Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jennifer Ann Harikrishna | -- | 2819 | 2023-09-07 08:06:07 | | | |
| 2 | Jessie Wu | Meta information modification | 2819 | 2023-09-07 08:19:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Akram, H.; Hussain, S.; Mazumdar, P.; Chua, K.O.; Butt, T.E.; Harikrishna, J.A. Mangrove Forest Functions and Services. Encyclopedia. Available online: https://encyclopedia.pub/entry/48899 (accessed on 08 February 2026).
Akram H, Hussain S, Mazumdar P, Chua KO, Butt TE, Harikrishna JA. Mangrove Forest Functions and Services. Encyclopedia. Available at: https://encyclopedia.pub/entry/48899. Accessed February 08, 2026.
Akram, Hina, Shoaib Hussain, Purabi Mazumdar, Kah Ooi Chua, Talib E. Butt, Jennifer Ann Harikrishna. "Mangrove Forest Functions and Services" Encyclopedia, https://encyclopedia.pub/entry/48899 (accessed February 08, 2026).
Akram, H., Hussain, S., Mazumdar, P., Chua, K.O., Butt, T.E., & Harikrishna, J.A. (2023, September 07). Mangrove Forest Functions and Services. In Encyclopedia. https://encyclopedia.pub/entry/48899
Akram, Hina, et al. "Mangrove Forest Functions and Services." Encyclopedia. Web. 07 September, 2023.
Copy Citation
Mangroves stand out as one of the most diverse and biologically significant natural systems in the world. Playing critical roles in maintaining the health and productivity of coastal ecosystems, mangroves provide a range of services and functions, including habitat for local fauna and flora, food and other goods, carbon sequestration, and protection from natural disasters such as storm surges and coastal erosion.
carbon sequestration
climate change
coastal development
mangrove biodiversity
mangrove management
microbial communities
mitigation
1. Biotic Communities Associated with Mangroves
1.1. Habitat for Local Communities
Mangrove ecosystems are habitats for local fauna and flora, providing breeding places, shelter, nesting, and nursing areas [1] (Table 1 and Table 2). Mangrove canopies are home to several wild animals, such as monkeys, monitor lizards, snakes, and otters [2]. The canopy also provides shade and shelter to aquatic-based animals, including amphibians and larger reptiles such as crocodiles [3] and dugongs [2]. Several birds inhabit mangroves, notably eagles, kingfishers, herons, plovers, terns, cormorants, egrets, and ibises [4]. On tree trunks, the residing flora includes orchids, ferns, lilies, and vines [5], which are home to invertebrates such as spiders and various insects [6]. Other than that, mangrove roots are swarmed by arthropods (crabs, lobsters, and shrimp) [7]; Molluscs (barnacles, oysters, mussels, and snails) [8]; sponges [9]; worms [10]; jellyfish [2]; and fish such as sea trout, snappers, jacks, tarpon, sea bass, red drums, and snook [11]. Moreover, mangroves host diverse epibiont macroalgal communities on their prop roots, trunks, and mud surfaces [12]. Mangrove habitats provide shallow water and, in many cases, high turbidity and fine sediment suitable for burrowing animals [13]. These factors act to protect animals from their predators by reducing their visibility and lowering their encounter rate with potential predators [14]. Mangrove plants, along with kelps, seagrasses, oysters, and corals, are key foundation species of coastal ecosystems [15]. Foundation species are crucial for maintaining the structure and resilience of an ecosystem [16].
Table 1. List of fauna associated with mangroves.
| Group | Common Name | Genus/Species | References |
|---|---|---|---|
| Sponges | Common Mangrove Sponge | Tedania sp. Mycale sp. Dysidea sp. Haliclona sp. |
[17] |
| Worms | Segmented worms | Sabellastarte sp. | [18] |
| Insects | Ant | Polyrachis bicolor sp. | [19] |
| Weevils | Rhynchites sp. | [20] | |
| Bettles | Monolepta sp. | [21] | |
| Crustaceans | Crabs | Ilyogynis microcheirum Portunus pelagicus Uca sp. Hippidea sp. |
[22][23] |
| Prawns | Penaeus monodon Exopalaemon styliferus Metapenaeus affinis Parapenaeopsis sculptilis |
[24][25] | |
| Barnacles | Balanus sp. Euraphia sp. Tetraclita sp. |
[26][27] | |
| Mollusks | Oyster | Crassostrea sp. | [28] |
| Clam | Tridacna derasa Tridacna maxima nodontia edentula |
[29][30][31] | |
| Sea slug/sea hares | Dolobella sp. | [32] | |
| Venus clam | Bursa sp. Paphia amabilis Venus clam Paphia Haliotis asinina Tectus pyramis Echininus cumingii Terebralia sulcata Rhinoclavis sinensis Rhinoclavis vertegus Ficus gracilis Plicacularia pullus Fasciolaria trapezium Oliva reticulata Mitra mitra Trisodos tortuosa Anadara maculosa Chicoreus brunneus |
[33][34][35][36][37] | |
| Echinoderms | Sea urchin | Protoreaster sp. Archaster sp. Linckia sp. Clypeaster sp. Cerithium sp. Tripneustes sp. Holothuria sp. Oreaster albeolatus Ophiarachna incrasala Echinocardium cordatum Diadema setosum Laganum laganum Echinometra mathaei |
[33][38][39][40] |
| Star fish | Astropecten sp. Protoreaster nodosus Linkia laevigata |
[40][41] | |
| Feather star | Comanthina bennetti Comanthina schlegeli |
[42] | |
| Sea star | Luidia sp. Culcita novaeguineae |
[43] | |
| Tunicates | Sea squirt | Didemnum molle Atriolum robustum Polycarpa aurata Rhopalea sp. |
[44] |
| Fishes | Rabbitfish | Siganid sp. | [45] |
| Mudskipper | Periophthalmodon Periophthalmus |
[45] | |
| Spot-tail needlefish | Strongylura strongylura | [46] | |
| Amphibians | Mangrove frog | Fejervarya cancrivora Rana cancrivora |
[47] |
| Reptiles | Snake | Cerberus rhybchos | [33] |
| Lizard | Tupinambis indicus | [48] | |
| Crocodiles | Crocodylus porosus | [49] | |
| Birds | Eagles | Haliastur indus Pitta megarhyncha |
[50][51] |
| Kingfishers | Halcyon senegaloides Todiramphus sordidus |
[52] | |
| Herons | Nycticorax nycticorax Egretta gularis |
[53][54] | |
| Plovers | Charadrius sp. Pluvialis sp. Thinornis sp. |
[55][56] | |
| Terns | Sterna paradisaea | [56] | |
| Crow | Corvus splendens | [57] | |
| Green pigeon | Treron olax | [57] | |
| Egrets | Egretta garzetta Egretta immaculata Egretta nigripes |
[58][59] | |
| Mammals | Bats | Cynopterus brachyotis Acerodon jubatus |
[60][61] |
| Monkey | Nasalis larvatus | [62] | |
| Dugong | Dugong dugon | [63] | |
| Otters | Lutrinae sp. | [64] |
Table 2. List of flora associated with mangrove.
| Group | Common Name | Genus/Species | References |
|---|---|---|---|
| Angiosperm | Seagrasses | Cymodocea sp. Thalassia sp. Halodule sp. Halophila sp. Enhalus sp. |
[65][66] |
| Orchids | Acampe sp. Agrostophyllum sp. Apotasi sp. Ascocentrum sp. Bulbophyllum sp. Ceratostylis sp. Cleisostoma sp. Cymbidium sp. Dendrobium sp. Flickingeria sp. Grosourdya sp. Habenaria sp. Liparis sp. Malaxis sp. Podochilus sp. Pomatocalpa sp. Thelasis sp. |
[67][68][69][70][71] | |
| Lilies | Crinum sp. Hymenocallis sp. Nymphaeaceae sp. Lycoris sp. |
[72][73] | |
| Vines | Cryptostegia grandiflora | [12] | |
| Bryophytes | Ferns | Acrostichum sp. Waterhousea sp. |
[74][75] |
| Algae | Marine algae | Padina sp. Ulva sp. Ventricaria ventricosa |
[76][77] |
1.2. Mangroves Association with Corals and Seagrass
Mangrove ecosystems are partly linked with and support corals and seagrasses [78]. Mangrove ecosystems have a positive impact on seagrass meadow traits such as shoot length, width, and height, shoot density, root length, number of leaves, leaf biomass, and population dynamics [79]. Mangrove roots trap the fine sediments coming from terrestrial sources and intercept turbid water, preventing it from reaching coral and seagrass systems [80]. On the other hand, coral reefs provide tranquil conditions that increase the deposition of fine sediments in adjusting areas, which supports the growth and development of seagrass beds and mangrove forests [81]. Likewise, corals and seagrasses maintain the balance between organic and inorganic carbon contents in coastal areas, subsequently establishing carbon sinks and sources in the mangrove ecosystem [82]. As mangrove forests, coral reefs, and seagrasses are interdependent ecosystems, to effectively store and export blue carbon in tropical coastal areas, it is essential to maintain the health of each of these coexisting ecosystems [83].
1.3. Reservoir of Microbial Communities
Mangroves are reservoirs of diverse microbial communities that include bacteria and fungi [84]. Organic sediments swept into mangroves by tides are inhabited by bacteria that decompose the organic debris and are primary contributors to carbon cycling [85]. Diverse bacteria in these populations are involved in many other essential ecological functions such as nitrogen fixation [86], photosynthesis [87], phosphate solubilisation [88], enzyme production [89], sulfate reduction [90], antibiotic production [91], anoxygenesis [92], and methanogenesis [93] (Table 3). Among fungi, the dominant fungal phyla are Ascomycetes and Basidiomycetes, which have been reported to be primarily associated with the survival of mangrove plants in waterlogged and nutrient-restricted environments [94] (Table 3). The microbial communities of mangroves improve nutrient availability, support the growth of vegetation, and provide protection from pathogenic bacteria, thereby positively impacting species diversity [95].
Table 3. Major microbial groups inhabiting the mangrove forests.
| Group | Phyla | Functions | References |
|---|---|---|---|
| Bacteria | Actinobacteria |
|
[96] |
| Chloroflexota |
|
[84][85] | |
| Asgardarchaeota |
|
[97] | |
| Bacteroidetes |
|
[16] | |
| Thermoproteota |
|
[98] | |
| Calditrichota |
|
[99] | |
| Bacillota |
|
[100] | |
| Thermodesulfobacteriota |
|
[95] | |
| Euryarchaeota |
|
[84] | |
| Firmicutes |
|
[101][102] | |
| Halobacterota |
|
[103] | |
| Nitrososphaerota |
|
[98] | |
| Nitrospirota |
|
[93] | |
| Planctomycetota |
|
[104] | |
| Pseudomonadota |
|
[105][106] | |
| Thaumarchaeota |
|
[93] | |
| Zixibacteria |
|
[107] | |
| Cyanobacteria | Cyanobacteriota |
|
[108][109] |
| Fungi | Ascomycota |
|
[94][110] |
| Basidiomycota |
|
[111] |
2. Mangrove Ecosystem and Economic Functions and Services
There are several functions of mangrove forests other than as habitats for flora and fauna: They act as a carbon sink (blue carbon storage) [112], maintain water quality [113], protect coastal land from natural disasters [114], and support coral and seagrass ecosystems [115] (Figure 1). In addition, mangroves provide livelihood opportunities for coastal communities through aquaculture, fodder, timber, and ecotourism [116].
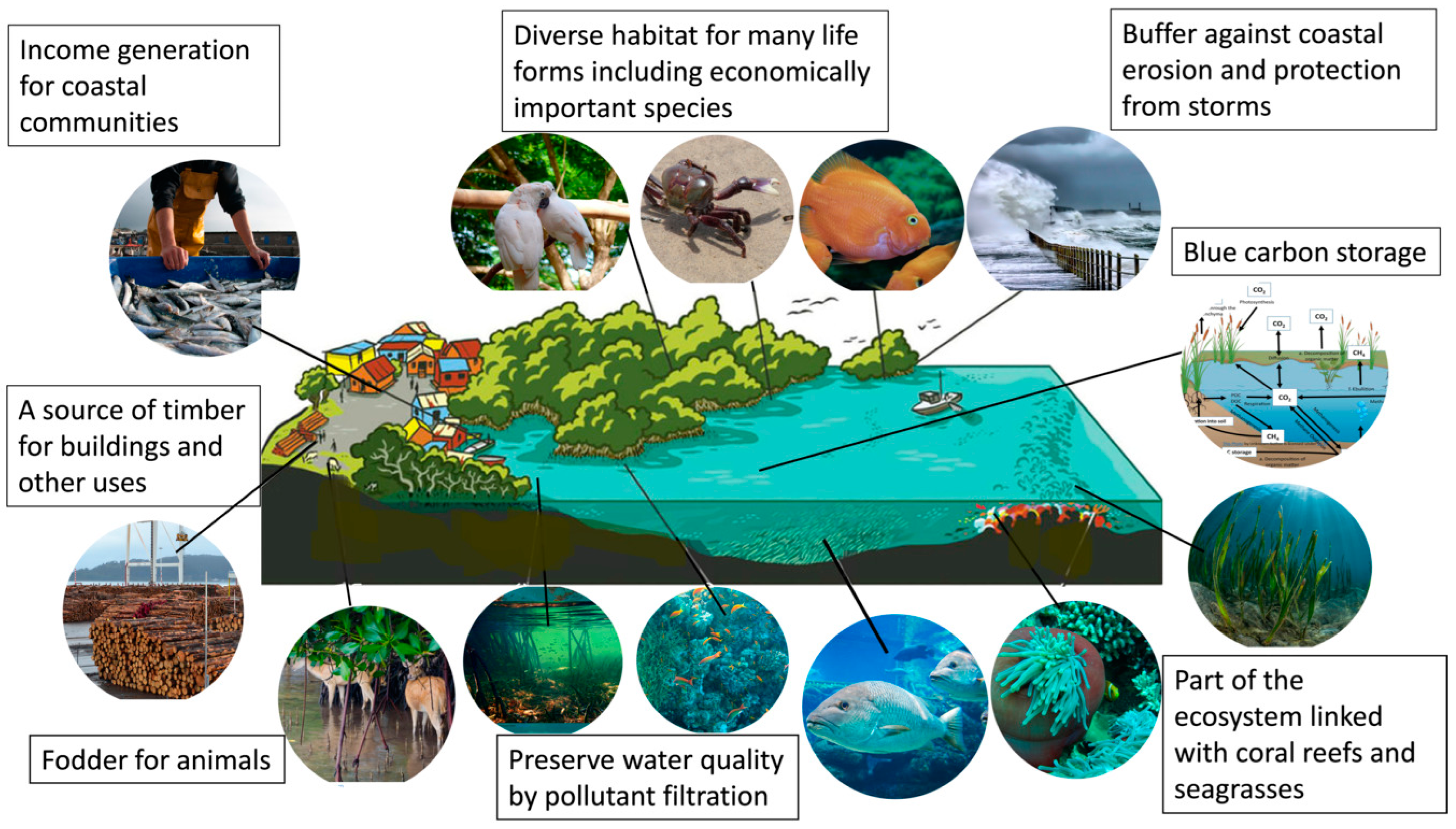
Figure 1. Functions and services of an intact mangrove ecosystem.
2.1. Carbon Sink
Mangroves play an important role in mitigating the effects of greenhouse gases generated by anthropogenic activities such as deforestation, agriculture, and industrial processes. This mitigation involves removing CO2 from the atmosphere, after which mangrove flora sequester carbon in their above- and below-ground biomass [112]. Mangroves, as a carbon sink, can hold an estimated 1023 Mg/hectare of carbon [117]. Various studies have confirmed that mangroves have a faster carbon sequestering capacity than other ecosystems, such as grasslands or tropical rainforests [118]. According to a report from the Global Mangrove Alliance (GMA) 2022 [119], the total organic carbon stored in mangrove forests at a global level is estimated at around 21,896.56 Mt CO2e with 2817.23 Mt CO2e stored in above-ground biomass and 19,079.32 Mt CO2e stored in the upper 1 m of soil [120]. It can be seen from Figure 2 that the carbon storage capacity varies quite considerably for different countries, with Indonesia having a relatively strong capacity compared to the other countries. In mangroves, carbon-rich soils extend from 0.5 m to ~3 m in depth and accommodate 49%–98% of the carbon stored by the mangrove ecosystem [121]. Figure 2 represents the organic carbon storage capacity of mangrove forests in various countries as above-ground biomass (data derived from GMW version 0.3, 2020) [122]. As mangroves store a considerable amount of carbon, the destruction of this habitat disturbs the carbon sink and emits huge amounts of carbon back into the atmosphere, significantly contributing to climate change. Therefore, protecting and restoring mangrove habitats can reduce the impact of climate change [123]. Although it would be great to consider many more countries in this discussion, due to the brevity of the paper, only 12 countries have been included that have the most robust data, as shown in Figure 2.
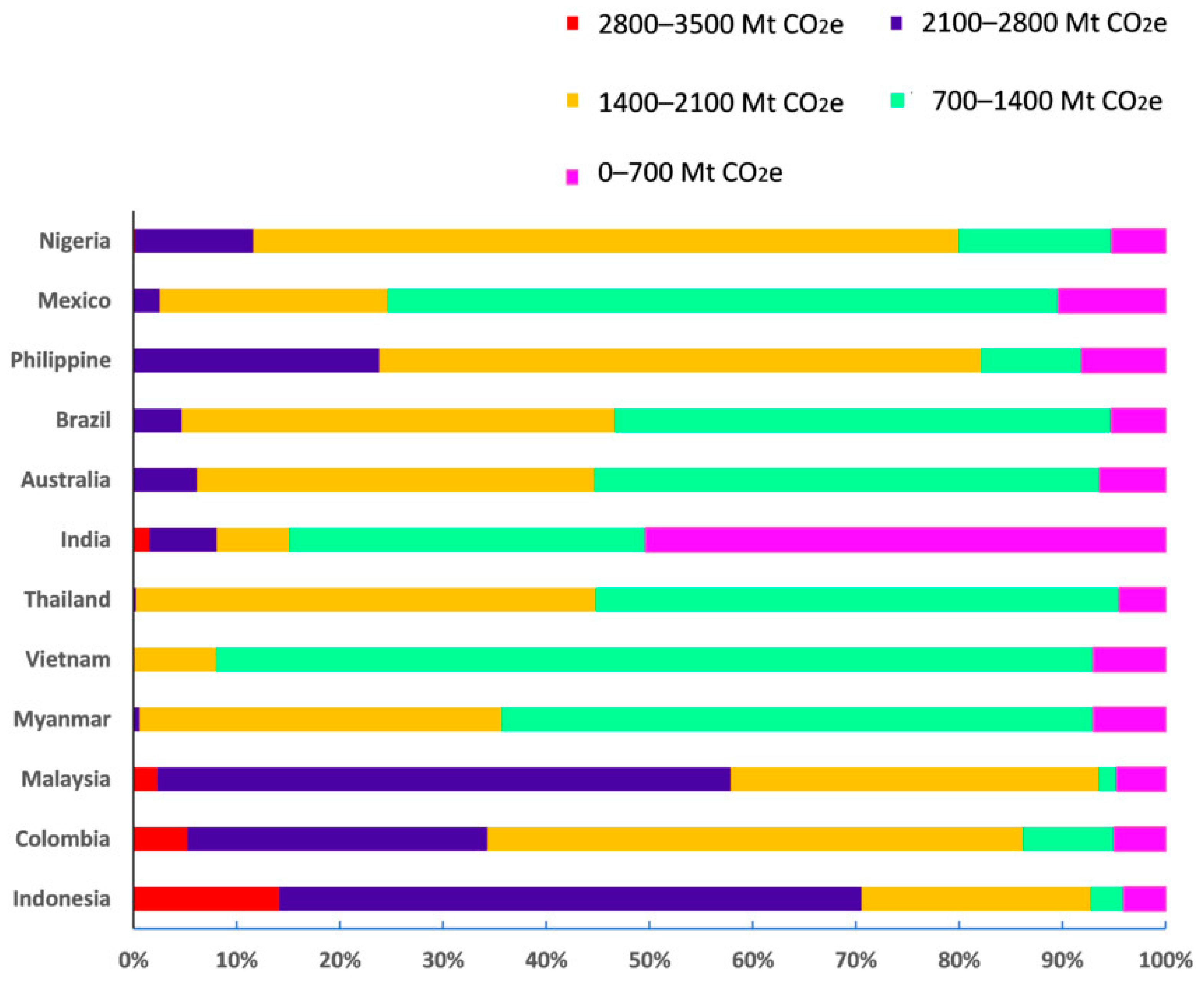
Figure 2. Above-ground carbon storage capacity of mangrove forests in different countries in 2020. Each country has mangrove forests with different carbon storage capacities, which are presented in ranges of carbon storage measured in metric tons of equivalent carbon (Mt CO2e) with each range represented by a different colour. The x-axis is a scale bar of the percentages of the total forests in each country that fall into each carbon storage range. (Data sourced from GMW, 2022).
2.2. Natural Water Filters
Mangrove forests act as natural water filters for coastal areas, improving the water quality by trapping sediments and other solid impurities with their roots [113]. This reduces the flow of sediments into offshore waters, thereby reducing erosion [124], maintaining clean habitats for seagrass beds and coral reefs, and contributing to SDG 14, which talks about life below water [125]. Mangroves can grow in saline water and filter 90% of sodium ions (Na+) from the surrounding seawater [126]. Their roots comprise a three-layered pore structure in the root epidermis, which facilitates Na+ filtration [127]. Additionally, mangrove roots, such as pneumatophores and prop roots, create a low-energy environment, allowing wastewater-containing contaminants to reside for an extended period [128]. Mangrove plants also sequester other metals, including the heavy metals Zn, Mn, and Cu [129]. The study of the mechanisms by which mangrove plants filter water has led to novel water treatment technology: Researchers at Virginia Tech (Virginia Polytechnic Institute and State University, USA) [130] have developed a “synthetic tree” water purifier system inspired by the water filtration technique used in mangrove plants. Specifically, a synthetic tree is composed of a nano-porous “leaf” to produce suction via evaporation, a vertical column of glass tubes similar to the xylem vessels of the tree, and filters attached to the tube inlets, mimicking roots [130]. In another recent study, a group of engineers from Yale University (New Haven, CT, USA) invented a water purification device that mimics the desalinisation ability of mangrove trees based on the principle of cohesion-tension theory in mangroves. In this technique, synthetic leaves can generate highly negative pressures that allow desalination through a reverse osmosis (RO) membrane [131].
2.3. Barriers to Natural Disasters
Mangroves not only prevent soil and coastal erosion by retaining sediments in their aerial roots [124] but also act as barriers against natural disasters. The canopy, trunk, and roots of mangrove plants restrain storm surges [114] and waves [132]. In the aftermath of the Asian tsunami on 26 December 2004 [133], Hurricane Katrina on 23 August 2005, on the US Gulf Coast [134], and the Transoceanic tsunami on 23 January 2022 [135], persuasive evidence emerged from field studies in several countries justifying the role of mangroves as natural barriers protecting coastal habitats and communities. It is quite evident after the tsunami survey that the intact and dense mangroves with higher structural complexity near coastal areas offered fewer fatalities and minimal damage to assets as compared to the areas where mangroves had either been destroyed or transformed to alternate land uses [136][137].
2.4. Livelihood Opportunities for Coastal Communities
About 90% of the global mangrove forests grow in economically less privileged countries [138]. Approximately 100 million people live within a 10 km range of mangrove forests and directly benefit from this ecosystem as a source of livelihood opportunities [139].
2.4.1. Aquaculture
Mangroves are considered hotspot locations for aquaculture [140]. The species commonly reared include various fish, shrimp/prawns, crabs, molluscs, and other invertebrates [141]. Approximately 80 million tonnes of fish were produced globally through aquaculture in 2022 [142]. Extensive mangrove-associated aquaculture has been observed in Indonesia, Malaysia, and the Philippines [143]. Mangrove-associated aquaculture accounts for 21% (1.4 million tons annually) of the coastline fisheries of the ASEAN (Association of South East Asian Nations) region [144]. Of the annual fish and seafood resources, fin fish alone contribute around 1.09 million tons [145], while shrimp/prawn contribute around 0.4 million tons [146]. In addition, fish products from these aquaculture activities are a principal source of food for coastal communities.
Large-scale aquaculture [147], fish farming in cages or in ponds [148], and integrated rice-fish farming [149] have reduced pressure on overexploited fisheries by diversifying fish production other than wild stocks. Small-scale aquaculture, in particular, enables fish farmers to provide food for their families while generating income from the sale of surplus stock [150]. Such activities also create employment opportunities through various enterprises ranging from the processing, distribution, and sale of fish linked to the aquaculture value chain [151]. These livelihood opportunities facilitate the sustainable mangrove ecosystem’s ability to successfully contribute to the outcomes of various sustainable development goals set by the United Nations, such as SDG 1, SDG 2, SDG 8, SDG 11, SDG 13, SDG 14, and SDG 15. (The detailed agenda of these SDGs can be seen at https://www.un.org/development/desa/disabilities/envision2030.html, accessed on 11 July 2023) [152].
2.4.2. Fodder, Timber and Traditional Medicines
Mangroves also provide fodder, timber, and medicine resources for coastal indigenous communities (Figure 1). Cattle, sheep, goats, and buffaloes are domestic animals that are generally fed on mangrove foliage [153]. Mangrove foliage, particularly from Avicennia marina, is considered healthy fodder for domestic animals (Mitra, 2020). Mangrove wood, being highly resistant to rot and insects, is frequently utilised as timber as well as for fuel wood [154]. Rhizophora spp., Xylocarpus sp., Bruguiera sp., and Sonneratia sp. are significantly important for timber due to the durability of their wood and their large trunk size [155]. The timber of these species is used for small watercraft, shipbuilding, and for making utensil handles, furniture, poles, piles, and other building materials [156]. Mangrove firewood has been widely used as an energy source by rural communities.
Mangrove services also include the provision of traditional medicine for treating skin ailments and stomach issues [157]. Extracts from mangrove-associated species, for example, Abonnema and Nypa fruticans, have shown antimicrobial activity against some plant and animal pathogens [158]. The bioactive compound ecteinascidin, extracted from the mangrove tunicate Ecteinascidia turbinate, has been reported to show strong in vivo activity against various cancerous cells [159]. Furthermore, the bark of Ceriops sp. is a good source of tannin, and its decoction is used in Vedic medicine to stop haemorrhage and in the treatment of malignant ulcers [160].
2.4.3. Ecotourism
Ecotourism refers to the form of tourism that focuses on responsible travel that minimises environmental impact and supports local communities [161]. Ecotourism in mangrove regions places a strong emphasis on mangrove conservation, education of visitors about the mangrove forest, and providing economic benefit to local communities [162]. Ecotourism syndicates three key aspects, viz., (i) ecology, which includes the existence of the elements upon which the mangrove ecosystem depends and also its conservation efforts [163], (ii) financial revenue generated as a result of ecotourism activities in sustainable mangroves, a share of which is expended to maintain the ecosystem [164], and (iii) empowerment and engagement of the local community in the ecotourism business [165]. The species diversity of both fauna and flora and the unique characteristics of mangrove plants have been a great attraction for ecotourism [166]. Mangrove areas offer several forms of ecotourism activities, such as sports and recreational activities such as fishing, boating, and camping [167]; educational and research tourism in the form of field trips to mangroves to observe and study the mangrove vegetation and life inside the mangroves [168]; and health tourism as sites for self-meditation and other therapy [169]. Many mangrove forests have been established as tourist attractions by governmental or non-governmental organisations in different regions [170]. For example, areas of mangrove forest in Bali, Indonesia, have been established by local communities for the purpose of ecotourism and to maintain the conservation of biodiversity, landscapes, and the ecosystem overall [171]. Ecotourism activities carried out by these community groups are supported and fostered by the relevant stakeholders of the region and/or the state government and have been incorporated as a part of their CSR (corporate social responsibility) program [172]. The use of mangroves for ecotourism is in accordance with the development directions of the Sustainable Development Goals (SDGs), 12, 13, 14, 15, and 17 [173].
References
- Nunoo, F.K.; Agyekumhene, A. Mangrove Degradation and Management Practices along the Coast of Ghana. Agric. Sci. 2022, 13, 1057–1079.
- Spalding, M.; Parrett, C.L. Global patterns in mangrove recreation and tourism. Mar. Policy 2019, 110, 103540.
- Aung, T.T. Mangroves in Myanmar. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Pullaiah, T., Ashton, E.C., Eds.; Springer Nature: Singapore, 2022; pp. 331–371.
- Hassan, H.U.; Ali, Q.M.; Ahmad, N.; Attaullah, M.; Chatta, A.M.; Farooq, U.; Ali, A. Study of vertebrate diversity and associated threats in selected habitats of Sindh and Baluchistan, Pakistan. Int. J. Biol. Biotechnol. 2020, 17, 163–175.
- Aburto-Oropeza, O.; Burelo-Ramos, C.M.; Ezcurra, E.; Ezcurra, P.; Henriquez, C.L.; Vanderplank, S.E.; Zapata, F. Relict inland mangrove ecosystem reveals Last Interglacial sea levels. Proc. Natl. Acad. Sci. USA 2021, 118, e2024518118.
- Arceo-Carranza, D.; Chiappa-Carrara, X.; Chávez López, R.; Yáñez Arenas, C. Mangroves as feeding and breeding grounds. In Mangroves: Ecology, Biodiversity and Management; Springer: Singapore, 2021; pp. 63–95.
- Davie, P.J. Crabs: A Global Natural History; Princeton University Press: Princeton, NJ, USA, 2021.
- Vozzo, M.L.; Bishop, M.J.; Dafforn, K.A.; Mayer-Pinto, M. Volvo Cars Australia-Sydney Institute of Marine Science ‘Living Seawall’ Biodiversity Assessment. 2021. Available online: https://static1.squarespace.com/static/60efa1626de4b55189f0d735/t/62c4dfe9e3afc61414478f89/1657069552808/SIMS_Volvo+Living+Seawall_24+Month+Report.pdf (accessed on 11 July 2023).
- Wulff, J.L. Targeted predator defenses of sponges shape community organization and tropical marine ecosystem function. Ecol. Monogr. 2021, 91, e01438.
- Le Huy Baa, N.X.H.; Nguyenb, T.T.; Van Namb, T. The Bio-Habit and Role of Peanut Worm (Sipuculus Nudus) in Mangrove Ecosystems of Thanh Phu, Ben Tre Province and Can Gio, Ho Chi Minh City, Viet Nam. Chem. Eng. 2021, 84, 1–6. Available online: https://www.researchgate.net/profile/Van-Thai/publication/361228677_The_bio-habit_and_role_of_peanut_worm_sipuculus_nudus_in_mangrove_ecosystems_of_Thanh_Phu_Ben_Tre_province_and_Can_Gio_Ho_Chi_Minh_City_Viet_Nam/links/62a4876a55273755ebe31c05/The-bio-habit-and-role-of-peanut-worm-sipuculus-nudus-in-mangrove-ecosystems-of-Thanh-Phu-Ben-Tre-province-and-Can-Gio-Ho-Chi-Minh-City-Viet-Nam.pdf (accessed on 11 March 2023).
- Jane, S.F.; Smith, K.M.; Baker, D.; Saroni, A.; Cutler, E.; Carvalho, P. News media and fisheries-independent data reveal hidden impacts of hurricanes. Ambio 2022, 51, 2169–2181.
- Goudkamp, K.; Chin, A. Mangroves and Saltmarshes. 2006. Available online: https://elibrary.gbrmpa.gov.au/jspui/retrieve/b16abcf6-52fb-445d-b7fd-9af2e7ffb4bc/State-of-the-Reef-Report-2006-Mangroves-and-saltmarshes.pdf (accessed on 2 February 2023).
- Egawa, R.; Sharma, S.; Nadaoka, K.; MacKenzie, R.A. Burrow dynamics of crabs in subtropical estuarine mangrove forest. Estuar. Coast. Shelf Sci. 2021, 252, 107244.
- Min, W.W.; Kandasamy, K.; Balakrishnan, B. Crab species-specific excavation and architecture of burrows in restored mangrove habitat. J. Mar. Sci. Eng. 2023, 11, 310.
- Ridlon, A.D.; Marks, A.; Zabin, C.J.; Zacherl, D.; Allen, B.; Crooks, J.; Fleener, G.; Grosholz, E.; Peabody, B.; Toft, J. Conservation of marine foundation species: Learning from native oyster restoration from California to British Columbia. Estuar. Coast 2021, 44, 1723–1743.
- Coe, M.A.; Gaoue, O.G. Cultural keystone species revisited: Are we asking the right questions? J. Ethnobiol. Ethnomed. 2020, 16, 70.
- Bettcher, L.; Fernandez, J.C.; Gastaldi, M.; Bispo, A.; Leal, C.V.; Leite, D.; Avelino-Alves, D.; Clerier, P.H.; Rezende, D.; Gulart, C.M. Checklist, diversity descriptors and selected descriptions of a highly diverse intertidal sponge (Porifera) assemblage at Costa do Descobrimento (Bahia, Brazil). Zootaxa 2023, 5277, 443–489.
- Brown, S. Revising the Taxonomy and Biology of Ornamental Worms (Polychaeta: Sabellidae) around the Arabian Peninsula. 2020. Available online: https://repository.kaust.edu.sa/handle/10754/662775 (accessed on 2 April 2023).
- Hardiwinoto, S.; Syahbudin, A. Changes in insect biodiversity on rehabilitation sites in the southern coastal areas of Java Island, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 1.
- Husain, P.; Al Idrus, A.; Ihsan, M.S. The ecosystem services of mangroves for sustainable coastal area and marine fauna in Lombok, Indonesia: A review. J. Inov. Pendidik. Dan Sains 2020, 1, 1–7.
- Setyawan, Y.P. The denser canopy of mangrove drives the structure of insect communities. Trop. Life Sci. Res. 2020, 31, 77–90.
- Hajializadeh, P.; Safaie, M.; Naderloo, R.; Shojaei, M.G. Spatial and temporal distribution of brachyuran crabs in mangroves of the Persian Gulf. Wetlands 2022, 42, 99.
- Sharifian, S.; Kamrani, E.; Saeedi, H. Global biodiversity and biogeography of mangrove crabs: Temperature, the key driver of latitudinal gradients of species richness. J. Therm. Biol. 2020, 92, 102692.
- Sinage, A.A.L. Distribuição Longitudinal e Sazonal da População do Camarão Explorado Pela Pesca Artesanal no Estuário de Macuse, Moçambique. 2019. Available online: http://monografias.uem.mz/handle/123456789/2874 (accessed on 11 March 2023).
- Nandi, N. Aquaculturable fishery resources in wetlands of West Bengal. J. Aquac. Mar. Biol. 2023, 12, 80–84.
- Williams, G.A.; Chan, B.K.; Dong, Y.-W.; Hawkins, S.; Bohn, K.; Firth, L. Rocky Shores of Mainland China, Taiwan and Hong Kong: Past, Present and Future. Interact. Mar. Benthos Glob. Patterns Process. 2019, 87, 360–390. Available online: https://www.researchgate.net/profile/Yunwei-Dong-2/publication/335676645_Rocky_Shores_of_Mainland_China_Taiwan_and_Hong_Kong_Past_Present_and_Future/links/5d7857fb4585151ee4adfbe6/Rocky-Shores-of-Mainland-China-Taiwan-and-Hong-Kong-Past-Present-and-Future.pdf (accessed on 15 March 2023).
- Pitriana, P.; Valente, L.; von Rintelen, T.; Jones, D.S.; Prabowo, R.E.; von Rintelen, K. An annotated checklist and integrative biodiversity discovery of barnacles (Crustacea, Cirripedia) from the Moluccas, East Indonesia. ZooKeys 2020, 945, 17.
- Azevedo, J.A.M.; Barros, A.B.; Miranda, P.R.B.d.; Costa, J.G.D.; Nascimento, V.X. Biomonitoring of heavy metals (Fe, Zn, Cu, Mn, Cd and Cr) in oysters: Crassostrea rhizophorae of mangrove areas of Alagoas (Brazil). Braz. Arch. Biol. Technol. 2019, 62, e19180211.
- Zvonareva, S.S.; Mekhova, E.S.; Hà, V.T.; Kantor, Y.I. Checklist of bivalve molluscs in mangroves of Khánh Hòa Province, Vietnam. Molluscan Res. 2019, 39, 296–312.
- Bahari, N.A.; Jaafar, N.S.N.; Nor, S.M.M.; Omar, W.B.W. Habitat preferences of mangrove clam (Geloina expansa) in East coast of Peninsular Malaysia. Aquac. Aquar. Conserv. Legis. 2021, 14, 3776–3781.
- Argente, F.; Ilano, A. Population dynamics and aquaculture potential of the mud clam, Geloina expansa (Mousson, 1849) (Bivalvia: Cyrenidae) in Loay-Loboc River, Bohol, Central Philippines. J. Sustain. Sci. Manag. 2021, 16, 43–55.
- Zhang, H.; Zou, J.; Yan, X.; Chen, J.; Cao, X.; Wu, J.; Liu, Y.; Wang, T. Marine-derived macrolides 1990–2020: An overview of chemical and biological diversity. Mar. Drugs 2021, 19, 180.
- Picardal, J.P.; Avila, S.T.R.; Tano, M.F.; Marababol, M.S. The species composition and associated fauna of the mangrove forest in Tabuk and Cabgan Islets, Palompon Leyte, Philippines. CNU J. High. Educ. 2011, 5, 1–18.
- Islami, M.M.; Dody, D.G.B.a.S. Spatial variation in population characteristics of tumid venus clam Gafrarium tumidum Röding, 1798 (Bivalvia: Veneridae) in Ambon Bay, Maluku. Mar. Res. Indones. 2018, 43, 63–70.
- Peck, H. The Application of Ecological Models and Trophic Analyses to Archaeological Marine Fauna Assemblages: Towards Improved Understanding of Prehistoric marine Fisheries and Ecosystems in Tropical Australia. Ph.D. Thesis, James Cook University, Douglas, QLD, Australia, 2016.
- Dolorosa, R.G.; Dangan-Galon, F. Population dynamics of the mangrove clam Polymesoda erosa (Bivalvia: Corbiculidae) in Iwahig, Palawan, Philippines. Int. J. Fauna Biol. Stud. 2014, 1, 11–15.
- da Silva Mourão, J.; Baracho, R.L.; de Faria Lopes, S.; Medeiros, M.C.; Diele, K. The harvesting process and fisheries production of the venus clam Anomalocardia flexuosa in a Brazilian extractive reserve, with implications for gender-sensitive management. Ocean Coast. Manag. 2021, 213, 105878.
- Alves, R.R.N.; Pinto, M.F.; Borges, A.K.M.; Oliveira, T.P.R. Fisheries and Uses of Coastal Aquatic Fauna in the Northernmost Brazilian Atlantic Forest. In Animal Biodiversity and Conservation in Brazil’s Northern Atlantic Forest; Springer: Berlin/Heidelberg, Germany, 2023; pp. 229–255.
- Cunha-Lignon, M.; Mendonça, J.T.; Conti, L.A.; de Souza Barros, K.V.; Magalhães, K.M. Mangroves and Seagrasses. In Blue Economy: An Ocean Science Perspective; Springer: Berlin/Heidelberg, Germany, 2022; pp. 55–85.
- Lidour, K.; Béarez, P.; Beech, M.; Charpentier, V.; Méry, S. Intensive exploitation of marine crabs and sea urchins during the middle holocene in the eastern Arabian peninsula offers new perspectives on ancient maritime adaptations. J. Isl. Coast. Archaeol. 2021, 18, 498–526.
- Tawasil, S.I.; Alibon, R.D.; Bensali, S.L. Species diversity of Echinoderms in Manubul Island, Sulu Pro-vince, Southern Philippines. Biodivers. J. 2021, 12, 301–311.
- Madin, J.; Angkirim, M.A.; Yassine, M.; Nor’azlan, S.N. Feather Star Community in Sepanggar Bay, Sabah. In The Marine Ecosystems of Sabah; Universiti Malaysia Sabah Press: Sabah, Malaysia, 2022; p. 153.
- Keable, S.J.; Mah, C.L. Range extension of the Regulus seastar Pentaceraster regulus (Müller & Troschel, 1842) (Echinodermata: Asteroidea: Oreasteridae): Evidence of tropicalization of the east Australian coast. Tech. Rep. Aust. Mus. Online 2021, 35, 1–10.
- Brant, C. The Sentience of Sea Squirts. In Life Writing in the Posthuman Anthropocene; Springer: Berlin/Heidelberg, Germany, 2021; pp. 123–156.
- Habib, K.A.; Neogi, A.K.; Nahar, N.; Oh, J.; Lee, Y.-H.; Kim, C.-G. An overview of fishes of the Sundarbans, Bangladesh and their present conservation status. J. Threat. Taxa 2020, 12, 15154–15172.
- Nair, R.J.; Dinesh Kumar, S. Overview of the fish diversity of Indian waters. In Proceedings of the DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals, Kochi, Japan, 2 February 2015–31 March 2018.
- Satheeshkumar, P. First record of a mangrove frog Fejervarya cancrivora (Amphibia: Ranidae) in the Pondicherry mangroves, Bay of Bengal-India. World J. Zool. 2011, 6, 328–330.
- Weijola, V. Case 3676 Tupinambis indicus Daudin, 1802 (currently Varanus indicus; Reptilia, Squamata): Proposed conservation of usage of the specific name by replacement of the neotype. Bull. Zool. Nomencl. 2015, 72, 134–141.
- Saragih, G.; Hidayatullah, M.; Hadi, D. A preliminary study on the population and habitat of saltwater crocodile (Crocodylus porosus) in Timor Island, East Nusa Tenggara. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Tangerang, Indonesia, 24 October 2019; p. 012044.
- FOREST, S. World Biodiversity Congress. Available online: https://d1wqtxts1xzle7.cloudfront.net/47312793/Density_and_diversity_of_bird_species_at20160717-24890-kv1fd8-libre.pdf?1468819714=&response-content-disposition=inline%3B+filename%3DDensity_and_diversity_of_bird_species_at.pdf&Expires=1689590275&Signature=WSKCOeqFMlLlWoA2oz1sEFNjazexNOX2VKzCgZ1yeqharlnjepWJS1ml9LD7M~C0viHEoCgnvYMPEb~cJAv4KdU567R3DM3f031eJr1H9U5h1wJfj~wBPhDHk3KBQ9PopBRHNSn-mlkh5p2vrwsqMcjMlOe07C6whNXyIv-Aay-wJ4CGxtEpVt7gJRsUXRxs1g6bCkO1wlIm4TtJZT~yIUkAuwoeMKMAuBWkYuOBZx4Rf6e5MGQmOnq9Hg1HOVRL2HLo0PJciuRDjYKjFh8EhEPrs0Xs2Ip-y7VL2wpKpdzNNE54axEoVeFzyre3KaxPL6llJ3QcOAtls1YhpqfayQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 15 March 2023).
- Khaleghizadeh, A.; Anuar, S. Nest tree selection by the Brahminy Kite Haliastur indus in a Rhizophora mangrove forest. Trop. Zool. 2014, 27, 40–52.
- Davies, G.B.; Symes, C.T.; Peek, J.R. Mangrove kingfishers (Halcyon senegaloides; Aves: Alcedinidae) nesting in arboreal Nasutitermes (Isoptera: Termitidae: Nasutitermitinae) termitaria in central Mozambique. Ann. Ditsong Natl. Mus. Nat. Hist. 2012, 2, 146–152.
- Etezadifar, F.; Barati, A. Nest-site selection of Western Reef Heron (Egretta gularis) in relation to mangrove (Avicennia marina) structure in the Persian Gulf: Implication for management. For. Ecol. Manag. 2013, 310, 74–79.
- Padmakumar, V.; Joseph, S.P. Understanding the mangrove-associated avifauna and their conservation status in the Gorai Creek, Western Mumbai, Maharashtra, India: A Recent Study. Int. J. For. Anim. Fish. Res. 2022, 6, 3.
- Crossland, A.C.; Sitorus, A.W.; Sitorus, A.S. Land use change impacts shorebird habitat at an important site for javan plover charadrius javanicus and sanderling calidris alba in java, indonesia. Stilt J. East Asian-Australas. Flyway 2014, 66, 30–36.
- Hale, J. Addendum to the Ecological Character Description for the Corner Inlet Ramsar Site. 2017. Available online: https://www.water.vic.gov.au/__data/assets/pdf_file/0025/85444/Corner-Inlet-Ramsar-Site-Ecological-Character-Description-Addendum.pdf (accessed on 14 April 2023).
- Tan, H.; Low, G.W.; Sadanandan, K.; Rheindt, F.E. Population assessment of the house crow, Corvus splendens, in Singapore. Malay. Nat. J. 2020, 72, 133–142.
- Hancock, J.; Kushlan, J.A. The Herons Handbook; A&C Black: London, UK, 2010.
- Johnstone, R.; van Balen, S. The birds of the Kai and Tayandu islands, Maluku region, Indonesia. West. Aust. Nat. 2013, 29, 11–56.
- Thong, V.D.; Denzinger, A.; Long, V.; Sang, N.V.; Huyen, N.T.T.; Thien, N.H.; Luong, N.K.; Tuan, L.Q.; Ha, N.M.; Luong, N.T. Importance of mangroves for bat research and conservation: A case study from Vietnam with notes on echolocation of Myotis hasselti. Diversity 2022, 14, 258.
- Morrison, R. Flying fox: Characteristics, Habitat, Reproduction, Feeding. Available online: https://warbletoncouncil.org/zorro-volador-902 (accessed on 14 April 2023).
- Shanida, S.S.; Lestari, T.H.; Partasasmita, R. The effect of total solar eclipse on the daily activities of Nasalis larvatus (Wurmb.) in Mangrove Center, Kariangau, East Kalimantan. In Proceedings of the Journal of Physics: Conference Series, Bandung, Indonesia, 3–4 June 2016; p. 012017.
- Akbar, N.; Marus, I.; Ridwan, R.; Baksir, A.; Paembonan, R.; Ramili, Y.; Tahir, I.; Ismail, F.; Wibowo, E.; Madduppa, H. Feeding ground indications are based on species, seagrass density and existence of Dugong dugon in Hiri Island Sea, North Maluku, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Ternate, Indonesia (Virtual), 15 July 2021; p. 012058.
- Li, F.; Chan, B.P.L. Past and present: The status and distribution of otters (Carnivora: Lutrinae) in China. Oryx 2018, 52, 619–626.
- Notified, I.S.F.D.C.R.; Bay, I.P.; Nadu, T. Sirenews. 2023. Available online: https://www.researchgate.net/profile/Prachi-Hatkar/publication/370561039_INDIA’S_FIRST_DUGONG_CONSERVATION_RESERVE_NOTIFIED_IN_PALK_BAY_TAMIL_NADU_BRINGING_HOPE_FOR_EXTINCTION-PRONE_DUGONGS/links/6455f98a4af78873525fb1ad/INDIAS-FIRST-DUGONG-CONSERVATION-RESERVE-NOTIFIED-IN-PALK-BAY-TAMIL-NADU-BRINGING-HOPE-FOR-EXTINCTION-PRONE-DUGONGS.pdf (accessed on 15 April 2023).
- Mendoza, A.R.R.; Patalinghug, J.M.R.; Divinagracia, J.Y. The benefit of one cannot replace the other: Seagrass and mangrove ecosystems at Santa Fe, Bantayan Island. J. Ecol. Environ. 2019, 43, 18.
- Latiff, A. Biodiversity in Malaysia. Glob. Biodivers. 2018, 1, 307–349.
- Karthigeyan, K.; Jayanthi, J.; Sumathi, R.; Jalal, J. A review of the orchid diversity of Andaman & Nicobar Islands, India. Richardiana 2014, 15, 9–85.
- Alanís-Méndez, J.L.; Ortiz-Santos, L.d.C.; Chamorro-Florescano, I.A.; Pech-Canché, J.M.; Limón, F. Pollinators and floral visitors of two orchids in a protected natural area in Tuxpan, Veracruz. Ecosistemas Y Recur. Agropecu. 2019, 6, 361–368.
- Antoh, A.A. Diversity of EPIFIT Orchid Types in the Mangrove Ecosystem in Ayari Village, Teluk Ampimoi Sub District, Yapen Islands District, Papua Province, Indonesia. Int. J. Multidiscip. Res. Anal. 2023, 6, 1328–1331.
- Nurtjahya, E.; Sari, E. Flora of Bangka-A Preliminary Check List. In Proceedings of the 10th Flora Malesiana Symposium, Edinburgh, UK, 11–15 July 2016.
- Tunnell, J.W., Jr.; Chávez, E.; Withers, K. Island biota. In Coral Reefs of the Southern Gulf of Mexico; Texas A&M Press: College Station, TX, USA, 2007; pp. 119–125. Available online: https://www.researchgate.net/profile/John-Tunnell-Jr/publication/293306381_Island_biota/links/58acbc0a92851c3cfda05a8f/Island-biota.pdf (accessed on 16 April 2023).
- Montero, J. Synergistic Effect of Seaweed (Phylum ocrophyta) and Water Lily (Nymphaeaceae alba) Extract as Catalysts for the Rapid Germination of Mangrove Seeds. Ascendens Asia J. Multidiscip. Res. Abstr. 2019, 3, 2.
- Melville, S. An Assessment of the Fauna Habitat along Kedron Brook. Rep. Wildl. Preserv. Soc. Qld. Brisb. 2001. Available online: http://kbcb.server101.com/references/references/Kedron_Brook_Fauna_Report.pdf (accessed on 14 April 2023).
- Kumar, M.; Srivastava, G.; Spicer, R.A.; Spicer, T.E.; Mehrotra, R.C.; Mehrotra, N.C. Sedimentology, palynostratigraphy and palynofacies of the late Oligocene Makum Coalfield, Assam, India: A window on lowland tropical vegetation during the most recent episode of significant global warmth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 342, 143–162.
- Valenzuela, H.Y.; Bacalso, A.D.; Gano, C.B.; Pilones, K.D.; Picardal, J.P. The species composition and associated flora and fauna of the mangrove forest in Badian, Cebu Island, Philippines. IAMURE Int. J. Mar. Ecol 2013, 1, 1–23.
- Zulpikar, F.; Handayani, T. Life form, diversity, and spatial distribution of macroalgae in Komodo National Park waters, East Nusa Tenggara. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 24–25 August 2021; p. 012026.
- Bastos, R.F.; Lippi, D.L.; Gaspar, A.L.B.; Yogui, G.T.; Frédou, T.; Garcia, A.M.; Ferreira, B.P. Ontogeny drives allochthonous trophic support of snappers: Seascape connectivity along the mangrove-seagrass-coral reef continuum of a tropical marine protected area. Estuar. Coast. Shelf Sci. 2022, 264, 107591.
- Mishra, A.K.; Apte, D. Ecological connectivity with mangroves influences tropical seagrass population longevity and meadow traits within an island ecosystem. Mar. Ecol. Prog. Ser. 2020, 644, 47–63.
- Jordan, P.; Fröhle, P. Bridging the gap between coastal engineering and nature conservation? A review of coastal ecosystems as nature-based solutions for coastal protection. J. Coast. Conserv. 2022, 26, 4.
- Watanabe, A.; Nakamura, T. Carbon dynamics in coral reefs. In Blue Carbon in Shallow Coastal Ecosystems: Carbon Dynamics, Policy, and Implementation; Kuwae, T., Hori, M., Eds.; Springer: Singapore, 2019; pp. 273–293.
- Akhand, A.; Watanabe, K.; Chanda, A.; Tokoro, T.; Chakraborty, K.; Moki, H.; Tanaya, T.; Ghosh, J.; Kuwae, T. Lateral carbon fluxes and CO2 evasion from a subtropical mangrove-seagrass-coral continuum. Sci. Total Environ. 2021, 752, 142190.
- Lovelock, C.E.; Reef, R. Variable impacts of climate change on blue carbon. One Earth 2020, 3, 195–211.
- Helfer, V.; Hassenrück, C. Microbial communities in mangrove sediments. Dyn. Sediment. Environ. Mangrove Coasts 2021, 141–175.
- Liu, M.; Huang, H.; Bao, S.; Tong, Y. Microbial community structure of soils in Bamenwan mangrove wetland. Sci. Rep. 2019, 9, 8406.
- Bag, S.; Sarkar, B.; Seal, M.; Chatterjee, A.; Mondal, A.; Chatterjee, S. Diversity and seasonal prevalence of starch hydrolysing, phosphate solubilizing and nitrogen-fixing bacterial groups of rooted and un-rooted regions of tropical mangrove sediments of Sundarbans, West Bengal, India. Mar. Biol. Res. 2022, 18, 531–543.
- Fu, W.; Chen, X.; Zheng, X.; Liu, A.; Wang, W.; Ji, J.; Wang, G.; Guan, C. Phytoremediation potential, antioxidant response, photosynthetic behavior and rhizosphere bacterial community adaptation of tobacco (Nicotiana tabacum L.) in a bisphenol A-contaminated soil. Environ. Sci. Pollut. Res. 2022, 29, 84366–84382.
- Fatimah, F.; Aula, N.; Salsabila, S.; Ramly, Z.A.; Rose, S.Y.; Surtiningsih, T.; Nurhariyati, T. Exploration of phosphate solubilizing bacteria from mangrove soil of Lamongan, East Java, Indonesia. Biodiversitas J. Biol. Divers. 2023, 24, 2.
- Mamangkey, J.; Suryanto, D.; Munir, E.; Mustopa, A.Z.; Sibero, M.T.; Mendes, L.W.; Hartanto, A.; Taniwan, S.; Ek-Ramos, M.J.; Harahap, A. Isolation and enzyme bioprospection of bacteria associated to Bruguiera cylindrica, a mangrove plant of North Sumatra, Indonesia. Biotechnol. Rep. 2021, 30, e00617.
- Zhou, J.; Zhang, C.-J.; Li, M. Desulfovibrio mangrovi sp. nov., a sulfate-reducing bacterium isolated from mangrove sediments: A member of the proposed genus “Psychrodesulfovibrio”. Antonie Van Leeuwenhoek 2023, 116, 499–510.
- Al Farraj, D.A.; Varghese, R.; Vágvölgyi, C.; Elshikh, M.S.; Alokda, A.; Mahmoud, A.H. Antibiotics production in optimized culture condition using low cost substrates from Streptomyces sp. AS4 isolated from mangrove soil sediment. J. King Saud. Univ. Sci. 2020, 32, 1528–1535.
- Nugraha, A.P.; Sibero, M.T.; Nugraha, A.P.; Puspitaningrum, M.S.; Rizqianti, Y.; Rahmadhani, D.; Kharisma, V.D.; Ramadhani, N.F.; Ridwan, R.D.; Ernawati, D.S. Anti-Periodontopathogenic Ability of Mangrove Leaves (Aegiceras corniculatum) Ethanol Extract: In silico and in vitro study. Eur. J. Dent. 2022, 17, 46–56.
- Kulkarni, S.O.; Shouche, Y.S. Mangrove Ecosystem and Microbiome. In Microbiome-Host Interactions; Sankaranarayanan, A., Dhanasekaran, D., Paul, D., Amaresan, N., Yogesh, S.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 259–273.
- Palit, K.; Rath, S.; Chatterjee, S.; Das, S. Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations. Environ. Sci. Pollut. Res. 2022, 29, 32467–32512.
- Fusi, M.; Booth, J.M.; Marasco, R.; Merlino, G.; Garcias-Bonet, N.; Barozzi, A.; Garuglieri, E.; Mbobo, T.; Diele, K.; Duarte, C.M. Bioturbation Intensity Modifies the Sediment Microbiome and Biochemistry and Supports Plant Growth in an Arid Mangrove System. Microbiol. Spectr. 2022, 10, e01117–e01122.
- Thye, A.Y.-K.; Letchumanan, V.; Tan, L.T.-H.; Law, J.W.-F.; Lee, L.-H. Malaysia’s Breakthrough in Modern Actinobacteria (MOD-ACTINO) Drug Discovery Research. Prog. Microbes Mol. Biol. 2022, 5, 1.
- Zhang, Z.; Nie, S.; Sang, Y.; Mo, S.; Li, J.; Kashif, M.; Su, G.; Yan, B.; Jiang, C. Effects of Spartina alterniflora invasion on nitrogen fixation and phosphorus solubilization in a subtropical marine mangrove ecosystem. Microbiol. Spectr. 2022, 10, e00682-21.
- Nimnoi, P.; Pongsilp, N. Insights into Bacterial Communities and Diversity of Mangrove Forest Soils along the Upper Gulf of Thailand in Response to Environmental Factors. Biology 2022, 11, 1787.
- Begmatov, S.; Savvichev, A.S.; Kadnikov, V.V.; Beletsky, A.V.; Rusanov, I.I.; Klyuvitkin, A.A.; Novichkova, E.A.; Mardanov, A.V.; Pimenov, N.V.; Ravin, N.V. Microbial communities involved in methane, sulfur, and nitrogen cycling in the sediments of the Barents Sea. Microorganisms 2021, 9, 2362.
- Yahaya, E.; Lim, S.W.; Yeo, W.S.; Nandong, J. A review on process modeling and design of biohydrogen. Int. J. Hydrogen Energy 2022, 47, 30404–30427.
- Halls, A. Wetlands, Biodiversity and the Ramsar Convention: The Role of the Convention on Wetlands in the Conservation and Wise Use of Biodiversity. In Proceedings of the Ramsar Convention Bureau, Gland, Switzerland; Available online: https://www.ramsar.org/sites/default/files/documents/library/wetlands_biodiversity_and_the_ramsar_convention.pdf (accessed on 11 March 2023).
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586.
- Barreto, C.R.; Morrissey, E.; Wykoff, D.; Chapman, S. Co-occurring mangroves and salt marshes differ in microbial community composition. Wetlands 2018, 38, 497–508.
- Araújo, J.E.d.; Taketani, R.G.; Pereira e Silva, M.d.C.; Lourenço, M.V.d.M.; Andreote, F.D. Draft genome sequence of Rhodopirellula baltica Strain BR-MGV, a planctomycete isolated from Brazilian mangrove soil. Microbiol. Resour. Announc. 2018, 7, e01102–e01118.
- de Araujo, J.E.; Taketani, R.G.; Pylro, V.S.; Leite, L.R.; Pereira e Silva, M.d.C.; Lemos, L.N.; de Mello Lourenço, M.V.; Andreote, F.D. Genomic analysis reveals the potential for hydrocarbon degradation of Rhodopirellula sp. MGV isolated from a polluted Brazilian mangrove. Braz. J. Microbiol. 2021, 52, 1397–1404.
- Zhang, C.-J.; Pan, J.; Duan, C.-H.; Wang, Y.-M.; Liu, Y.; Sun, J.; Zhou, H.-C.; Song, X.; Li, M. Prokaryotic diversity in mangrove sediments across southeastern China fundamentally differs from that in other biomes. Msystems 2019, 4, e00442-19.
- De Santana, C.O.; Spealman, P.; Melo, V.M.M.; Gresham, D.; De Jesus, T.B.; Chinalia, F.A. Effects of tidal influence on the structure and function of prokaryotic communities in the sediments of a pristine Brazilian mangrove. Biogeosciences 2021, 18, 2259–2273.
- Raut, Y.; Capone, D.G. Macroalgal detrital systems: An overlooked ecological niche for heterotrophic nitrogen fixation. Environ. Microbiol. 2021, 23, 4372–4388.
- Rahim, N.A.A.; Merican, F.M.M.S.; Radzi, R.; Omar, W.M.W.; Nor, S.A.M.; Broady, P.; Convey, P. Unveiling The Diversity of Periphytic Cyanobacteria (Cyanophyceae) from Tropical Mangroves in Penang, Malaysia. Trop. Life Sci. Res. 2023. Available online: https://nora.nerc.ac.uk/id/eprint/534309/ (accessed on 11 March 2023).
- Acharya, S.; Patra, D.K.; Mahalik, G.; Mohapatra, P.K. Quantitative Ecological Study of Rhizophoraceae Mangroves of Bhitarkanika Wildlife Sanctuary Regions of Odisha Coast, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 897–908.
- Ceccon, D.M.; Faoro, H.; da Cunha Lana, P.; de Souza, E.M.; de Oliveira Pedrosa, F. Metataxonomic and metagenomic analysis of mangrove microbiomes reveals community patterns driven by salinity and pH gradients in Paranaguá Bay, Brazil. Sci. Total Environ. 2019, 694, 133609.
- Askari, M.; Homaei, A.; Kamrani, E.; Zeinali, F.; Andreetta, A. Estimation of carbon pools in the biomass and soil of mangrove forests in Sirik Azini creek, Hormozgan province (Iran). Environ. Sci. Pollut. Res. 2022, 29, 23712–23720.
- Sultana, S.; Huang, R.; Van Zwieten, L.; Wang, H.; Wu, J. Trapping effect of mangrove and saltmarsh habitats on geochemical elements: A case study in Ximen Island, Zhejiang, China. J. Soils Sediments 2023, 23, 2327–2343.
- Soanes, L.; Pike, S.; Armstrong, S.; Creque, K.; Norris-Gumbs, R.; Zaluski, S.; Medcalf, K. Reducing the vulnerability of coastal communities in the Caribbean through sustainable mangrove management. Ocean Coast. Manag. 2021, 210, 105702.
- Edwards, A.J. Impact of climatic change on coral reefs, mangroves, and tropical seagrass ecosystems. In Climate Change; CRC Press: Boca Raton, FL, USA, 2021; pp. 209–234.
- Das, S.C.; Das, S.; Tah, J. Mangrove Forests and People’s Livelihoods. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Pullaiah, T., Ashton, E.C., Eds.; Springer Nature: Singapore, 2022; pp. 153–173.
- Song, S.; Ding, Y.; Li, W.; Meng, Y.; Zhou, J.; Gou, R.; Zhang, C.; Ye, S.; Saintilan, N.; Krauss, K.W. Mangrove reforestation provides greater blue carbon benefit than afforestation for mitigating global climate change. Nat. Commun. 2023, 14, 756.
- Zhu, J.-J.; Yan, B. Blue carbon sink function and carbon neutrality potential of mangroves. Sci. Total Environ. 2022, 822, 153438.
- Leal, M.; Spalding, M.D. The State of the World’s Mangroves 2022; Leal, M., Spalding, M.D., Eds.; Global Mangrove Alliance: 2022; Available online: https://www.mangrovealliance.org/wp-content/uploads/2022/09/The-State-of-the-Worlds-Mangroves-Report_2022.pdf (accessed on 2 February 2023).
- Sumarmi, S.; Purwanto, P.; Bachri, S. Spatial Analysis of Mangrove Forest Management to Reduce Air Temperature and CO2 Emissions. Sustainability 2021, 13, 8090.
- Alsumaiti, T.S.; Shahid, S.A. Mangroves Among Most Carbon-rich Ecosystem Living in Hostile Saline Rich Environment and Mitigating Climate Change–A Case of Abu Dhabi. J. Agri. Crop Res. 2019, 7, 1–8.
- Bunting, P.; Rosenqvist, A.; Hilarides, L.; Lucas, R.M.; Thomas, N. Global mangrove extent change 1996–2020: Global Mangrove Watch version 3.0. Remote Sens. 2022, 14, 3657.
- Jennerjahn, T.C. Relevance and magnitude of ‘Blue Carbon’ storage in mangrove sediments: Carbon accumulation rates vs. stocks, sources vs. sinks. Estuar. Coast. Shelf Sci. 2020, 247, 107027.
- Maza, M.; Lara, J.L.; Losada, I.J. Predicting the evolution of coastal protection service with mangrove forest age. Coast. Eng. 2021, 168, 103922.
- Liu, C.; Liu, G.; Yang, Q.; Luo, T.; He, P.; Franzese, P.P.; Lombardi, G.V. Emergy-based evaluation of world coastal ecosystem services. Water Res. 2021, 204, 117656.
- Hastuti, E.D.; Izzati, M.; Prihastanti, E. Water uptake and salt accumulation under Rhizophora stylosa seedling planted in controlled salinity and inundation levels. AACL Bioflux 2023, 16, 1069–1076.
- Li, H. Learning from Halophytes to Survive from Salt: Elucidating the Salt Tolerance Mechanisms of Schrenkiella parvula; Wageningen University: Wageningen, The Netherlands, 2022.
- Jusoff, K. Malaysian Mangrove Forests and their Significance to the Coastal Marine Environment. Pol. J. Environ. Stud. 2013, 22, 979–1005.
- Wang, Q.; Mei, D.; Chen, J.; Lin, Y.; Liu, J.; Lu, H.; Yan, C. Sequestration of heavy metal by glomalin-related soil protein: Implication for water quality improvement in mangrove wetlands. Water Res. 2019, 148, 142–152.
- Eyegheleme, N.L.; Umashankar, V.; Miller, D.N.; Kota, A.K.; Boreyko, J.B. Oil–Water Separation using Synthetic Trees. Langmuir 2023, 39, 2520–2528.
- Hayes, M.A.; Chapman, S.; Jesse, A.; O’Brien, E.; Langley, J.A.; Bardou, R.; Devaney, J.; Parker, J.D.; Cavanaugh, K.C. Foliar water uptake by coastal wetland plants: A novel water acquisition mechanism in arid and humid subtropical mangroves. J. Ecol. 2020, 108, 2625–2637.
- Menéndez, P.; Losada, I.J.; Torres-Ortega, S.; Narayan, S.; Beck, M.W. The global flood protection benefits of mangroves. Sci. Rep. 2020, 10, 4404.
- Josiah, N.; Laknath, D.; Araki, S. Assessment of Tsunami Preparedness Measures in East Coast of Sri Lanka Based on 2004 Tsunami Event. In Proceedings of the 22nd Congress of International Association for Hydro Environment Engineering and Research and Asia Pacific Division, Sapporo, Japan; Available online: https://iahrapd2020.xsrv.jp/proceedings/pdf/6-5-2.pdf (accessed on 5 April 2023).
- LaVeist, T.A. Katrina’s Lesson: Time to Imagine an After COVID-19. Am. J. Public Health 2020, 110, 1445.
- Pakoksung, K.; Suppasri, A.; Imamura, F. The near-field tsunami generated by the 15 January 2022 eruption of the Hunga Tonga-Hunga Ha’apai volcano and its impact on Tongatapu, Tonga. Sci. Rep. 2022, 12, 15187.
- Dahdouh-Guebas, F.; Hugé, J.; Abuchahla, G.M.; Cannicci, S.; Jayatissa, L.P.; Kairo, J.G.; Arachchilage, S.K.; Koedam, N.; Nijamdeen, T.W.M.; Mukherjee, N. Reconciling nature, people and policy in the mangrove social-ecological system through the adaptive cycle heuristic. Estuar. Coast. Shelf Sci. 2021, 248, 106942.
- Temmerman, S.; Horstman, E.M.; Krauss, K.W.; Mullarney, J.C.; Pelckmans, I.; Schoutens, K. Marshes and mangroves as nature-based coastal storm buffers. Ann. Rev. Mar. Sci. 2023, 15, 95–118.
- Arora, P.; Arora, N.K. COP27: A summit of more misses than hits. Environ. Sustain. 2023, 6, 99–105.
- Eger, A.M.; Marzinelli, E.M.; Beas-Luna, R.; Blain, C.O.; Blamey, L.K.; Byrnes, J.E.; Carnell, P.E.; Choi, C.G.; Hessing-Lewis, M.; Kim, K.Y. The value of ecosystem services in global marine kelp forests. Nat. Commun. 2023, 14, 1894.
- McSherry, M.; Davis, R.P.; Andradi-Brown, D.A.; Ahmadia, G.N.; Van Kempen, M.; Brian, S.W. Integrated mangrove aquaculture: The sustainable choice for mangroves and aquaculture? Front. For. Glob. Chang. 2023, 6, 1094306.
- Gentry, R.R.; Rassweiler, A.; Ruff, E.O.; Lester, S.E. Global pathways of innovation and spread of marine aquaculture species. One Earth 2023, 6, 20–30.
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization: Rome, Italy, 2023.
- Estante-Superio, E.; Panizales, J.; Arganioza, H.M.; Baliao, D.D. Issues and challenges in sustainable development of fisheries and aquaculture of the Southeast Asian Region: Aquaculture development: Impacts of intensification of aquaculture on the environment. In The Southeast Asian State of Fisheries and Aquaculture 2022; Seafdec, Ed.; Secretariat, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2022; pp. 182–187.
- Broszeit, S.; Langmead, O.; Praptiwi, R.A.; Creencia, L.; Then, A.Y.H.; Lim, V.-C.; Hau, T.D.; Hattam, C.; Edwards-Jones, A. Ecosystem Service Provision by Marine Habitats in Southeast Asia; PML Publishing: Plymouth, UK, 2022.
- Zu Ermgassen, P.S.; Mukherjee, N.; Worthington, T.A.; Acosta, A.; da Rocha Araujo, A.R.; Beitl, C.M.; Castellanos-Galindo, G.A.; Cunha-Lignon, M.; Dahdouh-Guebas, F.; Diele, K. Fishers who rely on mangroves: Modelling and mapping the global intensity of mangrove-associated fisheries. Estuar. Coast. Shelf Sci. 2020, 247, 106975.
- Treviño, M.; Murillo-Sandoval, P.J. Uneven consequences: Gendered impacts of shrimp aquaculture development on mangrove dependent communities. Ocean Coast. Manag. 2021, 210, 105688.
- Malleret, D.; Simbua, J. The Occupational Structure of the Mnazi Bay Ruvuma Estuary Marine Park Communities; IUCN Nairobi: Nairobi City, Kenya, 2004.
- Kassam, L.; Dorward, A. A comparative assessment of the poverty impacts of pond and cage aquaculture in Ghana. Aquaculture 2017, 470, 110–122.
- Islam, A.H.M.S.; Barman, B.K.; Murshed-e-Jahan, K. Adoption and impact of integrated rice–fish farming system in Bangladesh. Aquaculture 2015, 447, 76–85.
- Lakra, W.; Krishnani, K. Circular bioeconomy for stress-resilient fisheries and aquaculture. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 481–516.
- Malorgio, G.; Mulazzani, L.; Pugliese, P.; Rota, C.; Zanasi, C.; Zuccaro, M. The role of small-scale fisheries in Mediterranean coastal communities. An analytical framework for their development. New Medit 2017, 16, 19–26.
- Koh, H.L.; Teh, S.Y. Ecological Modeling for Mitigating Environmental and Climate Shocks: Achieving the UNSDGs; World Scientific: Singapore, 2021.
- Zuhri, F.; Tafsin, M.R. Mangrove utilization as sources of ruminant feed in Belawan Secanang Subdistrict, Medan Belawan District. J. Sylva Indones. 2022, 5, 1.
- Olorunnisola, A.O. The Past, Present and Future Outlook of the Wood Industry in Nigeria; IntechOpen: London, UK, 2023.
- Kusmana, C. Mangrove plant utilization by local coastal community in Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Selangor, Malaysia, 6–8 November 2017; p. 012028.
- Walters, B.B.; Rönnbäck, P.; Kovacs, J.M.; Crona, B.; Hussain, S.A.; Badola, R.; Primavera, J.H.; Barbier, E.; Dahdouh-Guebas, F. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236.
- Friess, D.A.; Yando, E.S.; Alemu, J.B.; Wong, L.-W.; Soto, S.D.; Bhatia, N. Ecosystem services and disservices of mangrove forests and salt marshes. An Annual Review. In Oceanography and Marine Biology, 1st ed.; Hawkins, S.J., Allcock, A.L., Bates, A.E., Evans, A.J., Firth, L.B., McQuaid, C.D., Russell, B.D., Smith, I.P., Swearer, S.E., Todd, P.A., Eds.; Taylor & Francis: New York, NY, USA, 2020.
- Vinoth, R.; Kumaravel, S.; Ranganathan, R. Therapeutic and traditional uses of mangrove plants. J. Drug Deliv. Ther. 2019, 9, 849–854.
- Kumar, A.; Naveen, B.; Abhilash, G.; Akila, C. Extraction and characterization of sea anemones compound and its Anti bacterial and hemolytic studies. Int. J. Rev. Life Sci. 2020, 10, 93–97.
- Habib, M.A.; Khatun, F.; Ruma, M.; Chowdhury, A.; Silve, A.; Rahman, A.; Hossain, M.I. A review on phytochemical constituents of pharmaceutically important mangrove plants, their medicinal uses and pharmacological activities. Vedic Res. Int. Phytomed. 2018, 6, 1–9.
- Blamey, R.K. Principles of ecotourism. In The Encyclopedia of Ecotourism; Cabi Publishing: Wallingford, UK, 2001; pp. 5–22.
- Friess, D.A. Ecotourism as a tool for mangrove conservation. Sumatra J. Disaster Geogr. Geogr. Educ. 2017, 1, 24–35.
- Satyanarayana, B.; Bhanderi, P.; Debry, M.; Maniatis, D.; Foré, F.; Badgie, D.; Jammeh, K.; Vanwing, T.; Farcy, C.; Koedam, N. A socio-ecological assessment aiming at improved forest resource management and sustainable ecotourism development in the mangroves of Tanbi Wetland National Park, The Gambia, West Africa. Ambio 2012, 41, 513–526.
- Uddin, M.S.; van Steveninck, E.d.R.; Stuip, M.; Shah, M.A.R. Economic valuation of provisioning and cultural services of a protected mangrove ecosystem: A case study on Sundarbans Reserve Forest, Bangladesh. Ecosyst. Serv. 2013, 5, 88–93.
- Basyuni, M.; Bimantara, Y.; Siagian, M.; Wati, R.; Slamet, B.; Sulistiyono, N.; Nuryawan, A.; Leidonad, R. Developing community-based mangrove management through eco-tourism in North Sumatra, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Medan, Indonesia, 11–12 October 2017; p. 012109.
- Willard, K.; Aipassa, M.I.; Sardjono, M.A.; Rujehan, R.; Ruslim, Y.; Kristiningrum, R. Locating the unique biodiversity of Balikpapan Bay as an ecotourism attraction in East Kalimantan, Indonesia. Biodiversitas J. Biol. Divers. 2022, 23, 5.
- Van Riper, C.J.; Kyle, G.T.; Sutton, S.G.; Barnes, M.; Sherrouse, B.C. Mapping outdoor recreationists’ perceived social values for ecosystem services at Hinchinbrook Island National Park, Australia. Appl. Geogr. 2012, 35, 164–173.
- Avau, J.; Cunha-Lignon, M.; De Myttenaere, B.; Godart, M.-F.; Dahdouh-Guebas, F. The Commercial Images Promoting Caribbean Mangroves to Tourists: Case Studies in Jamaica, Guadeloupe and Martinique. J. Coast. Res. 2011, 64, 1277–1281.
- Khoshtaria, T.; Chachava, N. Prospects of ecotourism development in recreation areas of South Georgia. Ann. Agrar. Sci. 2017, 15, 312–317.
- Sumarmi, S.; Arinta, D.; Suprianto, A.; Aliman, M. The development of ecotourism with community-based tourism (CBT) in clungup mangrove conservation (CMC) of tiga warna beach for sustainable conservation. Folia Geogr. 2021, 63, 123.
- Gaora, P.A.; Pedrason, R.; Herman, E. Indonesia’s Climate Diplomacy under Joko Widodo: Shaping Equitable and Sustainable Global Future. Nation State J. Int. Stud. 2023, 6, 34–48.
- Keith Gumede, T.; Thandi Nzama, A. Approaches toward Community Participation Enhancement in Ecotourism. Prot. Area Manag.-Recent Adv. 2022, 1–13.
- Das, S. Ecotourism, sustainable development and the Indian state. Econ. Political Wkly. 2011, 46, 60–67.
More
Information
Subjects:
Biodiversity Conservation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.9K
Revisions:
2 times
(View History)
Update Date:
12 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




