
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Orestes Martín Medina | -- | 4633 | 2023-09-07 08:52:27 | | | |
| 2 | Catherine Yang | Meta information modification | 4633 | 2023-09-07 09:36:58 | | | | |
| 3 | Catherine Yang | Meta information modification | 4633 | 2023-09-11 09:45:23 | | |
Video Upload Options
The sustainable development of biodiesel and oleochemical industries requires optimal recycling and reuse strategies for all the generated residues and by-products. The main residues from non-edible oilseeds are either lignocellulosic materials, such as fruit shells, pods, hulls, branches, and leaves, generated before oil extraction or a protein-rich material, e.g., the press cake or de-oiled meal, generated after oil extraction. Both lignocellulosic- and protein-rich materials have huge economic potential. However, since using non-edible oils for biodiesel production is still emerging, the valorization of non-edible oilseed residues is still underdeveloped compared to that of edible oil production residues. The utilization potential of non-edible oilseed residues goes far beyond the traditional energetic approaches. Thermochemical, biochemical, physico-chemical, and chemical approaches provide different utilization routes. Thermochemical approaches, such as gasification and pyrolysis, result in syngas, biochar, and biooil, which can then be converted into advanced biofuels or serve as raw materials for the chemical industry. In the biochemical conversion approach, by either anaerobic digestion, sugar-platform processes, or solid-state fermentation, microorganisms convert the starting substrates into gaseous or liquid biofuels, enzymes, or other compounds.
1. Bioconversion Processes for Valorization of Non-Edible Oilseed Residues
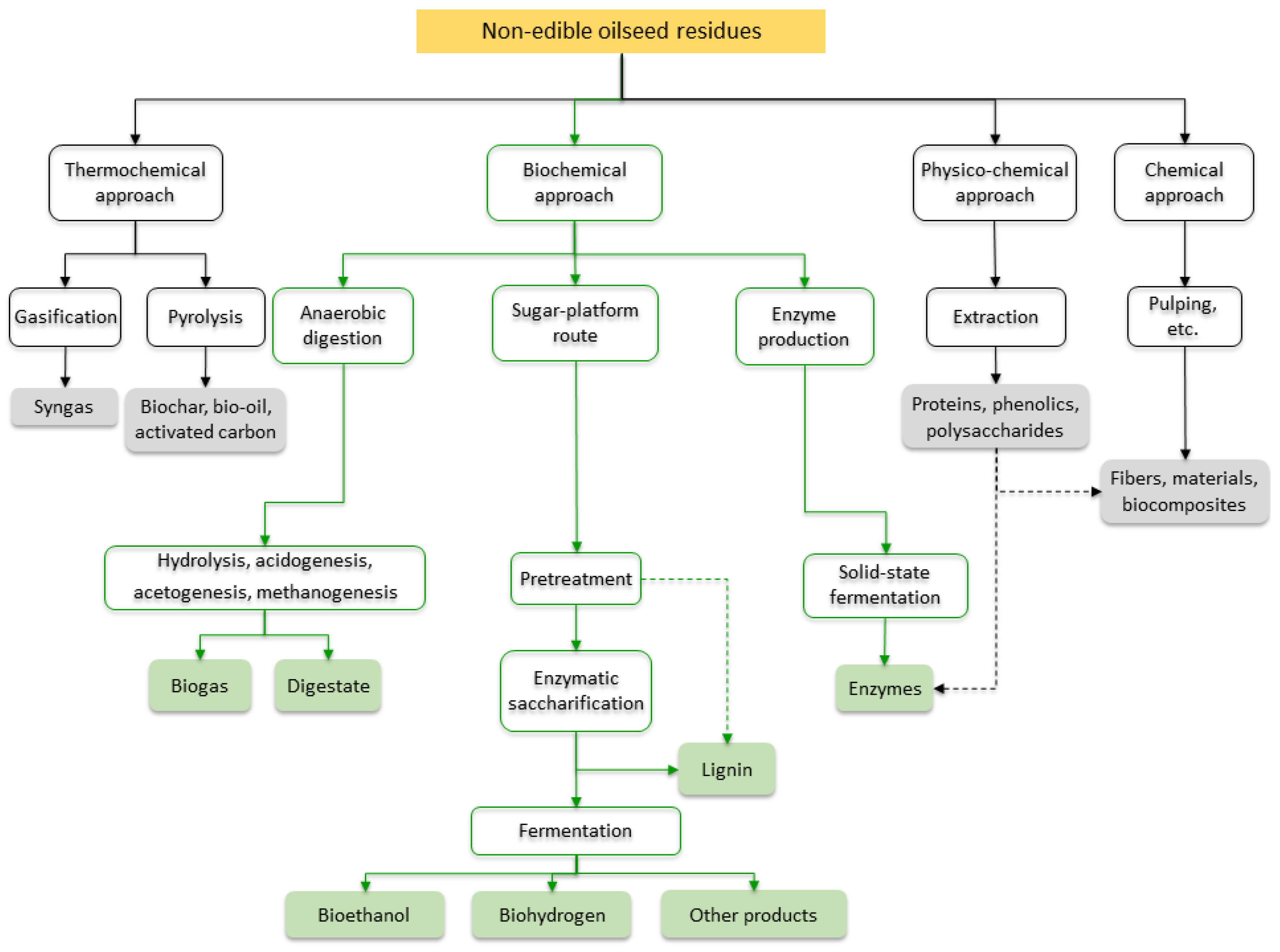
1.1. Anaerobic Digestion
| Material | Conditions | Results | Ref. |
|---|---|---|---|
| Jatropha seed cake | AD of a 1:20 cake/water slurry in a 5-L batch reactor at 30 °C for 60 days. | Methane yield: 156 L/kg of seed cake; COD removal: 52%. | [14] |
| Jatropha seed cake | Semi-continuous flow at 30 °C; COD range: 1.25–5 kg/m3 day. | Highest methane yield (340 L/kg COD degraded) was obtained at an OLR of 1.25 kg COD/m3 day. | [15] |
| Jatropha seed cake | AD of cow dung alone and mixed with jatropha cake in 2-L plastic jars for 40 days. | Biogas yield of jatropha cake (0.170 m3/kg) was higher than that of cow dung (0.166 m3/kg). The digestate was a suitable fertilizer for maize and tomato. | [16] |
| Jatropha seed cake | Jatropha cake alone or combined with cattle dung, 37 °C, 5-L glass fermenter | Biogas yield: 265 L/kg biomass; methane concentration: 65% | [17] |
| Jatropha seed cake | Co-digestion of jatropha cake and cattle dung in a 6-m3 floating-type digester for 60 days. | Methane concentration: 62.3–69.2% under mesophilic conditions and 65.2–69.2% for psychrophilic conditions. | [18] |
| Jatropha seed cake | Pilot-scale continuous 40-m3 stirred digester; co-digestion with cow dung (3:1) for 120 days | Within 5 days, the reactor started producing 20 m3 of biogas per day. | [19] |
| Jatropha seed cake | Co-digestion with sugarcane bagasse and addition of Fe2+ ions in 120-mL serum vials as digesters. | Co-digestion of jatropha cake (10% (w/v)) and bagasse (5% (w/v)) gave higher BPR than experiments with jatropha cake alone. Adding 10 mM of Fe2+ ions led to further improvement. | [20] |
| Jatropha seed cake | AD in the presence of an iron additive | H2S content in biogas was reduced. | [21] |
| Jatropha and karanja cakes | AD in a 20 m3/d floating drum under mesophilic temperature | Methane potential: 0.39 (for jatropha cake) and 0.43 m3/kg TS (for karanja cake); average methane concentration: 66.6% (for jatropha) and 62.5% (for karanja); higher methane concentration than in biogas from cattle dung. | [22] |
| Jatropha and karanja cakes, pods, and glycerol | Serum glass bottles (125 mL) fitted with rubber airtight stoppers were used as digesters. | The biogas potential of residues of karanja and jatropha was, respectively, 3.07 and 1.83 m3 per kg of produced biodiesel. | [23] |
| Karanja oil cake | Karanja cake mixed with cow dung in 75:25, 50:50, 25:75 and 0:100 (w/w) proportions | The 25:75 mixture gave the best results. Methane content was 73%, and the slurry had a higher fertilizer value. | [24] |
| Mahua and hingan cakes | A 20-L plastic bottle was used as single-phase digestion system | Biogas yield: 198–233 L/kg seedcake. The digestates had high fertilizer value due to high nitrogen content. | [25] |
| Castor cake | AD in 5-L capacity single-stage fermenters at 30 and 37 °C | Particle size 2.0–1.4 mm was favorable for BPR. High temperature resulted in higher yield. Conversion of the feed: 30–35% TS. | [26] |
| Castor cake, stem, and leaves | AD in 118-mL bottles | Seed cakes and leaves were suitable substrates for AD, but stems were unsuitable without pretreatment. The combined biogas yield from cake, stem, and leaves was 131 g/kg of initial plant biomass. Biodiesel yield is 155 g/kg, and ethanol yield is 85 g/kg. | [27] |
1.2. Sugar-Platform Processes
1.3. Production of Enzymes from Residues of Non-Edible Oilseeds
| Source | Microorganism(s) | Enzyme(s) | Application | Ref. |
|---|---|---|---|---|
| Jatropha seed cake | Pseudomonas aeruginosa | Protease, lipase | Industrial enzyme production | [61] |
| Aspergillus niger, Rhizomucor miehei | Lipase | Enzyme production | [57] | |
| Paecilomyces variotii | Cellulases | Biofuel production | [9] | |
| Scytadilium thermophilum | Xylanase | Biobleaching of paper pulp | [62] | |
| Thermoascus aurantiacus | Cellulases | Saccharification of sugarcane bagasse | [63] | |
| A. niger | Cellulase, xylanase | Biofuel production | [64] | |
| Jatropha seed husk | Bjerkandera adusta Pycnoporus sanguineus |
Cellulases, xylanases | Screening of inducible enzyme activity on lignocellulosic residues | [59] |
| Castor bean waste | Penicillium simplicissimum | Lipases | Ricin detoxification and biodiesel enzyme production | [65] |
| Penicillium simplicissimum | Lipases | Biodiesel enzyme production | [66] | |
| Aspergillus spp., Emericela spp., Rhodotorula spp. | CMCase, FPase, β-glucosidase | Screening of fungal isolates for cellulase activity | [67] | |
| Pa. varoitii | Tannase, phytase | Ricin detoxification, phytate phosphate release |
[68] | |
| Jojoba meal | Aspergillus spp. | Extracellular β-glucosidase | Biofuel production, fortification of T. reesei cellulases | [58] |
| Karanja seed residue | Spingomonas echinoides, Iprex lacteus | Endo- and exoglucanases, xylanase, laccase | Biofuel production | [42] |
| A. niger, Bacillus licheniformis, Acinetobacter pittii | Proteases | Enzyme production, gelatin film breakdown | [69] | |
| Moringa straw | Penicillium funiculosum, Fusarium verticillioides, Cladosporium cladosporoides | CMCase, FPase, β-glucosidase | Screening of fungal isolates for cellulase activity | [60] |
| Mahua seed cake | A. niger | Proteases | ANF detoxification | [9] |
| Enzyme | Microorganism | Substrate | SSF Length | Max. Activity (U/g Substrate) |
Ref. |
|---|---|---|---|---|---|
| Lipase (EC 3.1.1.3) | P. aeruginosa | Jatropha seed cake | 120 h | 620 | [61] |
| P. simplicissimum | Castor cake | 96 h | 44.8 | [65] | |
| P. simplicissimum | Castor cake | 120 h | 155 | [66] | |
| Tannase (EC 3.1.1.20) | Pa. varoitii | Castor cake | 48 h | 2600 | [67] |
| Phytase (EC 3.1.3.8/.26) | Pa. varoitii | Castor cake | 72 h | 260 | [68] |
| Cellulase (FPase 1) (EC 3.2.x.x) |
Th. aurantiacus | Jatropha seed cake | 6 days | 4.9 | [63] |
| Pa. variotii | Jatropha seed cake | 4 days | 27.3 | [9] | |
| Endoglucanse (CMCase 2) (EC 3.2.1.4) |
Th. aurantiacus | Jatropha seed cake | 6 days | 124.4 | [63] |
| Aspergillus niger FGSCA733 | Jatropha seed cake | 120 h | 3974 | [63] | |
| Spingomonas echinoides | Karanja seed residue | 8 days | 16.2 | [42] | |
| Iprex lacteus | Karanja seed residue | 8 days | 49.2 | [42] | |
| Exoglucanase (EC 3.2.1.9) | S. echinoides | Karanja seed residue | 8 days | 23.4 | [42] |
| Iprex lacteus | Karanja seed residue | 8 days | 31.2 | [42] | |
| β-glucosidase (EC 3.2.1.21) | Th. aurantiacus | Jatropha seed cake | 6 days | 28.9 | [63] |
| Aspergillus sp. DHE7 | Jojoba meal | 72 h | 153 | [71] | |
| Xylanase (EC 3.2.1.8) | Scytadilium thermophilum | Jatropha seed cake | 9 days | 1455 | [62] |
| A. niger FGSCA733 | Jatropha seed cake | 48 h | 6087 | [63] | |
| S. echinoides | Karanja seed residue | 8 days | 4.8 | [42] | |
| I. lacteus | Karanja seed residue | 8 days | 16.2 | [42] | |
| Protease (EC 3.4.x.x) | P. aeruginosa PseA | Jatropha seed cake | 72 h | 1800 | [61] |
| A. niger | Mahua deoiled seed cake | 2 days | 52.5 | [9] | |
| A. niger | Karanja seed residue | 7 days | 3.7 | [69] | |
| Acinetobacter pittii | Karanja seed residue | 7 days | 1.8 | [69] | |
| B. licheniformis | Karanja seed residue | 48 h | 2.1 | [69] |
2. Other Valorization Routes for Non-Edible Oilseed Residues
References
- Mohanty, A.; Rout, P.R.; Dubey, B.; Meena, S.S.; Pal, P.; Goel, M. A Critical Review on Biogas Production from Edible and Non-Edible Oil Cakes. Biomass Convers. Biorefinery 2022, 12, 949–966.
- Pasciucco, F.; Francini, G.; Pecorini, I.; Baccioli, A.; Lombardi, L.; Ferrari, L. Valorization of Biogas from the Anaerobic Co-Treatment of Sewage Sludge and Organic Waste: Life Cycle Assessment and Life Cycle Costing of Different Recovery Strategies. J. Clean. Prod. 2023, 401, 136762.
- Pecorini, I.; Peruzzi, E.; Albini, E.; Doni, S.; Macci, C.; Masciandaro, G.; Iannelli, R. Evaluation of MSW Compost and Digestate Mixtures for a Circular Economy Application. Sustainability 2020, 12, 3042.
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112.
- Gandla, M.L.; Tang, C.; Jönsson, L.J.; Martín, C. Enzymatic Saccharification of Lignocellulosic Biomass. In Enzymes in Agriculture and Industry; Agricultural Biocatalysis; Jenny Stanford Publishing: Singapore, 2022; Volume 9, pp. 413–469.
- Chacón-Navarrete, H.; Martín, C.; Moreno-García, J. Yeast Immobilization Systems for Second-Generation Ethanol Production: Actual Trends and Future Perspectives. Biofuels Bioprod. Biorefining 2021, 15, 1549–1565.
- dos Santos, J.R.A.; Souto-Maior, A.M.; Gouveia, E.R.; Martín, C. Comparison of SHF and SSF processes from sugar cane bagasse for ethanol production by Saccharomyces cerevisiae|Comparação entre processos em SHF e em SSF de bagaço de cana-de-açúcar para a produção de etanol por Saccharomyces cerevisiae. Quím. Nova 2010, 33, 904–908.
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834.
- Gupta, A.; Sharma, A.; Pathak, R.; Kumar, A.; Sharma, S. Solid State Fermentation of Non-Edible Oil Seed Cakes for Production of Proteases and Cellulases and Degradation of AntiNutritional Factors. J. Food Biotechnol. Res. 2018, 2, 1:4.
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1.
- Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods 2023, 12, 432.
- Staubmann, R.; Foidl, G.; Foidl, N.; Gübitz, G.M.; Lafferty, R.M.; Arbizu, V.M.; Steiner, W. Biogas Production from Jatropha curcas Press-Cake. Appl. Biochem. Biotechnol. 1997, 63–65, 457–467.
- Singh, R.N.; Vyas, D.K.; Srivastava, N.S.L.; Narra, M. SPRERI Experience on Holistic Approach to Utilize All Parts of Jatropha curcas Fruit for Energy. Renew. Energy 2008, 33, 1868–1873.
- Sinbuathong, N.; Munakata-Marr, J.; Sillapacharoenkul, B.; Chulalaksananukul, S. Effect of the Solid Content on Biogas Production from Jatropha curcas Seed Cake. Int. J. Glob. Warm. 2011, 3, 403–416.
- Sinbuathong, N.; Sillapacharoenkul, B.; Khun-Anake, R.; Watts, D. Optimum Organic Loading Rate for Semi-Continuous Operation of an Anaerobic Process for Biogas Production from Jatropha curcas Seed Cake. Int. J. Glob. Warm. 2010, 2, 179–188.
- Raheman, H.; Mondal, S. Biogas Production Potential of Jatropha Seed Cake. Biomass Bioenergy 2012, 37, 25–30.
- Chandra, R.; Vijay, V.; Subbarao, P. A Study on Biogas Generation from Non-Edible Oil Seed Cakes: Potential and Prospects in India. In Proceedings of the 2nd Joint International Conference on Sustainable Energy and Environment, Bangkok, Thailand, 21–23 November 2006.
- Sharma, A.K.; Sahoo, P.K.; Mukherjee, M.; Patel, A. Assessment of Sustainable Biogas Production from Co-Digestion of Jatropha De-Oiled Cake and Cattle Dung Using Floating Drum Type Digester under Psychrophilic and Mesophilic Conditions. Clean. Technol. 2022, 4, 529–541.
- Singhal, S.; Agarwal, S.; Singhal, N.; Sharma, R.; Sharma, R. Designing and Operation of Pilot Scale Continuous Stirred Tank Reactor for Continuous Production of Bio-Methane from Toxic Waste. Environ. Prog. Sustain. Energy 2019, 38, 198–200.
- Sen, K.; Mahalingam, S.; Sen, B. Rapid and High Yield Biogas Production from Jatropha Seed Cake by Co-Digestion with Bagasse and Addition of Fe2+. Environ. Technol. 2013, 34, 2989–2994.
- Schmidt, T. Anaerobic Digestion of Jatropha curcas L. Press Cake and Effects of an Iron-Additive. Waste Manag. Res. 2011, 29, 1171–1176.
- Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Khura, T.K. Production of Methane from Anaerobic Digestion of Jatropha and Pongamia Oil Cakes. Appl. Energy 2012, 93, 148–159.
- Khuntia, H.K.; Chanakya, H.N.; Siddiqha, A.; Thomas, C.; Mukherjee, N.; Janardhana, N. Anaerobic Digestion of the Inedible Oil Biodiesel Residues for Value Addition. Sustain. Energy Technol. Assess. 2017, 22, 9–17.
- Barik, D.; Murugan, S. Assessment of Sustainable Biogas Production from De-Oiled Seed Cake of Karanja-an Organic Industrial Waste from Biodiesel Industries. Fuel 2015, 148, 25–31.
- Deshpande, N.V.; Kale, N.W.; Deshmukh, S.J. A Study on Biogas Generation from Mahua (Madhuca indica) and Hingan (Balanites aegyaptiaca) Oil Seedcake. Energy Sustain. Dev. 2012, 16, 363–367.
- Gollakota, K.G.; Meher, K.K. Effect of Particle Size, Temperature, Loading Rate and Stirring on Biogas Production from Castor Cake (Oil Expelled). Biol. Wastes 1988, 24, 243–249.
- Bateni, H.; Karimi, K.; Zamani, A.; Benakashani, F. Castor Plant for Biodiesel, Biogas, and Ethanol Production with a Biorefinery Processing Perspective. Appl. Energy 2014, 136, 14–22.
- Gupta, A.; Kumar, A.; Sharma, S.; Vijay, V.K. Comparative Evaluation of Raw and Detoxified Mahua Seed Cake for Biogas Production. Appl. Energy 2013, 102, 1514–1521.
- Al-Widyan, M.I.; Al-Muhtaseb, M.A. Experimental Investigation of Jojoba as a Renewable Energy Source. Energy Convers. Manag. 2010, 51, 1702–1707.
- Muhammad, M.B.; Chandra, R. Enhancing Biogas and Methane Production from Leaf Litter of Neem by Co-Digestion with Vegetable Waste: Focus on the Effect of Tannin. Biomass Bioenergy 2021, 147, 106007.
- Tambone, F.; Pradella, M.; Bedussi, F.; Adani, F. Moringa oleifera Lam. as an Energy Crop for Biogas Production in Developing Countries. Biomass Convers. Biorefinery 2020, 10, 1083–1089.
- Gaul, M. An Analysis Model for Small-Scale Rural Energy Service Pathway—Applied to Jatropha-Based Energy Services in Sumbawa, Indonesia. Energy Sustain. Dev. 2012, 16, 283–296.
- Palit, D.; Malhotra, R.; Mande, S. Enhancing Viability of Biofuel-Based Decentralized Power Projects for Rural Electrification in India. Environ. Dev. Sustain. 2017, 19, 263–283.
- Momayez, F.; Hedenström, M.; Stagge, S.; Jönsson, L.J.; Martín, C. Valorization of Hydrolysis Lignin from a Spruce-Based Biorefinery by Applying γ-Valerolactone Treatment. Bioresour. Technol. 2022, 359, 127466.
- Martín, C.; García, A.; Schreiber, A.; Puls, J.; Saake, B. Combination of Water Extraction with Dilute-Sulphuric Acid Pretreatment for Enhancing the Enzymatic Hydrolysis of Jatropha curcas Shells. Ind. Crops Prod. 2015, 64, 233–241.
- Marasabessy, A.; Kootstra, A.M.J.; Sanders, J.P.; Weusthuis, R.A. Dilute H2SO4-Catalyzed Hydrothermal Pretreatment to Enhance Enzymatic Digestibility of Jatropha curcas Fruit Hull for Ethanol Fermentation. Int. J. Energy Environ. Eng. 2012, 3, 15.
- García, A.; López, Y.; Karimi, K.; Benítez, A.; Lundin, M.; Taherzadeh, M.; Martín, C. Chemical and Physical Characterization and Acid Hydrolysis of a Mixture of Jatropha curcas Shells and Husks. Cell. Chem. Technol. 2015, 49, 737–744.
- Kumar, G.; Sen, B.; Lin, C.-Y. Pretreatment and Hydrolysis Methods for Recovery of Fermentable Sugars from De-Oiled Jatropha Waste. Bioresour. Technol. 2013, 145, 275–279.
- Kumar, G.; Sen, B.; Sivagurunathan, P.; Lin, C.-Y. High Rate Hydrogen Fermentation of Cello-Lignin Fraction in de-Oiled Jatropha Waste Using Hybrid Immobilized Cell System. Fuel 2016, 182, 131–140.
- Garza, J.a.R.L.; Castillo-Quiroz, D.; Ríos-González, L.; Morales-Martínez, T.; González-Fuentes, J.A.; Valdez-Aguilar, L.; Medina-Morales, M.A. Autohydrolysis Pretreatment of Castor Plant Pruning Residues to Enhance Enzymatic Digestibility and Bioethanol Production. Bioresources 2020, 15, 6206–6216.
- Abada, E.; Al-Fifi, Z.; Osman, M. Bioethanol Production with Carboxymethylcellulase of Pseudomonas poae Using Castor Bean (Ricinus communis L.) Cake. Saudi J. Biol. Sci. 2019, 26, 866–871.
- Radhakumari, M.; Taha, M.; Shahsavari, E.; Bhargava, S.K.; Satyavathi, B.; Ball, A.S. Pongamia pinnata Seed Residue—A Low Cost Inedible Resource for on-Site/in-House Lignocellulases and Sustainable Ethanol Production. Renew. Energy 2017, 103, 682–687.
- Doshi, P.; Srivastava, G. Sustainable Approach to Produce Bioethanol from Karanja (Pongamia pinnata) Oilseed Residue. Turk. J. Agric. 2013, 37, 781–788.
- Hernández, E.; García, A.; López, M.; Puls, J.; Parajó, J.C.; Martín, C. Dilute Sulphuric Acid Pretreatment and Enzymatic Hydrolysis of Moringa oleifera Empty Pods. Ind. Crops Prod. 2013, 44, 227–231.
- Montaño, H.F.; Rincón, S.L.; Serrato, J.C. Study of the Influence of Dilute Acid Pre-Treatment Conditions on Glucose Recovery from Jatropha curcas Lam for Fuel-Ethanol Production. Int. J. Green Energy 2017, 14, 613–623.
- Harry-O’kuru, R.E.; Gordon, S.H.; Klokkenga, M. Bio-Generation of Succinic Acid by Fermentation of Physaria fendleri Seed Polysaccharides. Ind. Crops Prod. 2015, 77, 116–122.
- Visser, E.M.; Filho, D.O.; Martins, M.A.; Steward, B.L. Bioethanol Production Potential from Brazilian Biodiesel Co-Products. Biomass Bioenergy 2011, 35, 489–494.
- Kumar, G.; Sivagurunathan, P.; Lin, C.-Y. Influence of Various Combinations of Heat Pretreatment on Hydrogen Fermentation from Deoiled Jatropha Waste Using Microflora. Environ. Eng. Manag. J. 2019, 18, 1–7.
- Qureshi, N.; Harry-O’kuru, R.; Liu, S.; Saha, B. Yellow Top (Physaria fendleri) Presscake: A Novel Substrate for Butanol Production and Reduction in Environmental Pollution. Biotechnol. Prog. 2019, 35, e2767.
- Chang, P.-C.; Hsu, H.-Y.; Jang, G.-W. Biological Routes to Itaconic and Succinic Acids. Phys. Sci. Rev. 2016, 1, 20160052.
- Jiang, L.; Fang, Z.; Li, X.-K.; Luo, J. Production of 2,3-Butanediol from Cellulose and Jatropha Hulls after Ionic Liquid Pretreatment and Dilute-Acid Hydrolysis. AMB Express 2013, 3, 48.
- Oruganti, R.K.; Gungupalli, M.P.; Bhattacharyya, D. Alkaline Hydrolysis for Yield of Glucose and Kraft Lignin from De-Oiled Jatropha curcas Waste: Multiresponse Optimization Using Response Surface Methodology. Biomass Convers. Biorefinery 2022.
- Muktham, R.; Ball, A.S.; Bhargava, S.K.; Bankupalli, S. Bioethanol Production from Non-Edible de-Oiled Pongamia Pinnata Seed Residue-Optimization of Acid Hydrolysis Followed by Fermentation. Ind. Crops Prod. 2016, 94, 490–497.
- Radhakumari, M.; Ball, A.; Bhargava, S.K.; Satyavathi, B. Optimization of Glucose Formation in Karanja Biomass Hydrolysis Using Taguchi Robust Method. Bioresour. Technol. 2014, 166, 534–540.
- Martín, C.; Moure, A.; Martín, G.; Carrillo, E.; Domínguez, H.; Parajó, J.C. Fractional Characterisation of Jatropha, Neem, Moringa, Trisperma, Castor and Candlenut Seeds as Potential Feedstocks for Biodiesel Production in Cuba. Biomass Bioenergy 2010, 34, 533–538.
- Ancuța, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432.
- Ilmi, M.; Hidayat, C.; Hastuti, P.; Heeres, H.J.; van der Maarel, M.J.E.C. Utilisation of Jatropha Press Cake as Substrate in Biomass and Lipase Production from Aspergillus niger 65I6 and Rhizomucor miehei CBS 360.62. Biocatal. Agric. Biotechnol. 2017, 9, 103–107.
- El-Ghonemy, D.H. Optimization of Extracellular Ethanol-Tolerant β-Glucosidase Production from a Newly Isolated Aspergillus sp. DHE7 via Solid State Fermentation Using Jojoba Meal as Substrate: Purification and Biochemical Characterization for Biofuel Preparation. J. Genet. Eng. Biotechnol. 2021, 19, 45.
- Quiroz-Castañeda, R.E.; Pérez-Mejía, N.; Martínez-Anaya, C.; Acosta-Urdapilleta, L.; Folch-Mallol, J. Evaluation of Different Lignocellulosic Substrates for the Production of Cellulases and Xylanases by the Basidiomycete Fungi Bjerkandera adusta and Pycnoporus sanguineus. Biodegradation 2011, 22, 565–572.
- Vázquez-Montoya, E.L.; Castro-Ochoa, L.D.; Maldonado-Mendoza, I.E.; Luna-Suárez, S.; Castro-Martínez, C. Moringa Straw as Cellulase Production Inducer and Cellulolytic Fungi Source. Rev. Argent. Microbiol. 2020, 52, 4–12.
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of Protease and Lipase by Solvent Tolerant Pseudomonas aeruginosa PseA in Solid-State Fermentation Using Jatropha curcas Seed Cake as Substrate. Bioresour. Technol. 2008, 99, 1729–1735.
- Joshi, C.; Khare, S.K. Utilization of Deoiled Jatropha curcas Seed Cake for Production of Xylanase from Thermophilic Scytalidium thermophilum. Bioresour. Technol. 2011, 102, 1722–1726.
- Dave, B.R.; Sudhir, A.P.; Pansuriya, M.; Raykundaliya, D.P.; Subramanian, R.B. Utilization of Jatropha Deoiled Seed Cake for Production of Cellulases under Solid-State Fermentation. Bioprocess. Biosyst. Eng. 2012, 35, 1343–1353.
- Ncube, T.; Howard, R.L.; Abotsi, E.K.; van Rensburg, E.L.J.; Ncube, I. Jatropha Curcas Seed Cake as Substrate for Production of Xylanase and Cellulase by Aspergillus niger FGSCA733 in Solid-State Fermentation. Ind. Crops Prod. 2012, 37, 118–123.
- Godoy, M.G.; Gutarra, M.L.E.; Maciel, F.M.; Felix, S.P.; Bevilaqua, J.V.; Machado, O.L.T.; Freire, D.M.G. Use of a Low-Cost Methodology for Biodetoxification of Castor Bean Waste and Lipase Production. Enzym. Microb. Technol. 2009, 44, 317–322.
- Godoy, M.G.; Gutarra, M.L.E.; Castro, A.M.; Machado, O.L.T.; Freire, D.M.G. Adding Value to a Toxic Residue from the Biodiesel Industry: Production of Two Distinct Pool of Lipases from Penicillium simplicissimum in Castor Bean Waste. J. Ind. Microbiol. Biotechnol. 2011, 38, 945–953.
- Herculano, P.N.; Lima, D.M.M.; Fernandes, M.J.S.; Neves, R.P.; Souza-Motta, C.M.; Porto, A.L.F. Isolation of Cellulolytic Fungi from Waste of Castor (Ricinus communis L.). Curr. Microbiol. 2011, 62, 1416–1422.
- Madeira, J.V.; Macedo, J.A.; Macedo, G.A. Detoxification of Castor Bean Residues and the Simultaneous Production of Tannase and Phytase by Solid-State Fermentation Using Paecilomyces variotii. Bioresour. Technol. 2011, 102, 7343–7348.
- Seshagiri, S.; Parthiban, B.; Reddy, N. Production, Properties and Applications of Proteases from Pongamia Oil Seed Cakes. J. Appl. Polym. Sci. 2023, 140, e54280.
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent Advances in Production of Lignocellulolytic Enzymes by Solid-State Fermentation of Agro-Industrial Wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100407.
- Farobie, O.; Hartulistiyoso, E. Palm Oil Biodiesel as a Renewable Energy Resource in Indonesia: Current Status and Challenges. BioEnergy Res. 2022, 15, 93–111.
- Sharma, R.; Sheth, P.N.; Gujrathi, A.M. Kinetic Modeling and Simulation: Pyrolysis of Jatropha Residue de-Oiled Cake. Renew. Energy 2016, 86, 554–562.
- Pfeil, M.; Tobío-Pérez, I.; Denfeld, D.; Díaz, Y.; Pohl, S.; Piloto-Rodríguez, R. Characterization and Assessment of Jatropha curcas and Moringa oleifera Husk and Their Potential Use in Gasification. Energ. Ecol. Environ. 2021, 6, 170–182.
- Parascanu, M.M.; Sandoval-Salas, F.; Soreanu, G.; Valverde, J.L.; Sanchez-Silva, L. Valorization of Mexican Biomasses through Pyrolysis, Combustion and Gasification Processes. Renew. Sustain. Energy Rev. 2017, 71, 509–522.
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Hydrothermal Treatment of Pretreated Castor Residue for the Production of Bio-Oil. BioEnergy Res. 2023, 16, 517–527.
- Majhi, A.; Sharma, Y.K.; Naik, D.V.; Chauhan, R. The Production and Evaluation of Bio-Oil Obtained from the Jatropha curcas Cake. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 1782–1789.
- Sun, K.; Jiang, J. chun Preparation and Characterization of Activated Carbon from Rubber-Seed Shell by Physical Activation with Steam. Biomass Bioenergy 2010, 34, 539–544.
- Onorevoli, B.; da Silva Maciel, G.P.; Machado, M.E.; Corbelini, V.; Caramão, E.B.; Jacques, R.A. Characterization of Feedstock and Biochar from Energetic Tobacco Seed Waste Pyrolysis and Potential Application of Biochar as an Adsorbent. J. Environ. Chem. Eng. 2018, 6, 1279–1287.
- Sowmya Dhanalakshmi, C.; Madhu, P. Biofuel Production of Neem Wood Bark (Azadirachta indica) through Flash Pyrolysis in a Fluidized Bed Reactor and Its Chromatographic Characterization. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 428–443.
- Vinayaka, D.L.; Guna, V.; Madhavi, D.; Arpitha, M.; Reddy, N. Ricinus Communis Plant Residues as a Source for Natural Cellulose Fibers Potentially Exploitable in Polymer Composites. Ind. Crops Prod. 2017, 100, 126–131.
- Kumode, M.M.N.; Bolzon, G.I.M.; Magalhães, W.L.E.; Kestur, S.G. Microfibrillated Nanocellulose from Balsa Tree as Potential Reinforcement in the Preparation of ‘Green’ Composites with Castor Seed Cake. J. Clean. Prod. 2017, 149, 1157–1163.
- Rahman, M.M.; Netravali, A.N. Micro-Fibrillated Cellulose Reinforced Eco-Friendly Polymeric Resin from Non-Edible ‘Jatropha curcas’ Seed Waste after Biodiesel Production. RSC Adv. 2016, 6, 47101–47111.
- Patil, N.V.; Rahman, M.M.; Netravali, A.N. “Green” Composites Using Bioresins from Agro-Wastes and Modified Sisal Fibers. Polym. Compos. 2019, 40, 99–108.
- Rantheesh, J.; Indran, S.; Raja, S.; Siengchin, S. Isolation and Characterization of Novel Micro Cellulose from Azadirachta indica A. Juss Agro-Industrial Residual Waste Oil Cake for Futuristic Applications. Biomass Convers. Biorefinery 2023, 13, 4393–4411.
- Sajithkumar, K.J.; Visakh, P.M.; Ramasamy, E.V. Moringa Oleifera (Drum Stick Vegetable Fibre) Based Nanocomposites with Natural Rubber: Preparation and Characterizations. Waste Biomass Valor. 2016, 7, 1227–1234.
- Grigoriou, A.H.; Ntalos, G.A. The Potential Use of Ricinus communis L. (Castor) Stalks as a Lignocellulosic Resource for Particleboards. Ind. Crops Prod. 2001, 13, 209–218.
- Evon, P.; Kartika, I.A.; Rigal, L. New Renewable and Biodegradable Particleboards from Jatropha Press Cakes. J. Renew. Mater. 2014, 2, 52–65.
- Liu, C.; Wang, S.; Wang, B.; Song, G. Catalytic Hydrogenolysis of Castor Seeds C-Lignin in Deep Eutectic Solvents. Ind. Crops Prod. 2021, 169, 113666.
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “Ideal Lignin” Facilitates Full Biomass Utilization. Sci. Adv. 2018, 4, eaau2968.
- Jingura, R.M.; Kamusoko, R. Technical Options for Valorisation of Jatropha Press-Cake: A Review. Waste Biomass Valor. 2018, 9, 701–713.
- Biswal, A.K.; Lenka, C.; Panda, P.K.; Yang, J.-M.; Misra, P.K. Investigation of the Functional and Thermal Properties of Mahua Deoiled Cake Flour and Its Protein Isolate for Prospective Food Applications. LWT 2021, 137, 110459.
- Kandasamy, G.; Shaleh, S.R.M. Flotation Removal of the Microalga Nannochloropsis Sp. Using Moringa Protein–Oil Emulsion: A Novel Green Approach. Bioresour. Technol. 2018, 247, 327–331.
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of Microwave Assisted Extraction of Simmondsins and Polyphenols from Jojoba (Simmondsia chinensis) Seed Cake Using Box-Behnken Statistical Design. Food Chem. 2021, 356, 129670.
- Oskoueian, E.; Abdullah, N.; Ahmad, S.; Saad, W.Z.; Omar, A.R.; Ho, Y.W. Bioactive Compounds and Biological Activities of Jatropha curcas L. Kernel Meal Extract. Int. J. Mol. Sci. 2011, 12, 5955–5970.




