
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laurent Dufossé | -- | 4097 | 2023-09-07 09:24:44 | | | |
| 2 | Sanjay K Singh | -1 word(s) | 4096 | 2023-09-07 10:20:48 | | | | |
| 3 | Lindsay Dong | Meta information modification | 4096 | 2023-09-08 11:14:32 | | |
Video Upload Options
Synthetic dyes are generally unsafe for human health or the environment, leading to the continuous search and growing demand for natural pigments that are considered safer, biodegrade more easily, and are environmentally beneficial. Among microorganisms, fungi represent an emerging source of pigments due to their many benefits; therefore, they are readily viable on an industrial scale. Among all the bioactive pigments produced by fungi, melanin is an enigmatic, multifunctional pigment that has been studied for more than 150 years. This dark pigment, which is produced via the oxidative polymerization of phenolic compounds, has been investigated for its potential to protect life from all kingdoms, including fungi, from biotic and abiotic stresses.
1. Introduction
2. Types of Melanin
3. Biosynthesis
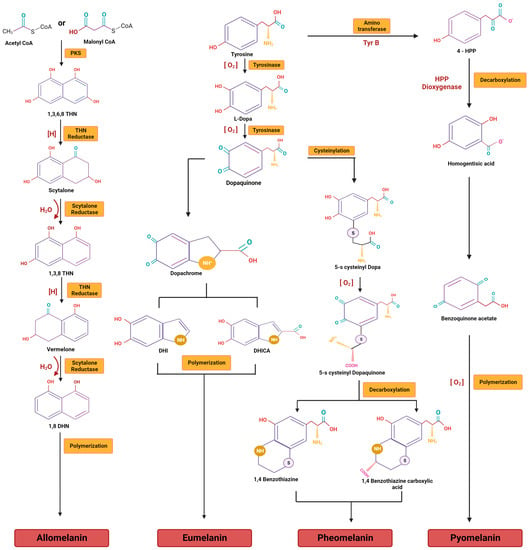
3.1. DHN Melanins
3.2. DOPA Melanins
4. Optimization for Enhancing Pigment Production
5. The Functional Role of Fungal Melanin
5.1. The Role of Melanin in Anti-Microbial Effects
5.2. The Role of Melanin in Photoprotection
5.3. The Role of Melanin in Thermoregulation
5.4. The Role of Melanin in Protection from Radiation
5.5. The Role of Melanin in Protecting against Oxidative Damage
5.6. The Role of Melanin in Metal Chelation
6. The Extraction and Purification of Melanin
6.1. Ultrasound-Assisted Extraction (UAE)
6.2. Negative Pressure Cavitation (NPC)
6.3. Microwave-Assisted Extraction (MWE)
6.4. Hydrodynamic Cavitation Extraction (HCE)
7. The Characterization of Melanin
7.1. UV–Visible Spectroscopy
7.2. Fourier Transform Infra-Red (FTIR) Spectroscopy
7.3. Elemental Analysis
7.4. Physicochemical Properties
7.5. Thermal Characterization
7.6. Nuclear Magnetic Resonance (NMR)
7.7. Electron Paramagnetic Resonance (EPR)
8. Applications of Melanins
8.1. Applications in Bio-Electronic Industries
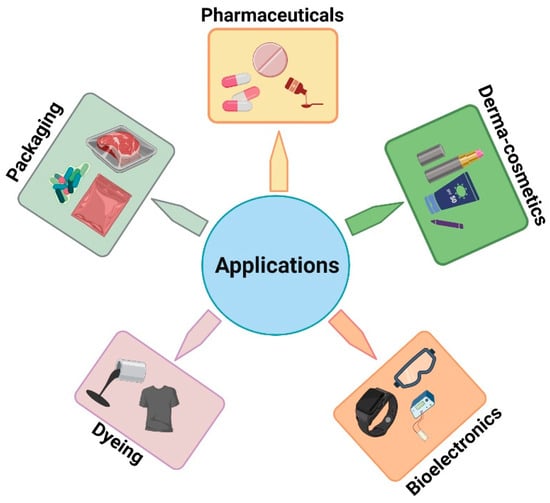
8.2. Applications in the Dyeing Industries
8.3. Applications in Pharmaceutical Industries
8.4. Applications in the Dermocosmetic Industry
8.5. Applications in Packaging Materials
9. Conclusions
References
- Rao, M.P.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113.
- Arora, S. Textile dyes: Its impact on environment and its treatment. J. Bioremed. Biodeg. 2014, 5, 1.
- Manzoor, J.; Sharma, M. Impact of textile dyes on human health and environment. In Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2020; pp. 162–169.
- Durán, N.; Teixeira, M.F.; De Conti, R.; Esposito, E. Ecological-friendly pigments from fungi. Crit. Rev. Food Sci. Nutr. 2002, 42, 53–66.
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369.
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604.
- Suthar, M.; Lagashetti, A.C.; Räisänen, R.; Singh, P.N.; Dufossé, L.; Robinson, S.C.; Singh, S.K. Industrial Applications of Pigments from Macrofungi. In Advances in Macrofungi; CRC Press: Boca Raton, FL, USA, 2021; pp. 223–251.
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61.
- Chambergo, F.S.; Valencia, E.Y. Fungal biodiversity to biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 2567–2577.
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239.
- Solano, F. Photoprotection versus photodamage: Updating an old but still unsolved controversy about melanin. Polym. Int. 2016, 65, 1276–1287.
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940.
- Chatragadda, R.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637.
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.; Lisboa, H.F.; Gonçalves, R.C. Production of melanin pigment by fungi and its biotechnological applications. Melanin 2017, 1, 47–75.
- Agustinho, D.P.; Nosanchuk, J.D. Functions of fungal melanins. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017.
- Płonka, P.; Grabacka, M. Melanin synthesis in microorganisms: Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443.
- Gómez, B.L.; Nosanchuk, J.D. Melanin and fungi. Curr. Opin. Infect. Dis. 2003, 16, 91–96.
- Butler, M.J.; Day, A.W. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136.
- Belozerskaya, T.A.; Gessler, N.N.; Aver’yanov, A.A. Melanin pigments of fungi. Fungal Metab. 2017, 2017, 263–291.
- Jolivet, S.; Arpin, N.; Wichers, H.J.; Pellon, G. Agaricus bisporus browning: A review. Mycol. Res. 1998, 102, 1459–1483.
- Thomas, M. Melanins. In Modern Methods of Plant Analysis/Moderne Methoden der Pflanzenanalyse; Springer: Berlin/Heidelberg, Germany, 1955.
- Raman, N.M.; Ramasamy, S. Genetic validation and spectroscopic detailing of DHN-melanin extracted from an environmental fungus. Biochem. Biophys. Rep. 2017, 12, 98–107.
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158.
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187.
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451.
- Xiao, M.; Chen, W.; Li, W.; Zhao, J.; Hong, Y.L.; Nishiyama, Y.; Dhinojwala, A. Elucidation of the hierarchical structure of natural eumelanins. J. R. Soc. Interface 2018, 15, 20180045.
- Bayram, S. A comparative characterization study between fungal and bacterial eumelanin pigments. Indian J. Microbiol. 2022, 62, 393–400.
- Vitiello, G.; Melone, P.; Silvestri, B.; Pezzella, A.; Di Donato, P.; D’Errico, G.; Di Napoli, M.; Zanfardino, A.; Varcamonti, M.; Luciani, G. Titanium based complexes with melanin precursors as a tool for directing melanogenic pathways. Pure Appl. Chem. 2019, 91, 1605–1616.
- Singh nee’ Nigam, P. Production of bioactive secondary metabolites. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Springer: Berlin/Heidelberg, Germany, 2009; pp. 129–145.
- Gadd, G.M. Effects of media composition and light on colony differentiation and melanin synthesis in Microdochium bolleyi. Trans. Br. Mycol. Soc. 1982, 78, 115–122.
- Said, F.M.; Chisti, Y.; Brooks, J. The effects of forced aeration and initial moisture level on red pigment and biomass production by Monascus ruber in packed bed solid state fermentation. Int. J. Environ. Sci. Dev. 2010, 1, 1.
- Singhania, R.R.; Soccol, C.R.; Pandey, A. Application of tropical agro-industrial residues as substrate for solid-state fermentation processes. In Current Developments in Solid-State Fermentation; Springer: Berlin/Heidelberg, Germany, 2008; pp. 412–442.
- Arun, G.; Eyini, M.; Gunasekaran, P. Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J. Exp. Biol. 2015, 53, 380–387.
- Surendirakumar, K.; Pandey, R.R.; Muthukumar, T.; Sathiyaseelan, A.; Loushambam, S.; Seth, A. Characterization and biological activities of melanin pigment from root endophytic fungus, Phoma sp. RDSE17. Arch. Microbiol. 2022, 204, 171.
- Bin, L.; Wei, L.; Xiaohong, C.; Mei, J.; Mingsheng, D. In vitro antibiofilm activity of the melanin from Auricularia auricula, an edible jelly mushroom. Ann. Microbiol. 2012, 62, 1523–1530.
- Oh, J.J.; Kim, J.Y.; Son, S.H.; Jung, W.J.; Seo, J.W.; Kim, G.H. Fungal melanin as a biocompatible broad-spectrum sunscreen with high antioxidant activity. RSC Adv. 2021, 11, 19682–19689.
- Maier, T.; Korting, H.C. Sunscreens–which and what for? Ski. Pharmacol. Physiol. 2005, 18, 253–262.
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Melanin pigments of fungi under extreme environmental conditions (Review). Appl. Biochem. Microbiol. 2014, 50, 105–113.
- Pacelli, C.; Cassaro, A.; Maturilli, A.; Timperio, A.M.; Gevi, F.; Cavalazzi, B.; Stefan, M.; Ghica, D.; Onofri, S. Multidisciplinary characterization of melanin pigments from the black fungus Cryomyces antarcticus. Appl. Microbiol. Biotechnol. 2020, 104, 6385–6395.
- Paolo, W.F.; Dadachova, E.; Mandal, P.; Casadevall, A.; Szaniszlo, P.J.; Nosanchuk, J.D. Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol. 2006, 6, 55.
- Prota, G.; D’Ischia, M.; Napolitano, A. The chemistry of melanins and related metabolites. In The Pigmentary System: Its Physiology and Pathophysiology; IRIS: Pisa, Italy, 1998; pp. 307–332.
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68.
- Wu, Y.; Shan, L.; Yang, S.; Ma, A. Identification and antioxidant activity of melanin isolated from Hypoxylon archeri, a companion fungus of Tremella fuciformis. J. Basic Microbiol. 2008, 48, 217–221.
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzym. Microb. Technol. 1996, 19, 311–317.
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276.
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615.
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31.
- Ghadge, V.A.; Singh, S.; Kumar, P.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Shinde, P.B. Extraction, Purification, and Characterization of Microbial Melanin Pigments. In Melanins: Functions, Biotechnological Production, and Applications; Springer: Cham, Switzerland, 2023; pp. 91–110.
- Borovansky, J.; Riley, P.A. (Eds.) Melanins and Melanosomes: Biosynthesis, Structure, Physiological and Pathological Functions; John Wiley & Sons: Hoboken, NJ, USA, 2011.
- Guo, X.; Chen, S.; Hu, Y.; Li, G.; Liao, N.; Ye, X.; Xue, C. Preparation of water-soluble melanin from squid ink using ultrasound-assisted degradation and its anti-oxidant activity. J. Food Sci. Technol. 2014, 51, 3680–3690.
- Kumar, C.G.; Mongolla, P.; Pombala, S.; Kamle, A.; Joseph, J. Physicochemical characterization and antioxidant activity of melanin from a novel strain of Aspergillus bridgeri ICTF-201. Lett. Appl. Microbiol. 2011, 53, 350–358.
- El-Naggar, N.E.A.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129.
- Mattoon, E.R.; Cordero, R.J.; Casadevall, A. Fungal melanins and applications in healthcare, bioremediation and industry. J. Fungi 2021, 7, 488.
- Gomez-Marin, A.M.; Sanchez, C.I. Thermal and mass spectroscopic characterization of a sulphur-containing bacterial melanin from Bacillus subtilis. J. Non-Cryst. Solids 2010, 356, 1576–1580.
- Oh, J.J.; Kim, J.Y.; Kwon, S.L.; Hwang, D.H.; Choi, Y.E.; Kim, G.H. Production and characterization of melanin pigments derived from Amorphotheca resinae. J. Microbiol. 2020, 58, 648–656.
- Buszman, E.; Pilawa, B.; Zdybel, M.; Wilczyński, S.; Gondzik, A.; Witoszyńska, T.; Wilczok, T. EPR examination of Zn2+ and Cu2+ binding by pigmented soil fungi Cladosporium cladosporioides. Sci. Total Environ. 2006, 363, 195–205.
- Zdybel, M.; Pilawa, B.; Drewnowska, J.M.; Swiecicka, I. Comparative EPR studies of free radicals in melanin synthesized by Bacillus weihenstephanensis soil strains. Chem. Phys. Lett. 2017, 679, 185–192.
- Ligonzo, T.; Ambrico, M.; Augelli, V.; Perna, G.; Schiavulli, L.; Tamma, M.A.; Capozzi, V. Electrical and optical properties of natural and synthetic melanin biopolymer. J. Non-Cryst. Solids 2009, 355, 1221–1226.
- Ambrico, M.; Ambrico, P.F.; Cardone, A.; Ligonzo, T.; Cicco, S.R.; Mundo, R.D.; Augelli, V.; Farinola, G.M. Melanin Layer on Silicon: An Attractive Structure for a Possible Exploitation in Bio-Polymer Based Metal–Insulator–Silicon Devices. Adv. Mater. 2011, 23, 3332–3336.
- Wu, W.; Xu, X.; Yang, H.; Hua, J.; Zhang, X.; Zhang, L.; Long, Y.; Tian, H. D–π–M–π–A structured platinum acetylide sensitizer for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 10666–10671.
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 170.
- Selvakumar, P.; Rajasekar, S.; Periasamy, K.; Raaman, N. Isolation and characterization of melanin pigment from Pleurotus cystidiosus (telomorph of Antromycopsis macrocarpa). World J. Microbiol. Biotechnol. 2008, 24, 2125–2131.
- Rajagopal, K.; Kathiravan, G.; Karthikeyan, S. Extraction and characterization of melanin from Phomopsis: A phellophytic fungi Isolated from Azadirachta indica A. Juss. Afr. J. Microbiol. Res. 2011, 5, 762–766.
- Marcovici, I.; Coricovac, D.; Pinzaru, I.; Macasoi, I.G.; Popescu, R.; Chioibas, R.; Zupko, I.; Dehelean, C.A. Melanin and melanin-functionalized nanoparticles as promising tools in cancer research—A review. Cancers 2022, 14, 1838.
- Araújo, M.; Viveiros, R.; Correia, T.R.; Correia, I.J.; Bonifácio, V.D.; Casimiro, T.; Aguiar-Ricardo, A. Natural melanin: A potential pH-responsive drug release device. Int. J. Pharm. 2014, 469, 140–145.
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90.
- Brand, R.M.; Pike, J.; Wilson, R.M.; Charron, A.R. Sunscreens containing physical UV blockers can increase transdermal absorption of pesticides. Toxicol. Ind. Health 2003, 19, 9–16.
- Kurian, N.K.; Bhat, S.G. Photoprotection and Anti-Inflammatory Properties of Non–Cytotoxic Melanin from Marine Isolate Providencia rettgeri Strain BTKKS1. Biosci. Biotechnol. Res. Asia 2017, 14, 1475–1484.
- Kurian, N.K.; Bhat, S.G. Food, cosmetic and biological applications of characterized DOPA-melanin from Vibrio alginolyticus strain BTKKS3. Appl. Biol. Chem. 2018, 61, 163–171.
- Singla, S.; Htut, K.Z.; Zhu, R.; Davis, A.; Ma, J.; Ni, Q.Z.; Burkart, M.D.; Maurer, C.; Miyoshi, T.; Dhinojwala, A. Isolation and characterization of allomelanin from pathogenic black knot fungus-a sustainable source of melanin. ACS Omega 2021, 6, 35514–35522.
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Polak-Śliwińska, M.; Kowalczyk, E.; Sienkiewicz, M. Preparation and characterization of carboxymethyl cellulose-based bioactive composite films modified with fungal melanin and carvacrol. Polymers 2021, 13, 499.




