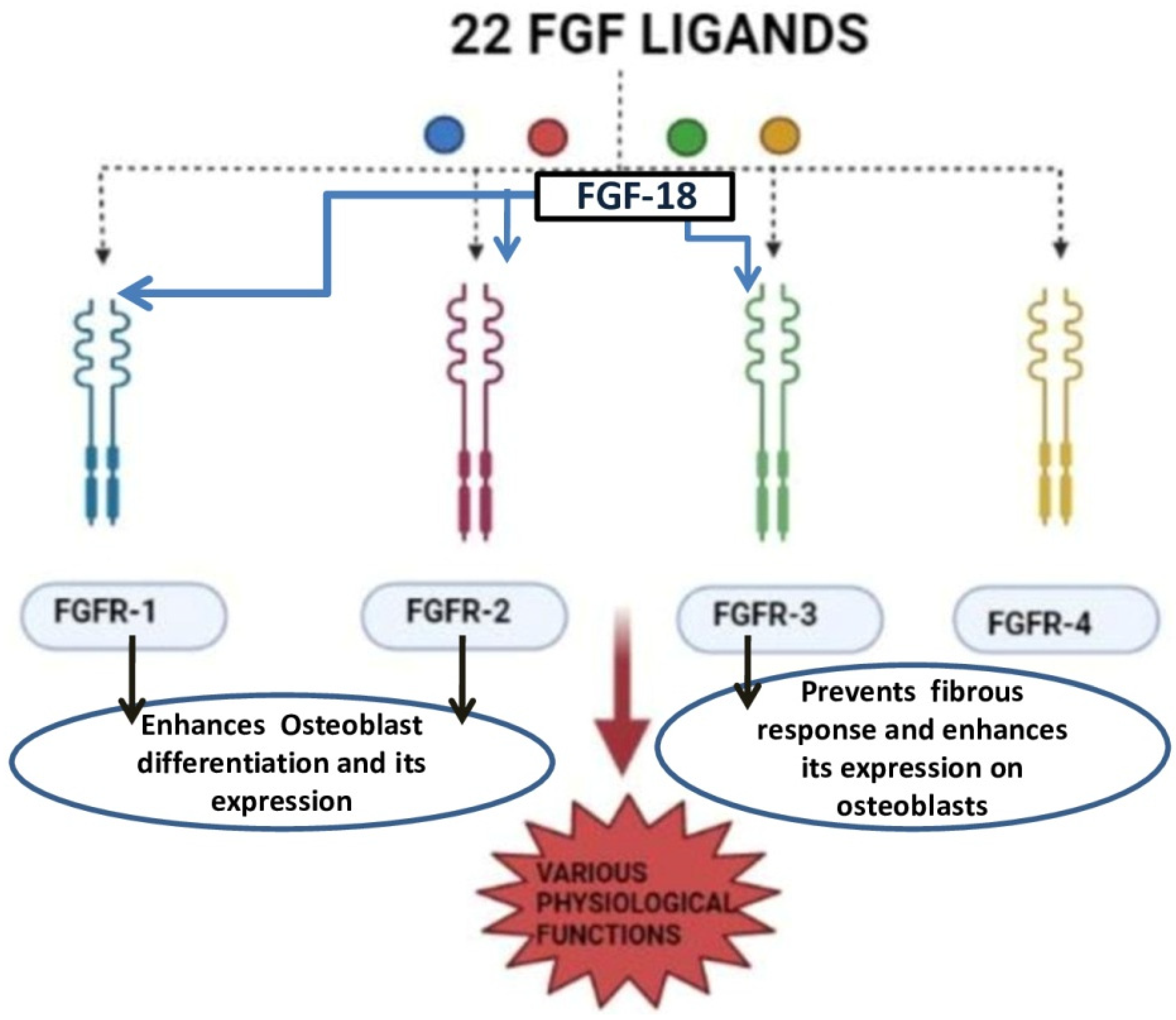
Liu et al. demonstrated that FGF-18 acts as a ligand for FGFR-3 and mice lacking FGF-18 and showed delayed ossification and the reduction in osteogenic gene expression [14]. Ohbayashi et al. proved that FGF-18 is required for skeletal development by using an FGF-18−/− deficient mouse model, which showed the delayed closure of sutures in calvarial bone [23]. Shimoka et al. also showed that FGF-18 may compensate for the role of FGF-2 on bone and cartilage since it is as equally potent as the latter [24]. FGF-18, along with other factors, regulates gene expression during skeletal development [25]. Recently, FGF-18 was immunostained in a developing fetal spine showing its importance in cellular proliferation and bone formation [26]. Recombinant FGF-18 improved the osseointegration of implants and prevented peri-implant fibrous response in FGFR-3−/− mice [20]. Behr et al. showed that decreased FGF-18 affected the expression of RUNX2 and osteocalcin in FGF-18+/− mice [27]. They also observed that the haploinsufficiency could not be compensated with other FGF ligands and bone morphogenic proteins (BMP)-2 in FGF-18+/− mice. Wan et al. proved that the suppression of noggin-stimulated osteogenesis in vitro accelerated the same in vivo [28]. FGF-18-suppressed noggin is a BMP antagonist which positively augments the action of the bone morphogenic protein towards osteogenic differentiation [29]. BMP-2 is a potential osteoinductive growth factor [30], and FGF-18 further helps it in enhancing this property by inhibiting noggin [29].
In a study conducted by Hamidouche et al., the fact that FGF-18 promotes osteoblastic differentiation was experimentally confirmed by checking for osteoblastic markers, such as RUNX-2, ALP, and COL1A1 in C3H10T1/2, after treating them with the recombinant fibroblastic growth factor (rh FGF)-18. This was further corroborated by checking for alkaline phosphate (ALP) activity and in vitro matrix mineralization in the same cells. The authors displayed that FGF-18 enhanced osteogenic differentiation through the activation of the Extracellular signal-regulated kinase 1/2(ERK1/2) and Phosphatidylinositol 3-kinase (PI3K) pathway acting through FGFR-1 and 2 [19]. Fujioka-Kobayashi et al. studied the effect of different FGFs-8, 9, 17 and 18 on MC3T3-E1 cells, which are immature murine osteoblastic cells from mice, where FGF-18 alone with BMP-2 was sufficient to show significant osteogenic potential [31]. Behr et al. looked at the bone regeneration potential of FGFs-2, 9, and 18 in the cranial bone defects of adult mice. The results showed that FGF-18 was able to show higher healing potential compared to other growth factors (FGF-2 and 9). After the injury of the cranial defects, FGF-18+/− mice showed no RUNX-2 expression, even though the weak expression was observed in fibroblastic growth factor (FGF)-9+/− mice [32]. Jeon et al. showed the early osteogenic differentiation of FGF-18 on rat bone marrow-derived stem cells (BMSCs) by upregulating genes such as RUNX-2, Col1, and BMP-4 [33]. Nagayama et al. demonstrated that FGF-18 not only promotes osteogenic differentiation through BMP-2 but also supports the expression of FGFR-1, 2, and 3 in osteoblasts in the fetal coronal suture of mice [21]. A summary of the aforementioned pathways is given here (see Figure 3).
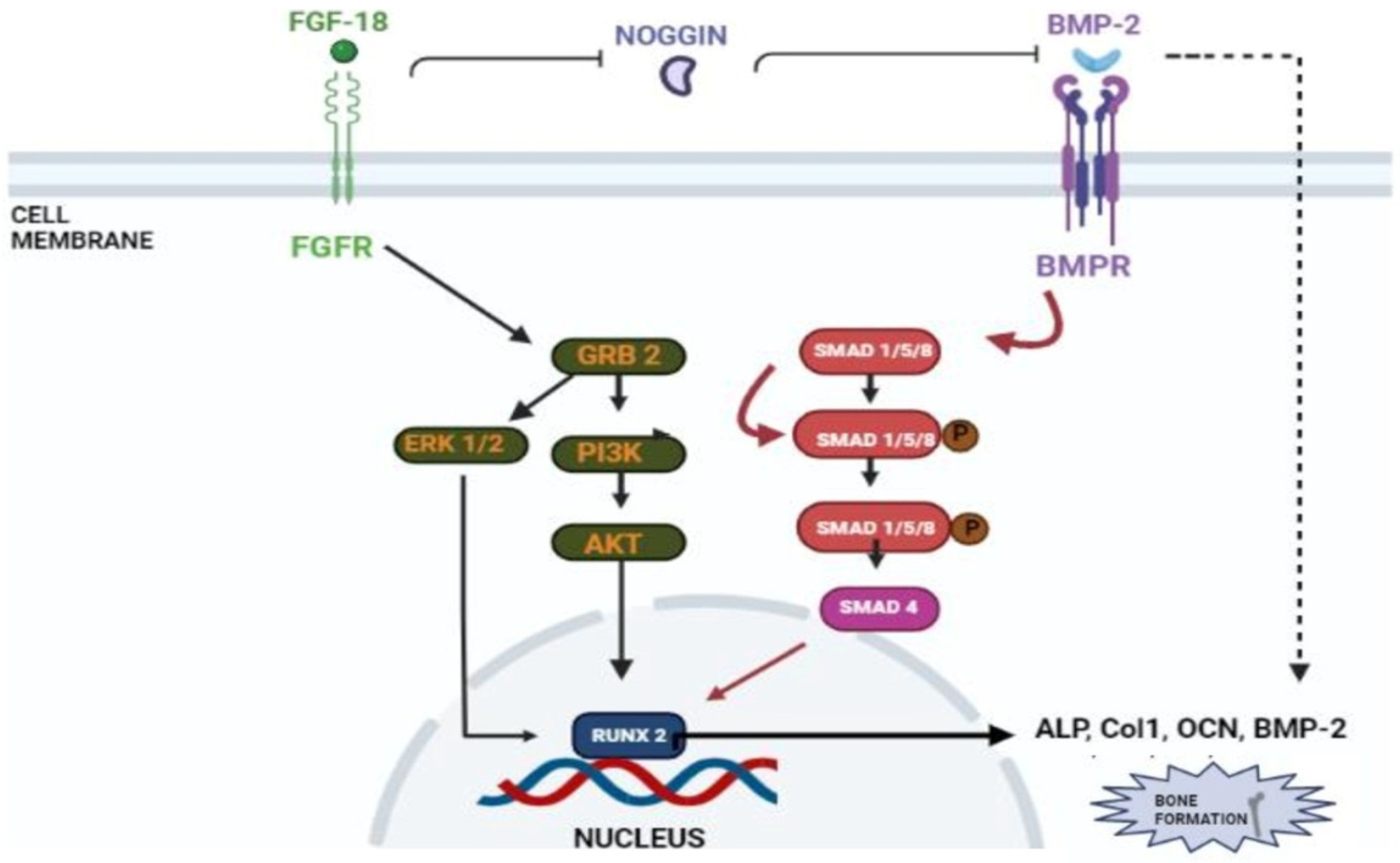
Figure 3. Schematic representation of FGF-18’s role in osteogenic differentiation. FGF-18 inhibits noggin which is a BMP-2 antagonist [27]. BMP activates the smad 1/5/8 pathway which activates early osteoblastic markers such as ALP, Col1, OCN and RUNX-2 [34][35]. FGF-18 themselves activate PI3K-ERK pathway which also activates the RUNX-2 gene [33] which is an important marker for osteogenic differentiation.
References
- Florencio-Silva, R.; da Sasso, G.R.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed. Res. Int. 2015, 2015, 421746.
- Barnes, G.L.; Kostenuik, P.J.; Gerstenfeld, L.C.; Einhorn, T.A. Growth Factor Regulation of Fracture Repair. J. Bone Miner. Res. 1999, 14, 1805–1815.
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213.
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40.
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 13, 467–479.
- Dang, M. Biomimetic Delivery of Signals for Bone Tissue Engineering. Bone Res. 2018, 6, 25.
- Yun, Y.-R.; Jang, J.H.; Jeon, E.; Kang, W.; Lee, S.; Won, J.-E.; Kim, H.W.; Wall, I. Administration of Growth Factors for Bone Regeneration. Regen. Med. 2012, 7, 369–385.
- Canalis, E.; McCarthy, T.L.; Centrella, M. Growth Factors and Cytokines in Bone Cell Metabolism. Ann. Rev. Med. 1991, 42, 17–24.
- Tumedei, M.; Savadori, P.; Del Fabbro, M. Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4221.
- Fillingham, Y.; Jacobs, J. Bone Grafts and Their Substitutes. Bone Jt. J. 2016, 98-B (Suppl. A), 6–9.
- Antonova, L.; Kutikhin, A.; Sevostianova, V.; Velikanova, E.; Matveeva, V.; Glushkova, T.; Mironov, A.; Krivkina, E.; Shabaev, A.; Senokosova, E.; et al. BFGF and SDF-1α Improve In Vivo Performance of VEGF-Incorporating Small-Diameter Vascular Grafts. Pharmaceuticals 2021, 14, 302.
- Lai, W.-Y.; Chen, Y.-J.; Lee, A.K.-X.; Lin, Y.-H.; Liu, Y.-W.; Shie, M.-Y. Therapeutic Effects of the Addition of Fibroblast Growth Factor-2 to Biodegradable Gelatin/Magnesium-Doped Calcium Silicate Hybrid 3D-Printed Scaffold with Enhanced Osteogenic Capabilities for Critical Bone Defect Restoration. Biomedicines 2021, 9, 712.
- Takei, Y.; Minamizaki, T.; Yoshiko, Y. Functional Diversity of Fibroblast Growth Factors in Bone Formation. Int. J. Endocrinol. 2015, 2015, 1–12.
- Liu, Z.; Xu, J.; Colvin, J.S.; Ornitz, D.M. Coordination of Chondrogenesis and Osteogenesis by Fibroblast Growth Factor 18. Genes Dev. 2002, 16, 859–869.
- Dailey, L.; Ambrosetti, D.; Mansukhani, A.; Basilico, C. Mechanisms Underlying Differential Responses to FGF Signaling. Cytokine Growth Factor Rev. 2005, 16, 233–247.
- Zhai, F.; Song, N.; Ma, J.; Gong, W.; Tian, H.; Li, X.; Jiang, C.; Wang, H. Fgf18 Inhibits Mc3t3-E1 Cell Osteogenic Differentiation Via the Erk Signaling Pathway. Mol. Med. Rep. 2017, 16, 4127–4132.
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of Fibroblast Growth Factors in Bone Regeneration. Inflamm. Regen. 2017, 37, 10.
- Hu, M.C.-T.; Qiu, W.R.; Wang, Y.; Hill, D.; Ring, B.D.; Scully, S.; Bolon, B.; DeRose, M.; Luethy, R.; Simonet, W.S.; et al. FGF-18, a Novel Member of the Fibroblast Growth Factor Family, Stimulates Hepatic and Intestinal Proliferation. Mol. Cell. Biol. 1998, 18, 6063–6074.
- Hamidouche, Z.; Fromigué, O.; Nuber, U.; Vaudin, P.; Pages, J.-C.; Ebert, R.; Jakob, F.; Miraoui, H.; Marie, P.J. Autocrine Fibroblast Growth Factor 18 Mediates Dexamethasone-Induced Osteogenic Differentiation of Murine Mesenchymal Stem Cells. J. Cell. Physiol. 2010, 224, 509–515.
- Carli, A.; Gao, C.; Khayyat-Kholghi, M.; Li, A.; Wang, H.; Ladel, C.; Harvey, E.; Henderson, J. FGF18 Augments Osseointegration of Intra-Medullary Implants in Osteopenic FGFR3-/- Mice. Eur. Cell. Mater. 2012, 24, 107–117.
- Nagayama, T.; Okuhara, S.; Ota, M.S.; Tachikawa, N.; Kasugai, S.; Iseki, S. FGF18 Accelerates Osteoblast Differentiation by Upregulating Bmp2 Expression: Acceleration of Osteogenesis by FGF18. Congenit. Anom. 2013, 53, 83–88.
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 1, 218142.
- Ohbayashi, N.; Shibayama, M.; Kurotaki, Y.; Imanishi, M.; Fujimori, T.; Itoh, N.; Takada, S. FGF18 Is Required for Normal Cell Proliferation and Differentiation during Osteogenesis and Chondrogenesis. Genes Dev. 2002, 16, 870–879.
- Shimoaka, T.; Ogasawara, T.; Yonamine, A.; Chikazu, D.; Kawano, H.; Nakamura, K.; Itoh, N.; Kawaguchi, H. Regulation of Osteoblast, Chondrocyte, and Osteoclast Functions by Fibroblast Growth Factor (Fgf)-18 in Comparison with Fgf-2 and Fgf-10. J. Biol. Chem. 2002, 277, 7493–7500.
- Marie, P.J. Fibroblast Growth Factor Signaling Controlling Osteoblast Differentiation. Gene 2003, 316, 23–32.
- Shu, C.; Smith, S.S.; Little, C.B.; Melrose, J. Comparative Immunolocalisation of Perlecan, Heparan Sulphate, Fibroblast Growth Factor-18, and Fibroblast Growth Factor Receptor-3 and Their Prospective Roles in Chondrogenic and Osteogenic Development of the Human Foetal Spine. Eur. Spine J. 2013, 22, 1774–1784.
- Behr, B.; Sorkin, M.; Manu, A.; Lehnhardt, M.; Longaker, M.T.; Quarto, N. Fgf-18 Is Required for Osteogenesis But Not Angiogenesis During Long Bone Repair. Tissue Eng. Part A 2011, 17, 2061–2069.
- Wan, D.C.; Pomerantz, J.H.; Brunet, L.J.; Kim, J.-B.; Chou, Y.-F.; Wu, B.M.; Harland, R.; Blau, H.M.; Longaker, M.T. Noggin Suppression Enhances in Vitro Osteogenesis and Accelerates in Vivo Bone Formation. J. Biol. Chem. 2007, 282, 26450–26459.
- Reinhold, M.I.; Abe, M.; Kapadia, R.M.; Liao, Z.; Naski, M.C. Fgf18 Represses Noggin Expression and Is Induced by Calcineurin. J. Biol. Chem. 2004, 279, 38209–38219.
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone Morphogenetic Proteins: A Powerful Osteoinductive Compound with Non-Negligible Side Effects and Limitations: Bone Morphogenetic Proteins in Bone Healing. BioFactors 2014, 40, 459–481.
- Fujioka-Kobayashi, M.; Ota, M.S.; Shimoda, A.; Nakahama, K.; Akiyoshi, K.; Miyamoto, Y.; Iseki, S. Cholesteryl Group- and Acryloyl Group-Bearing Pullulan Nanogel to Deliver BMP2 and FGF18 for Bone Tissue Engineering. Biomaterials 2012, 33, 7613–7620.
- Behr, B.; Panetta, N.J.; Longaker, M.T.; Quarto, N. Different Endogenous Threshold Levels of Fibroblast Growth Factor-Ligands Determine the Healing Potential of Frontal and Parietal Bones. Bone 2010, 47, 281–294.
- Jeon, E.; Yun, Y.-R.; Kang, W.; Lee, S.; Koh, Y.-H.; Kim, H.-W.; Suh, C.K.; Jang, J.-H. Investigating the Role of FGF18 in the Cultivation and Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2012, 7, e43982.
- Kokabu, S.; Katagiri, T.; Yoda, T.; Rosen, V. Role of Smad Phosphatases in BMP-Smad Signaling Axis-Induced Osteoblast Differentiation. J. Oral Biosci. 2012, 54, 73–78.
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP Signaling and Other Molecular Events: Regulation of Osteoblastogenesis and Bone Formation. Bone Res. 2015, 3, 15005.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
617
Revisions:
2 times
(View History)
Update Date:
11 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





