Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alfredo Iandolo | -- | 2773 | 2023-09-07 09:11:44 | | | |
| 2 | Sirius Huang | Meta information modification | 2773 | 2023-09-08 03:36:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iandolo, A.; Pisano, M.; Buonavoglia, A.; Giordano, F.; Amato, A.; Abdellatif, D. Root Canal Irrigation Methods. Encyclopedia. Available online: https://encyclopedia.pub/entry/48906 (accessed on 07 February 2026).
Iandolo A, Pisano M, Buonavoglia A, Giordano F, Amato A, Abdellatif D. Root Canal Irrigation Methods. Encyclopedia. Available at: https://encyclopedia.pub/entry/48906. Accessed February 07, 2026.
Iandolo, Alfredo, Massimo Pisano, Alessio Buonavoglia, Francesco Giordano, Alessandra Amato, Dina Abdellatif. "Root Canal Irrigation Methods" Encyclopedia, https://encyclopedia.pub/entry/48906 (accessed February 07, 2026).
Iandolo, A., Pisano, M., Buonavoglia, A., Giordano, F., Amato, A., & Abdellatif, D. (2023, September 07). Root Canal Irrigation Methods. In Encyclopedia. https://encyclopedia.pub/entry/48906
Iandolo, Alfredo, et al. "Root Canal Irrigation Methods." Encyclopedia. Web. 07 September, 2023.
Copy Citation
According to contemporary dental standards, the primary goal of endodontic therapy is the chemo-mechanical cleaning of the complex root canal system. Watering root canals with approved solutions and activating them are essential parts of this operation.
cleaning
endodontics
irrigant activation
NaOCl
root canal treatment
1. Introduction
Nowadays, there is increased use of the term “modern endodontics” to refer to contemporary applied science and actual materials that have been introduced and invented in recent years. Diverse endodontic tools and technological devices have been designed to facilitate and enhance treatments [1], for example, magnification using the operative microscope, sonic and ultrasonic appliances, and certain laser machines. Moreover, modified alloys for NI-Ti rotary files and potent irrigation techniques were invented for the cleaning and shaping phases. There has been huge developments in irrigant solutions, obturation materials, and cone-beam or 3D (three-dimensional) radiography [2][3]. New trends are arising in regenerative endodontics, which are conducted to replace inflamed and necrotic pulp tissue with a functional and healthy dentine–pulp complex [4].
Consequently, applying advanced technologies in endodontic therapy allows the secure and manageable treatment of even the most complicated cases.
It was documented that some areas of the complex root canal space remain unreachable during the mechanical phase, independently of the file type. For illustration, hand and rotary endodontic files only clean the centre of root canals [5][6], while the lateral anatomies, like isthmuses, lateral and furcation canals, apical deltas, and others, remain untouched after mechanical shaping. Thus, active cleaning is essential to adequately eliminate bacteria and necrotic tissue [7]. Recently, a new concept including a minimally invasive shaping approach utilising rotating files of a smaller size and taper combined with more efficient irrigation protocols was introduced, leading to safer, more conservative endodontic therapies.
Considering the irrigant solution, sodium hypochlorite (NaOCl) is the most typically used irrigant, owing to its elevated ability in tissue dissolution and superior antimicrobial activity. Multiple procedures can be used to maximise the action and effectiveness of NaOCl [8][9]. Some techniques for activating the irrigants used during an endodontic procedure can be listed as follows (Figure 1):
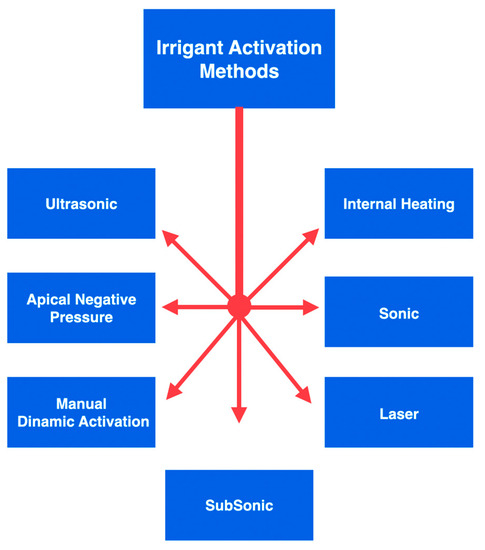
Figure 1. An overview of the graphic division of irrigant activation methods.
- Manual dynamic activation;
- Heating (internal or external);
- Negative apical pressure (EndoVac, Kerr Endodontics, Gilbert, AZ, USA; Rinsendo, Dürr Dental, Bietigheim, Germany);
- Subsonic technique (EndoAcivator, Dentsply Maillefer, Ballaigues, Switzerland);
- Sonic technique (EDDY, VDW, München, Germany);
- Passive ultrasonic irrigation (PUI);
- Laser techniques [10][11][12][13][14].
Bacteria and their byproducts are the main etiological agents of periapical pulp infection. The cleaning part is the most delicate phase, where the bacterial load is lowered [15][16][17][18]. The main factors that define the composition of the microbial flora are the anaerobic environment, the interactions among various bacteria, and the availability of nutrients [19]. The number of bacterial species is usually limited in the initial phase of endodontic infection. According to the literature, the number of bacterial species in an infected root canal can vary between 1 and 12. It also appears that there is a correlation between the size of the periapical lesion and the number of bacterial species present in the root canal [20][21]. It is generally believed that the persistence of endodontic infection is related to difficulties encountered during the initial treatment and thus to incomplete removal of damaged tissues or bacteria. Normally, only one or a few bacterial species are found in the root canals of dental elements with persistent pathology [19][22][23]. They are mostly Gram-positive microorganisms with equal distribution of facultative and obligate anaerobes [24]. The microbial flora is distinctly different from that typical of untreated tooth infections, which is polymicrobial with similar proportions of Gram-positive and Gram-negative species, and a predominance of obligate anaerobes [25][26][27][28]. Despite the differences between species isolated from different channels with the same clinical presentation (persistent periapical disease), many studies agree that enterococci and streptococci are highly prevalent [29]. Enterococcus faecalis is considered a specific opportunistic pathogen of persistent periapical pathology. While in the untreated infected canal, the microbial flora can find an abundance of nourishment, it can suffer famine in the endodontically well-treated canal. Ideally, all the necrotic pulp originally present will have been eliminated, leaving dry and poor conditions for the survival of the remaining microbial species. These species must endure hunger and a static environment but can sometimes find some nourishment in the exudate coming from the periapical tissue [30]. Persistent species survived antimicrobial treatments, entered the canal during treatment, and settled where other species could not. If the coronal seal has defects, there may be bacterial infiltration, and therefore, there is the possibility of new infection of the canal. Inside the endodontic space, there are free bacteria in planktonic form and microorganisms united in a very organized structure, the biofilm, which can oppose the removal action with different mechanisms [31]. The biofilm formation mechanism is known: on a protein layer that forms a conditioning film, the bacteria in planktonic form adhere, which subsequently stratify. The bacteria in the biofilm are immersed in a matrix called the glycocalyx, which acts as a mechanical barrier against antibacterial agents. Biofilm is 1000 times more resistant than bacteria in planktonic form. The biofilm can be broken up and removed thanks to the activation of the irrigating agents [32][33]. The endodontic space is not only composed of the main root canals, different and complex three-dimensional anatomies also form it (Figure 2).
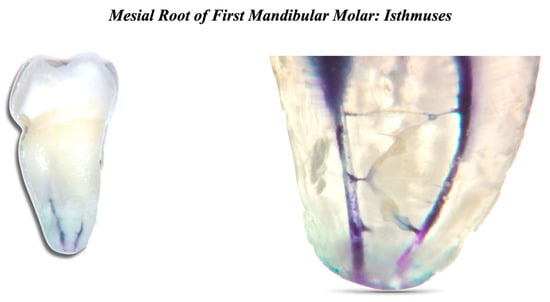
Figure 2. Mesial root of first mandibular molar. Two root canals and many interconnections can be seen.
2. Types of Irrigants and Irrigant Activation Methods
During the cleansing phase, 5.25% NaOCl, 17% EDTA, and 2% chlorhexidine (CHX) are available among the most commonly used irrigants [34].
2.1. Sodium Hypochlorite
NaOCl is the most widely used irrigant in endodontics thanks to its low cost and proven organoleptic and antiseptic power.
It was first used as a root canal irrigant in 1920 [35]. When in contact with water, sodium hypochlorite dissociates into sodium hydroxide and hypochlorous acid or active chlorine. Hypochlorous acid represents the active part of the irrigating solutions, and owing to its very small molecular structure, it is able to pass the bacterial cell membrane, causing its lysis easily. The concentration of active chlorine in commercial solutions varies a lot, and this is the reason that should push the dentist to buy hypochlorite solutions prepared by pharmaceutical companies exclusively for endodontic use. The lower the active chlorine concentration, the longer the solution will be required to dissolve the damaged tissues [36]. Sodium hypochlorite solutions must be stored at a temperature of around 4 °C and protected from light. The dissolving action of sodium hypochlorite is greater on necrotic tissues than on vital tissues. An ideal concentration of NaOCl is 5.25% because it represents the best compromise between toxicity and proteolytic power. Increasing the concentration would not only increase the dissolutive capacity but also increase the toxicity. However, thanks to the heating and agitation of this irrigant, it is possible to enhance its actions without increasing concentrations [37].
On the other hand, it must be said that it is not always active against Enterococcus faecalis, a protagonist in 70% of cases of post-treatment pathology. Its antibacterial characteristics are very valid for Gram-negative bacteria and microorganisms of primary infection. Another disadvantage is the inability to eliminate the smear layer [38]. The presence of exudate, pulp, and bacterial residues quickly weakens and consumes the active chlorine present in the irrigating solutions. Therefore, continuous turnover is essential to allow adequate effectiveness of the solution. If, on the other hand, its action on biofilm is considered, it is important that NaOCl is used at high concentrations (5.25–6%) and is activated to increase its effectiveness [39].
2.2. Chlorhexidine (CHX)
The CHX solutions used in endodontics are generally between 0.2% and 2.0%. High concentrations, such as those used for endodontic purposes, have a bactericidal action due to the destruction of bacterial cytoplasm by forming cross-links between proteins [40]. Initially, its effectiveness against various bacterial species, including E. Faecalis, the reduced cytotoxicity for periapical tissues, and the minimal consequences related to the possible extrusion of the irrigant beyond the apex has prompted the use of chlorhexidine as an alternative to the sodium hypochlorite. It also appears that heated chlorhexidine used as a root canal irrigant, but at a concentration of less than 2%, has greater antibacterial efficacy. Despite its effectiveness as an endodontic irrigant, chlorhexidine cannot be indicated as a reference irrigant during routine endodontic treatments because it cannot dissolve damaged tissues and is not active against the inorganic components of the smear layer, and is more active against Gram+ versus Gram−. CHX has a high adhesion capacity to dentinal surfaces, thus continuing its antibacterial action, which appears to be directly proportional to the time of application of the drug and its concentration. This feature is called “substantivity” [41]. Some commercially available chlorhexidine solutions dedicated to endodontics are added to a surfactant agent (cetrimide), capable of lowering the surface tension of the solution, thus allowing easier entry into the tubular system of the active ingredient, where it can perform, in addition due to the direct antibacterial action, also categorised as substantivity activity [42].
2.3. EDTA (Ethylene-Diamino-Tetra-Acetic Acid)
2.3.1. Smear Layer
The formation of the smear layer or smear layers is due to the mechanical cutting action of the dentin by manual and rotating endodontic instruments. The debris produced stratifies on the inner walls of the root canals [43]. The smear layer can also penetrate several microns into the dentinal tubules. It is made up of two components: organic particles and inorganic particles. The organic component is represented by necrotic or vital pulp tissue, odontoblastic processes, bacteria, and blood cells, while the inorganic component consists of calcified tissue [44]. It is evident that its removal is of fundamental importance to allow the irrigants to enter and act in the lateral macro and micro anatomies and obtain a good adaptation of the filling material to the canal walls [45].
2.3.2. EDTA
The 17% EDTA is very important for removing the inorganic component of the smear layer. EDTA can be used both during shaping and at its completion. EDTA was introduced in endodontics by Nygaard-Ostby in 1957. Today, 17% of EDTA solutions are associated with a detergent for greater flow [46][47].
2.3.3. Chemical Properties of EDTA
EDTA is a chelating substance; it can capture calcium ions through a coordinative or dative bond, forming so-called “chelate complexes”, giving rise to the ethylenediaminetetraacetate salt of calcium [48]. Therefore, this substance acts on the tooth’s hard tissues and the inorganic component of the smear layer. The most effective concentration is between 10% and 17% [49]. Given its strong demineralising action, directly proportional to the degree of acidity, EDTA is normally supplied in the form of buffered solutions with pH values of 6.5–7. Buffered EDTA is active not only on calcium ions but also on the non-collagen protein component of dentin [50].
2.4. Interactions
It is essential to avoid the combination of the various irrigants. For example, CHX mixed with NaOCl forms parachloroaniline, a potentially carcinogenic substance. EDTA together with CHX forms a non-carcinogenic substance but is capable of occluding the dentinal tubules. Finally, the union between NaOCl and EDTA should be avoided, as they deactivate each other [51]. Mixing chelators and NaOCl reduces the pH, affecting the amount of free chlorine in the solution and causing an increase in hypochlorous acid and chlorine gas with a consequent decrease in hypochlorite ion. This union leads to a loss of reactive free chlorine in the NaOCl solution. It is advisable to use a physiological solution or sterile water between one irrigating agent and another to solve these problems [52]. Finally, during endodontic treatments, gaseous blocks called vapour locks can form inside the root canals, compromising the cleansing phase if not removed because they prevent contact with the irrigants with the tissues and bacteria [53].
2.5. Activation of Irrigants
Among the most used techniques for activating irrigants are the following:
Activation of irrigants utilising ultrasonic inserts (25–40 kHz): Through a phenomenon called acoustic streaming, this technique allows an intense agitation of the irrigant, ensuring better antibacterial activity and greater tissue dissolution action. The limits of this technique are two: passivity, and the extrusion of the irrigant beyond the apex. By passivity, it means that the insert should work freely inside the channel without having many contacts. If, in addition to contact with the walls, non-smooth and pointed inserts are added, the conditions worsen [54].
Activation of irrigants using sonic inserts (<20 kHz): This technique allows a remarkable degree of cleansing of the endodontic space but is less powerful than ultrasonic activation except that the use times are not prolonged. One of the advantages of this technique is the lesser extrusion of irrigant beyond the apex [55].
The activation of irrigants can employ subsonic inserts (frequencies lower than sonic activation). With this technique, good results are obtained but inferior to the irrigant’s sonic and ultrasonic activation [56].
Controlled intracanal heating: The heated NaOCl increases its antibacterial, dissolving, and sliding properties. It has always been recommended to preheat it to 50 °C and then introduce it inside the root canal. Preheating it is useless because it quickly stabilises at body temperature in a few seconds. Precisely, it must be heated directly inside the channel through controlled heat sources to make the most of the above features [57]. Between 96 and 120 °C is where NaOCl boils [58]. A heated NaOCl solution has a stronger ability than the solution at room temperature to dissolve the pulp tissue and clean the root canal [37].
Further heating of sodium hypochlorite improves its effectiveness against E. faecalis and its capacity to disintegrate necrotic pulp tissue [59]. Heat carriers (System-B—Endodontic Heat Source, Kerr Endodontics, Gilbert, AZ, USA, or comparable) can be used for intracanal heating procedures. Mainly, the technique is performed by adjusting the device’s maximum temperature between 150 and 180 degrees Celsius, and the tip’s dimension is 30/04. An endodontic needle is used to inject sodium hypochlorite directly into the root canal. Afterwards, the heat carrier is inserted up to 3 mm from the working length before being turned on. This technique is repeated five times; in each cycle, the heat carrier’s activation lasts 5 s and is followed by a 5 s interval. During the procedure, the NaOCl solution carriers make brief upward and downward movements with a few mm of amplitude to empower the activation process. The irrigation solution is changed with a brand-new one following each of the abovementioned cycles [60][61]. Clinicians advise heated NaOCl intracanal ultrasonic activation because it achieves superior dentin tubule penetration and canal purity compared to syringe activation or ultrasonic activation alone [53]. In the side dentin tubules that remained contaminated after using either ultrasonic activation or thermal activation alone, better pulp dissolution was obtained when thermal and ultrasonic activation were combined [62].
Laser: An adequate strategy in cleansing the root canal space; however, it comes with particular limitations: the elevated equipment cost and the hazards of irrigant extrusion beyond the apex. Chiefly, the lasers employed for irrigant activation in endodontics are diode laser, neodymium-doped yttrium aluminium garnet (ND: YAG) laser, erbium-doped yttrium aluminium garnet (Er: YAG) laser, and erbium, chromium-doped yttrium, scandium, gallium, and garnet (Er, Cr: YSGG) laser.
The activation strategies of lasers are assorted depending on the tip position: Laser-Activated Irrigation (LAI) where the laser tip is placed within the canal, while in Photon-Induced Photoacoustic Streaming (PIPS), the laser tip is limited to the pulp chamber. Regarding the latter method, Er: YAG laser is especially utilised [63][64].
Negative pressure: This technique allows for better cleansing in the apical third than positive pressure systems. A further advantage is almost no extrusion beyond the tip of the irrigant. The only limitation of this technique is a preparation with an apical diameter of a minimum equal to 0.35 ISO and a taper of at least 0.4, which is only sometimes obtainable, especially in long, narrow, and curved canals [65][66].
MDA (Manual Dynamic Activation): This technique uses a gutta-percha cone inserted 1 mm prior to the working length, followed by a moderate pumping motion into the irrigated root canal. This motion creates a series of short vertical strokes with a 2 mm amplitude at a rate of 100 strokes/minute. Dynamic movements increase intracanal pressures, which cancels out the vapour lock and significantly improves the replacement rate of the irrigant. Consequently, it allows fresh parts of the solution to be used and their beneficial effect to be extended to a larger part of the system. After root canal preparation, root canal activation (NaOCl or EDTA solution) is recommended. A very simple technique to perform but not comparable to previous techniques in terms of effectiveness [67].
Moreover, the activation of the irrigants can be performed mechanically, utilising different types of files mounted on the micromotor. Very encouraging results are being obtained using these techniques.
These techniques allow us to have satisfactory results (removal of vapour locks, biofilm disintegration). Above all, they allow the irrigants to travel the main canals and the different anatomies that compose them—shaping the main canal and irrigating it simply without the aid of these techniques results in not removing tissues and bacteria present in the three-dimensional anatomies [68].
References
- Wong, J.; Cheung, G.S.P.; Lee, A.H.C.; McGrath, C.; Neelakantan, P. PROMs following Root Canal Treatment and Surgical Endodontic Treatment. Int. Dent. J. 2023, 73, 28–41.
- Baumgartner, J.C.; Cuenin, P.R. Efficacy of Several Concentrations of Sodium Hypochlorite for Root Canal Irrigation. J. Endod. 1992, 18, 605–612.
- Iandolo, A.; Pisano, M.; Scelza, G.; Abdellatif, D.; Martina, S. Three-Dimensional Evaluation of the Root Apex of Permanent Maxillary Premolars: A Multicentric Study. Appl. Sci. 2022, 12, 6159.
- Kumar, N.; Maher, N.; Amin, F.; Ghabbani, H.; Zafar, M.S.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Biomimetic Approaches in Clinical Endodontics. Biomimetics 2022, 7, 229.
- Ali, A.; Bhosale, A.; Pawar, S.; Kakti, A.; Bichpuriya, A.; Agwan, M.A. Current Trends in Root Canal Irrigation. Cureus 2022, 14, e24833.
- Vivekananda Pai, A.R. Factors Influencing the Occurrence and Progress of Sodium Hypochlorite Accident: A Narrative and Update Review. J. Conserv. Dent. 2023, 26, 3–11.
- Peters, O.A.; Boessler, C.; Zehnder, M. Effect of Liquid and Paste-Type Lubricants on Torque Values during Simulated Rotary Root Canal Instrumentation. Int. Endod. J. 2005, 38, 223–229.
- Wong, S.; Mundy, L.; Chandler, N.; Upritchard, J.; Purton, D.; Tompkins, G. Antibacterial Properties of Root Canal Lubricants: A Comparison with Commonly Used Irrigants. Aust. Endod. J. 2014, 40, 111–115.
- Liu, R.; Hou, B.X.; Wesselink, P.R.; Wu, M.-K.; Shemesh, H. The incidence of root microcracks caused by 3 different single-file systems versus the ProTaper system. J. Endod. 2013, 39, 1054–1056.
- Park, E.; Shen, Y.A.; Haapasalo, M. Irrigation of the apical root canal. Endod. Top. 2012, 27, 54–73.
- Boessler, C.; Peters, O.A.; Zehnder, M. Impact of Lubricant Parameters on Rotary Instrument Torque and Force. J. Endod. 2007, 33, 280–283.
- Jahromi, M.Z.; Fathi, M.H.; Zamiran, S. Experimental Study of Smear Layer and Debris Remaining following the Use of Four Root Canal Preparation Systems Using Scanning Electron Microscopy. J. Islam. Dent. Assoc. Iran 2013, 25, 235–241.
- Jena, A.; Sahoo, S.K.; Govind, S. Root Canal Irrigants: A Review of Their Interactions, Benefits, and Limitations. Compend. Contin. Educ. Dent. 2015, 36, 256–261.
- Pisano, M.; Di Spirito, F.; Martina, S.; Sangiovanni, G.; D’Ambrosio, F.; Iandolo, A. Intentional Replantation of Single-Rooted and Multi-Rooted Teeth: A Systematic Review. Healthcare 2022, 11, 11.
- Narayanan, L.L.; Vaishnavi, C. Endodontic microbiology. J. Conserv. Dent. 2010, 13, 233–239.
- Siqueira, J.F., Jr. Microbiology of apical periodontitis. In Essential Endodontology; Pitt Ford, T., Ed.; Blackwell: Oxford, UK, 2008; pp. 135–139.
- Olivieri, J.G.; García Font, M.; Stöber, E.; de Ribot, J.; Mercadé, M.; Duran-Sindreu, F. Effect of Manual Dynamic Activation with Citric Acid Solutions in Smear Layer Removal: A Scanning Electron Microscopic Evaluation. J. Dent. Sci. 2016, 11, 360–364.
- Williamson, A.E.; Cardon, J.W.; Drake, D.R. Antimicrobial Susceptibility of Monoculture Biofilms of a Clinical Isolate of Enterococcus Faecalis. J. Endod. 2009, 35, 95–97.
- Siqueira, J.F., Jr.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55, 512–530.
- Haupt, F.; Meinel, M.; Gunawardana, A.; Hülsmann, M. Effectiveness of Different Activated Irrigation Techniques on Debris and Smear Layer Removal from Curved Root Canals: A SEM Evaluation. Aust. Endod. J. 2020, 46, 40–46.
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7.
- Zamany, A.; Safavi, K.; Spångberg, L.S.W. The Effect of Chlorhexidine as an Endodontic Disinfectant. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 578–581.
- Iandolo, A.; Amato, A.; Martina, S.; Abdellatif, D.A.; Pantaleo, G. Management of severe curvatures in root canal treatment with the new generation of rotating files using a safe and predictable protocol. Open Dent J. 2020, 14, 421–425.
- Iandolo, A.; Simeone, M.; Riccitiello, F. The preparation of coronal isthmus is a fundamental step for long term success. G. Ital. Endod. 2012, 26, 150–154.
- Boutsioukis, C.; Arias-Moliz, M.T. Present Status and Future Directions—Irrigants and Irrigation Methods. Int. Endod. J. 2022, 55 (Suppl. S3), 588–612.
- Rito Pereira, M.; Silva, G.; Semiao, V.; Silverio, V.; Martins, J.N.R.; Pascoal-Faria, P.; Alves, N.; Dias, J.R.; Ginjeira, A. Experimental Validation of a Computational Fluid Dynamics Model Using Micro-Particle Image Velocimetry of the Irrigation Flow in Confluent Canals. Int. Endod. J. 2022, 55, 1394–1403.
- Pladisai, P.; Ampornaramveth, R.S.; Chivatxaranukul, P. Effectiveness of Different Disinfection Protocols on the Reduction of Bacteria in Enterococcus Faecalis Biofilm in Teeth with Large Root Canals. J. Endod. 2016, 42, 460–464.
- Stojicic, S.; Zivkovic, S.; Qian, W.; Zhang, H.; Haapasalo, M. Tissue Dissolution by Sodium Hypochlorite: Effect of Concentration, Temperature, Agitation, and Surfactant. J. Endod. 2010, 36, 1558–1562.
- Boutsioukis, C.; Lambrianidis, T.; Kastrinakis, E. Irrigant Flow within a Prepared Root Canal Using Various Flow Rates: A Computational Fluid Dynamics Study. Int. Endod. J. 2009, 42, 144–155.
- Abou-Rass, M.; Piccinino, M.V. The Effectiveness of Four Clinical Irrigation Methods on the Removal of Root Canal Debris. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 323–328.
- Boutsioukis, C.; Lambrianidis, T.; Kastrinakis, E.; Bekiaroglou, P. Measurement of Pressure and Flow Rates during Irrigation of a Root Canal Ex Vivo with Three Endodontic Needles. Int. Endod. J. 2007, 40, 504–513.
- Silva, P.V.; Guedes, D.F.C.; Pécora, J.D.; da Cruz-Filho, A.M. Time-Dependent Effects of Chitosan on Dentin Structures. Braz. Dent. J. 2012, 23, 357–361.
- Nair, P.N. On the causes of persistent apical periodontitis, a review. Int. Endod. J. 2006, 39, 249–281.
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303.
- Clarkson, R.M.; Moule, A.J. Sodium hypochlorite and its use as an endodontic irrigant. Aust. Dent. J. 1998, 43, 250–256.
- Orlowski, N.B.; Schimdt, T.F.; Teixeira, C.D.S.; Garcia, L.D.F.R.; Savaris, J.M.; Tay, F.R.; Bortoluzzi, E.A. Smear Layer Removal Using Passive Ultrasonic Irrigation and Different Concentrations of Sodium Hypochlorite. J. Endod. 2020, 46, 1738–1744.
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398.
- Cunningham, W.T.; Balekjian, A.Y. Effect of temperature on collagen-dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 175–177.
- Plotino, G.; Cortese, T.; Grande, N.M.; Leonardi, D.P.; Di Giorgio, G.; Testarelli, L.; Gambarini, G. New Technologies to Improve Root Canal Disinfection. Braz. Dent. J. 2016, 27, 3–8.
- Gomes, B.P.; Vianna, M.E.; Zaia, A.A.; Almeida, J.F.; Souza-Filho, F.J.; Ferraz, C.C. Chlorhexidine in endodontics. Braz. Dent. J. 2013, 24, 89–102.
- Basrani, B.; Santos, J.M.; Tjäderhane, L.; Grad, H.; Gorduysus, O.; Huang, J.; Lawrence, H.P.; Friedman, S. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 240–245.
- Bernardi, A.; Teixeira, C.S. The properties of chlorhexidine and undesired effects of its use in endodontics. Quintessence Int. 2015, 46, 575–582.
- Caron, G.; Nham, K.; Bronnec, F.; MacHtou, P. Effectiveness of Different Final Irrigant Activation Protocols on Smear Layer Removal in Curved Canals. J. Endod. 2010, 36, 1361–1366.
- Iandolo, A.; Pisano, M.; Abdellatif, D.; Amato, A.; Giordano, F.; Buonavoglia, A.; Sangiovanni, G.; Caggiano, M. Effectiveness of Different Irrigation Techniques on Post Space Smear Layer Removal: SEM Evaluation. Prosthesis 2023, 5, 539–549.
- Torabinejad, M.; Handysides, R.; Khademi, A.A.; Bakland, L.K. Clinical implications of the smear layer in endodontics: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 658–666.
- Jaiswal, S.; Gupta, S.; Nikhil, V.; Bhadoria, A.; Raj, S. Effect of Intracanal and Extracanal Heating on Pulp Dissolution Property of Continuous Chelation Irrigant. J. Conserv. Dent. 2021, 24, 544–548.
- Teixeira, C.S.; Felippe, M.C.S.; Felippe, W.T. The Effect of Application Time of EDTA and NaOCI on Intracanal Smear Layer Removal: An SEM Analysis. Int. Endod. J. 2005, 38, 285–290.
- Taweewattanapaisan, P.; Jantarat, J.; Ounjai, P.; Janebodin, K. The Effects of EDTA on Blood Clot in Regenerative Endodontic Procedures. J. Endod. 2019, 45, 281–286.
- Wright, P.P.; Kahler, B.; Walsh, L.J. The Effect of Heating to Intracanal Temperature on the Stability of Sodium Hypochlorite Admixed with Etidronate or EDTA for Continuous Chelation. J. Endod. 2019, 45, 57–61.
- Violich, D.R.; Chandler, N.P. The smear layer in endodontics—A review. Int. Endod. J. 2010, 43, 2–15.
- Krishnan, U.; Saji, S.; Clarkson, R.; Lalloo, R.; Moule, A.J. Free active chlorine in sodium hypochlorite solutions admixed with octenidine, smearoff, chlorhexidine, and EDTA. J. Endod. 2017, 43, 1354–1359.
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in endodontics. Dent. Clin. N. Am. 2010, 54, 291–312.
- Wright, P.P.; Kahler, B.; Walsh, L.J. Alkaline Sodium Hypochlorite Irrigant and Its Chemical Interactions. Materials 2017, 10, 1147.
- Paixão, S.; Rodrigues, C.; Grenho, L.; Fernandes, M.H. Efficacy of sonic and ultrasonic activation during endodontic treatment: A Meta-analysis of in vitro studies. Acta Odontol. Scand. 2022, 80, 588–595.
- Macedo, R.G.; Verhaagen, B.; Rivas, D.F.; Versluis, M.; Wesselink, P.; Van Der Sluis, L. Cavitation Measurement during Sonic and Ultrasonic Activated Irrigation. J. Endod. 2014, 40, 580–583.
- Mortman, R.E. Technologic advances in endodontics. Dent. Clin. N. Am. 2011, 55, 461–480.
- Sirtes, G.; Waltimo, T.; Schaetzle, M.; Zehnder, M. The Effects of Temperature on Sodium Hypochlorite Short-Term Stability, Pulp Dissolution Capacity, and Antimicrobial Efficacy. J. Endod. 2005, 31, 669–671.
- Bukiet, F.; Soler, T.; Guivarch, M.; Camps, J.; Tassery, H.; Cuisinier, F.; Candoni, N. Factors affecting the viscosity of sodium hypochlorite and their effect on irrigant flow. Int. Endod. J. 2013, 46, 954–961.
- Yared, G.; Al Asmar Ramli, G. Antibacterial Ability of Sodium Hypochlorite Heated in the Canals of Infected Teeth: An Ex Vivo Study. Cureus 2020, 12, e6975.
- Iandolo, A.; Simeone, M.; Orefice, S.; Rengo, S. 3D cleaning, a perfected technique: Thermal profile assessment of heated NaOCl. G. Ital. Endod. 2017, 31, 58–61.
- Jain, S.; Patni, P.M.; Jain, P.; Raghuwanshi, S.; Pandey, S.H.; Tripathi, S.; Soni, A. Comparison of Dentinal Tubular Penetration of Intracanal Heated and Preheated Sodium Hypochlorite Through Different Agitation Techniques. J. Endod. 2023, 49, 686–691.
- Damade, Y.; Kabir, R.; Gaddalay, S.; Deshpande, S.; Gite, S.; Bambale, S.; Dubey, N. Root canal debridement efficacy of heated sodium hypochlorite in conjunction with passive ultrasonic agitation: An ex vivo study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 235–238.
- Korkut, E.; Torlak, E.; Gezgin, O.; Özer, H.; Sener, Y. Antibacterial and Smear Layer Removal Efficacy of Er:YAG Laser Irradiation by Photon-Induced Photoacoustic Streaming in Primary Molar Root Canals: A Preliminary Study. Photomed. Laser Surg. 2018, 36, 480–486.
- Koch, J.D.; Jaramillo, D.E.; DiVito, E.; Peters, O.A. Irrigant Flow during Photon-Induced Photoacoustic Streaming (PIPS) Using Particle Image Velocimetry (PIV). Clin. Oral Investig. 2016, 20, 381–386.
- Loroño, G.; Zaldivar, J.R.; Arias, A.; Cisneros, R.; Dorado, S.; Jimenez-Octavio, J.R. Positive and negative pressure irrigation in oval root canals with apical ramifications: A computational fluid dynamics evaluation in micro-CT scanned real teeth. Int. Endod. J. 2020, 53, 671–679.
- Konstantinidi, E.; Psimma, Z.; Chávez de Paz, L.E.; Boutsioukis, C. Apical negative pressure irrigation versus syringe irrigation: A systematic review of cleaning and disinfection of the root canal system. Int. Endod. J. 2017, 50, 1034–1054.
- Hemalatha, S.; Srinivasan, A.; Srirekha, A.; Santhosh, L.; Champa, C.; Shetty, A. An in vitro radiological evaluation of irrigant penetration in the root canals using three different irrigation systems: Waterpik WP-100 device, passive irrigation, and manual dynamic irrigation systems. J. Conserv. Dent. 2022, 25, 403–408.
- Susila, A.; Minu, J. Activated Irrigation vs. Conventional non-activated Irrigation in Endodontics—A Systematic Review. Eur. Endod. J. 2019, 4, 96–110.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
948
Revisions:
2 times
(View History)
Update Date:
08 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




