
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paweł Kafarski | -- | 2400 | 2023-09-06 18:25:01 | | | |
| 2 | Lindsay Dong | -2 word(s) | 2398 | 2023-09-08 03:29:03 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2398 | 2023-09-08 10:48:18 | | |
Video Upload Options
Nisin is a readily available and cheap lanthipeptide and thus serves as a good model in the search for the tools to engineer lantibiotics with improved pharmacological properties. There are basically two general means to obtain nisin analogs—protein engineering and chemical functionalization of this antibiotic. Although bioengineering techniques have been well developed and enable the creation of nisin mutants of variable structures and properties, they are lacking spectacular effects so far. Chemical modifications of nisin based on utilization of the reactivity of its free amino and carboxylic moieties, as well as reactivity of the double bonds of its dehydroamino acids, are in their infancy.
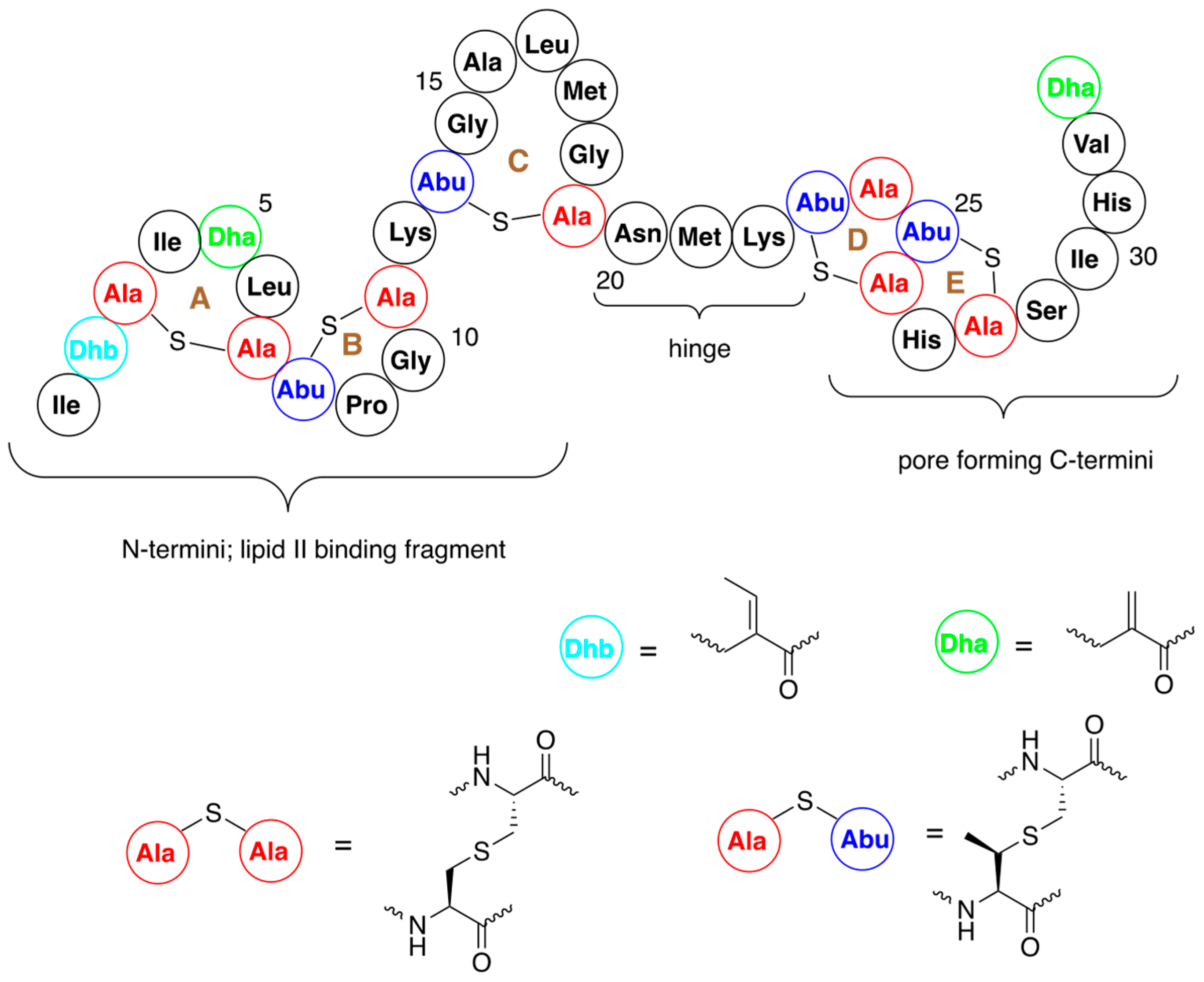
1. Nisin A
2. Design of Novel Antibacterials by Modification of Nisin Structure
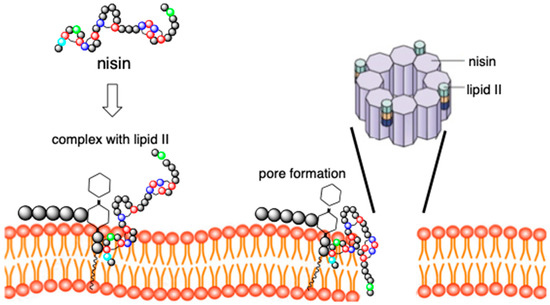
The structural features and mode of action make nisin suitable for its structure engineering. These modifications are performed in order to generate nisin variants with enhanced antimicrobial activity, better solubility, and ability to evade resistance. Enhancement of activity against Gram-negative bacteria is one of the major driving forces of these studies. Thus, the obtained results should allow progress to be made in overcoming the inherent shortcomings of nisin. The two major means that have been used for this purpose are bioengineering and chemical modifications of the structure of this antibiotic.
2.1. Bioengeeneering by Mutagenesis
2.2. Variants Containing Non-Canonical Amino Acids
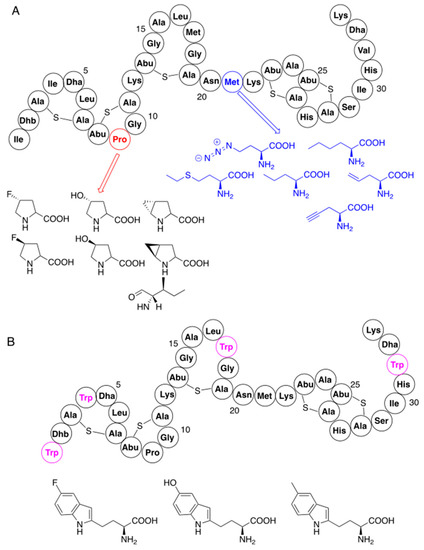
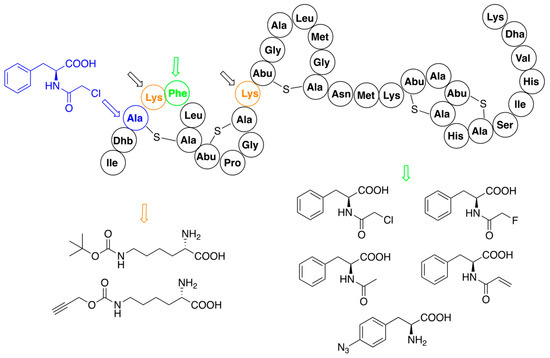
2.3. Hybrid Molecules
3. Chemical Modifications of Nisin
3.1. Click Reaction as a Tool for Nisin Modification
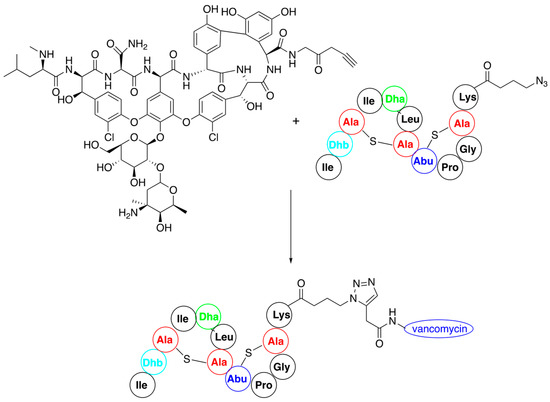
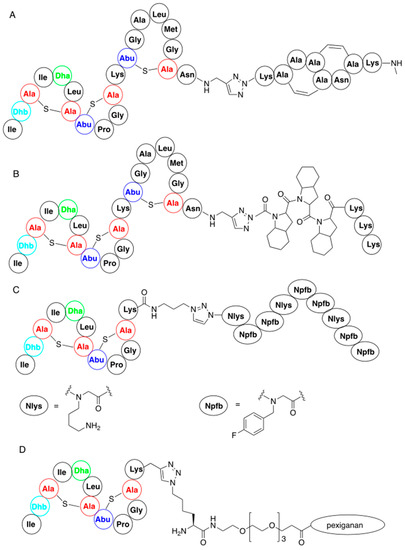
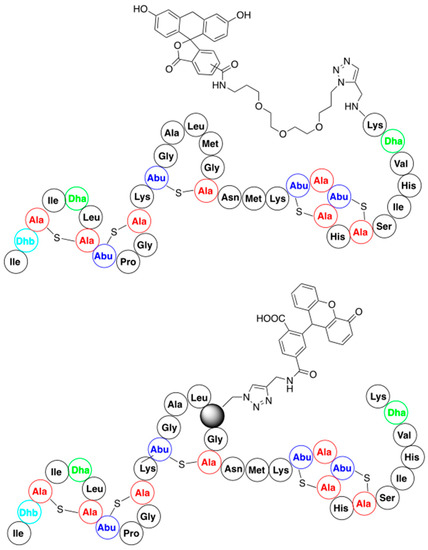
3.2. Late-Stage Functionalization of Nisin
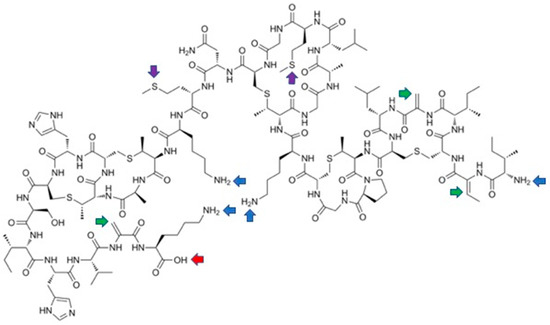
References
- Rogers, L.A.; Whittier, E.O. Limiting factors in lactic fermentation. J. Bacteriol. 1928, 16, 211–229.
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Invited review: Advances in nisin use for preservation of dairy products. J. Dairy Airy Sci. 2020, 103, 2041–2052.
- Animudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent advances in the application of the antimicrobial eptide nisin in the inactivation of spore-forming bacteria in foods. Molecules 2021, 26, 5552.
- Khan, F.; Singh, P.; Joshi, A.S.; Tabassum, N.; Jeong, G.-J.; Bamunuarachchi, N.I. Multiple potential strategies for the application of nisin and derivatives. Crit. Rev. Microbiol. 2022, 1–30.
- Shin, J.M.; Gwak, J.W.; Kamarayan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.J. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465.
- Małaczewska, J.; Kaczorek-Łukowska, E. Nisin—A lantibiotic with immunomodulatory properties: A review. Peptides 2021, 137, 170479.
- Gross, E.; Morell, J.E. Structure of Nisin. J. Am. Chem. Soc. 1971, 93, 4634–4635.
- Auliffe, O.; Ross, R.P.; Hill, C. Lantibiotics: Structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 2001, 25, 285–308.
- Hsu, S.-T.D.; Breukink, E.; Tischenko, E.; Lutters, M.A.; de Kruijff, B.; KAptein, R.; Bonwinn, A.M.J.J.; van Nuland, N.A.J. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 2004, 11, 963–967.
- T’Hart, P.; Oppendijk, S.F.; Breukink, E.; Martin, N.I. New insights into nisin’s antibacterial mechanism revealed by binding studies with synthetic llipid II analogues. Biochemistry 2016, 55, 232–237.
- Fields, D.; Cotter, P.D.; Ross, R.P.; Hill, C. Bioengineering of the model lantibiotic nisin. Bioengineered 2015, 6, 187–192.
- Zheng, Y.; Du, Y.; Qiu, Z.; Liu, Z.; Qiao, J.; Li, Y.; Caiyin, Q. Nisin variants generated by protein engineering and their properties. Bioengineering 2022, 9, 251.
- Fields, D.; Fernandez de Ullivarri, M.; Ross, R.P.; Hill, C. After a century of nisin research, where are we now and where are we going? FEMS Microbiol. Rev. 2023, 47, fuad023.
- Rouse, S.; Field, R.; Daly, K.M.; O’Connor, P.M.; Cotter, P.D.; Ross, R.P. Bioengineered nisin derivatives with enhanced activity in complex matrices. Microb. Biotech. 2012, 5, 501–508.
- Piper, C.; Hill, C.; Cotter, P.D.; Ross, R.P. Bioengineering of a Nisin A-producing Lactococcu slactisto create isogenic strains producing the natural variants Nisin F., Q and Z. Microb. Biotech. 2010, 4, 375–382.
- Kuwano, K.; Tanaka, N.; Shimizu, T.; Nagatoshi, K.; Nou, S.; Sonomoto, K. Dual antibacterial mechanisms of nisin Z against Gram-positive and Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 26, 396–402.
- Yuan, J.; Zhang, Z.Z.; Chen, X.Z.; Yang, W.; Huan, L.D. Site-directed mutagenesis of the hinge region of nisin Z and properties of nisin Z mutants. Appl. Microbiol. Biotechnol. 2004, 64, 806–815.
- Field, D.; O’Connor, P.M.; Cotter, P.D.; Hill, C.; Ross, R.P. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol. Microbiol. 2008, 69, 218–230.
- Zhou, L.; van Heel, A.J.; Kuipers, O.P. The length of a lantibiotic hinge region has profound influence on antimicrobial activity and host specificity. Front. Microbiol. 2015, 6, 11.
- Zaschke-Kriesche, J.; Reiners, J.; Lagedroste, M.; Smits, S.H.J. Influence of nisin hinge-region variants on lantibiotic immunity and resistance proteins. Bioorg. Med. Chem. 2019, 27, 3947–3953.
- Rink, R.; Wierenga, J.; Kuipers, A.; Kluskens, L.D.; Driessen, A.J.M.; Kuipers, O.P.; Moll, G.N. Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl. Environ. Microbiol. 2007, 73, 5809–5816.
- Van Heel, A.J.; Kloostermann, T.G.; Montalban-Lopez, M.; Deng, J.; Plat, A.; Baudu, B.; Hendriks, D.; Moll, G.N.; Kuipers, O.P. Discovery, production and modification of five novel lantibiotics using the promiscuous nisin modification machinery. ACS Synth. Biol. 2016, 5, 1146–1154.
- O’Connor, M.; Field, D.; Grainger, A.; O’Connor, P.M.; Draper, L.; Ross, R.P.; Hill, C. Nisin M: A Bioengineered nisin A variant that retains full induction capacity but has significantly reduced antimicrobial activity. Appl. Environ. Microbiol. 2016, 86, e00984-20.
- Medeiros-Silva, J.; Jekhmane, S.; Lucini Paioni, A.; Gawarecka, K.; Baldus, M.; Świeżewska, E.; Breukink, E.; Weingarth, M. High-resolution NMR studies of antibiotics in cellular membranes. Nat. Commun. 2018, 9, 3963.
- Du, Y.; Li, L.; Zheng, Y.; Liu, J.; Gong, J.; Qiu, Z.; Qiao, J.; Huo, Y.-X. Incorporation of non-canonical amino acids into antimicrobial peptides: Advances, challenges, and perspectives. Biotechnology 2022, 18, e0161722.
- Shi, Y.; Yang, X.; Garg, N.; van der Donk, W.A. Production of lantipeptides in Escherichia coli. J. Am. Chem. Soc. 2010, 133, 2338–2341.
- Bindman, N.A.; Bobeica, S.C.; Liu, W.R.; van der Donk, W.A. Facile removal of leader peptides from lanthipeptides by incorporation of a hydroxy acid. J. Am. Chem. Soc. 2015, 137, 6975–6978.
- Zambaldo, C.; Luo, X.; Mehta, A.P.; Schulz, P.G. Recombinant macrocyclic lanthipeptides incorporating non-canonical amino acids. J. Am. Chem. Soc. 2017, 139, 11646–11649.
- Kakkar, N.; Perez, J.G.; Lliu, W.R.; Jewett, M.C.; van der Donk, W.A. Incorporation of nonproteinogenic amino acids in class I and II lantibiotics. ACS Chem. Biol. 2018, 13, 951–957.
- Nickling, J.H.; Baumann, T.; Schmitt, F.-J.; Barthomolae, M.; Kuipers, O.P.; Friedrich, T.; Budisa, N. Antimicrobial peptides produced by selective pressure incorporation of non-canonical amino acids. J. Vis. Exp. 2018, 135, 57551.
- Nickling, J.H.; Baumann, T.; Bartholomae, M.; Buivydas, A.; Kuipers, O.P.; Budisa, N. Prospects of in vivo incorporation of non-canonical amino acids for the chemical diversification of antimicrobial peptides. Front. Microbiol. 2017, 8, 124.
- Guo, L.; Wang, C.; Broos, J.; Kuipers, O.P. Lipidated variants of the antimicrobial peptide nisin produced via incorporation of methionine analogs for click chemistry show improved bioactivity. J. Biol. Chem. 2023, 299, 104845.
- Zhao, L.; Shao, J.; Li, Q.; van Heel, A.J.; De Vries, M.P.; Broos, J.; Kuipers, O.P. Incorporation of tryptophan analogues into the lantibiotic nisin. Amino Acids 2016, 48, 1309–1318.
- Yuan, X.-Y.; Xu, W.-T.; Huang, K.-L.; Luo, Y.-B.; Gu, X.-X.; Tian, H.T. Construction of nisin-rbLF-N fusion gene and its expression in Escherichia coli. Food Sci. 2010, 31, 194–197.
- Zhou, L.; van Heel, A.J.; Montalban-Lopez, M.; Kuipers, O.P. Potentiating the activity of nisin against Escherichia coli. Front. Cell Dev. Biol. 2016, 4, 7.
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the antimicrobial activity of nisin-based lantibiotics against Gram-negative pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18.
- Bartholomae, M.; Baumann, T.; Nickling, J.H.; Peterhoff, D.; Wagner, R.; Budisa, N.; Kuipers, O.P. Expanding the genetic code of Lactococcus lactis and Escherichia coli to incorporate non-canonical amino acids for production of modified lantibiotics. Front. Microbiol. 2018, 9, 657.
- Etayash, H.; Azmi, S.; Dangeti, R.; Kaur, K. Peptide bacteriocins-structure activity relationships. Curr. Top. Med. Chem. 2016, 16, 220–241.
- Deng, J.; Viel, J.H.; Kubyshkin, V.; Busdisa, N.; Kuipers, O.P. Conjugation of synthetic polyproline moietes to lipid II binding fragments of nisin yields active and stable antimicrobials. Front Microbiol. 2020, 11, 575334.
- Bolt, H.L.; Kleijn, L.H.J.; Martin, N.I.; Cobb, S.L. Synthesis of antibacterial nisin–peptoid hybrids using click methodology. Molecules 2018, 23, 1566.
- Slootweg, J.V.; van der Wal, S.; van Ufford, H.C.Q.; Breukink, E.; Liskamp, R.M.J.; Rijkers, D.T.S. Synthesis, antimicrobial activity, and membrane permeabilizing properties of C-terminally modified nisin conjugates accessed by CuAAC. Bioconjugate Chem. 2013, 24, 2058–2066.
- Deng, J.; Viel, J.H.; Chen, J.; Kuipers, O.P. Synthesis and Characterization of heterodimers and fluorescent nisin species by incorporation of methionine analogues and subsequent click chemistry. ACS Synth. Biol. 2020, 9, 2525–2536.
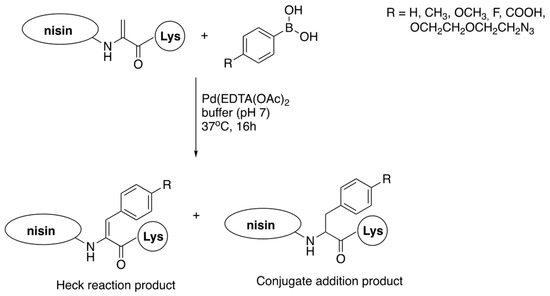
- De Bruijn, A.D.; Roelfes, G. Atalytic Modification of dehydroalanine in peptides and proteins by palladium-mediated cross-coupling. Chem. Eur. J. 2018, 24, 12728–12733.
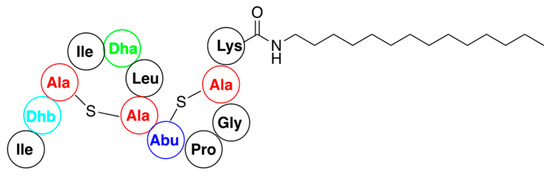
- Koopmans, T.; Wood, M.T.; t’Hart, P.; Klein, L.H.; Hendricks, A.P.A.; Willems, R.J.L.; Breukink, E.; Martin, N.I. Semisynthetic lipopeptides derived from nisin display antibacterial activity and lipid II binding on par with that of the parent compound. J. Am. Chem. Soc. 2015, 137, 9382–9389.




