Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Inés Errea | -- | 3464 | 2023-09-06 15:54:22 | | | |
| 2 | Sirius Huang | Meta information modification | 3464 | 2023-09-07 02:58:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Díaz Bukvic, G.; Rossi, E.; Errea, M.I. Polysaccharides for Preparation of Adsorbents for Water Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/48882 (accessed on 08 February 2026).
Díaz Bukvic G, Rossi E, Errea MI. Polysaccharides for Preparation of Adsorbents for Water Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/48882. Accessed February 08, 2026.
Díaz Bukvic, Gema, Ezequiel Rossi, María Inés Errea. "Polysaccharides for Preparation of Adsorbents for Water Treatment" Encyclopedia, https://encyclopedia.pub/entry/48882 (accessed February 08, 2026).
Díaz Bukvic, G., Rossi, E., & Errea, M.I. (2023, September 06). Polysaccharides for Preparation of Adsorbents for Water Treatment. In Encyclopedia. https://encyclopedia.pub/entry/48882
Díaz Bukvic, Gema, et al. "Polysaccharides for Preparation of Adsorbents for Water Treatment." Encyclopedia. Web. 06 September, 2023.
Copy Citation
Adsorption processes, due to their technical simplicity and cost-effectiveness, have arisen as one of the most well-known, straightforward solutions to water pollution. In this context, polysaccharides, due to their abundance, biodegradability, and biocompatibility, are appealing raw materials for the design of adsorbents.
adsorption
polysaccharides’ chemical modification
water pollutants
water treatment
1. Introduction
Water is a scarce natural resource globally, not only due to its limited physical access but also to the progressive deterioration in its quality caused by pollution [1]. Over the last century, freshwater use has increased by a factor of six and has been growing at a rate of one per cent per year since 1980, which is a result of population growth, economic development, and consumption habits. Some concerning facts reported by the United Nations are that eight out of ten people lack drinking water access in rural areas, and by 2030, only 81% of the need for clean water will be met, which means that 1.6 billion people would not manage to have safe water by then [2]. Thus, one of the greatest challenges of the XXI century is guaranteeing access to safe drinking water. Hence, technologies aiming to pursue this goal intend to make water affordable and accessible for all by developing efficient systems for polluted water remediation [3][4].
Depending on the target pollutant and its concentration, contaminated water can be treated via biological, physical, and chemical processes. These approaches include several technologies such as aeration [5], filtration [6][7][8], flotation [9][10][11], coagulation [12][13][14], flocculation [15], skimming [16][17], chlorination [18][19], membrane technologies [20][21][22], ozonation [23], oxidation and advanced oxidation processes [24][25], neutralization [26], and adsorption [27][28][29], among others.
Moreover, adsorption processes have famously provoked interest among the scientific community since they provide a simple, feasible, cost-effective, and straightforward solution to water pollution [30][31].
Adsorption is a superficial phenomenon in which one or more fluid phase components interact with a solid surface, adhering to it. Based on interaction forces between the adsorbate (the substance attached to the surface) and the adsorbent (which provides the surface where adsorbate adheres), adsorption can be either physical (physisorption) or chemical (chemisorption) [32].
One of the main differences between physisorption and chemisorption lies in enthalpy values of 40 kJ mol−1 or lower for physical adsorption and higher values for chemically governed adsorption processes [33][34]. Adsorbents’ capacity, selectivity, and adsorption rate is determined by their structure and properties [34][35][36][37][38][39]. It is therefore crucial to study the materials to select the most adequate material according to the pollutants found.
There is a wide variety of adsorbents, which include conventional materials such as inorganic materials (e.g., zeolites, aluminas, silica gel, iron oxides, and clays), activated carbons, ion-exchange resins; and non-conventional adsorbents such as polysaccharides and their derivatives (e.g., chitosan, cellulose, starch, alginate, or cyclodextrin), biosorbents (e.g., algae, fungi, or yeasts) and either by-products or waste from agricultural and industrial activities (e.g., sawdust, corn cob, sugar bagasse, wheat straw, or sewage sludges) [40].
In this context, polysaccharides are materials that are remarkable for their low cost and environmentally friendliness. Unfortunately, as it will be discussed throughout this manuscript, due to either their low adsorption capacities, physicochemical properties or both, polysaccharides are not suitable for water treatment in their native form. However, their polyhydroxylated structure provides them enough versatility to be subjected to chemical modifications in order to impart specific physicochemical properties depending on the products’ final application.
2. Polysaccharides Involved in Water Remediation
Among naturally existing polysaccharides, cellulose, alginate, chitosan, and starch are the most studied in this field because of their remarkable relative abundance and availability.
2.1. Cellulose
It is well known that cellulose, one of the most abundant organic compounds on Earth, is composed of D-glucopyranose units linked by β-(1→4) bonds (Figure 1).
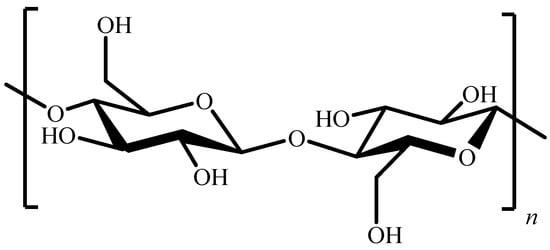
Figure 1. Chemical structure of cellulose.
Although traditionally cellulose has been mainly obtained from wood, over the past few decades, alternative vegetable sources have been increasingly researched to relieve the demand on wood-based biomass and improve process sustainability [41]. In this context, grasses such as bamboo as well as agricultural residues and agro-industrial crop by-products are among the most explored sources due to their low initial cost and fast regeneration. In the case of waste, their use also contributes to their valorisation [41], but they have as a drawback a lower content of cellulose than wood due to the higher content of lignin and hemicellulose [42].
The search for alternatives beyond vegetable sources has led to the study of bacterial cellulose. Bacterial cellulose is produced via fermentation with specific bacteria under suitable conditions and is obtained as a highly hydrated gelatinous pellicle that grows on the aerated surface of the fermentation vessel [43]. Bacterial cellulose is recognized for its high crystallinity, polymerization degree, and chemical purity, as well as for its unique intrinsic nanofibrillar structure. Furthermore, while the isolation of cellulose from plant sources implies the use of reagents that can have a negative environmental impact, bacterial cellulose is produced free of lignin, hemicelluloses, and pectin, thus, avoiding the need for aggressive chemical treatments for the removal of these compounds [38].
The production of bacterial cellulose has the disadvantage of being more expensive than plant cellulose since until now, it has mainly been produced using specific bacteria, typically Komagataeibacter xylinus (formerly called Gluconacetobacter xylinus and Acetobacter xylinum), which require axenic conditions and strict control of fermentation variables [44]. However, for certain applications, it could be alternatively obtained from the floating pellicle developed during kombucha tea production, in a much simpler process which does not require laboratory conditions, complex growth media, or sophisticated cultivation and processing equipment [44][45]. Given the proliferation in recent years of not only classical home-scale breweries, but also larger-scale kombucha enterprises, this production method offers an attractive alternative to obtain pure cellulose by valorising this by-product of kombucha tea.
As far as cellulose tridimensional structure is concerned, the D-glucopyranose units of its chains have a linear arrangement that allows for the formation of numerous intercatenary hydrogen bonds, which result in the polymer’s water insolubility [46] (Figure 2). Additionally, this cellulose arrangement is responsible for its low surface area and consequent low adsorption efficiency of pollutants when employed unmodified, necessitating the functionalization of cellulose [47][48].
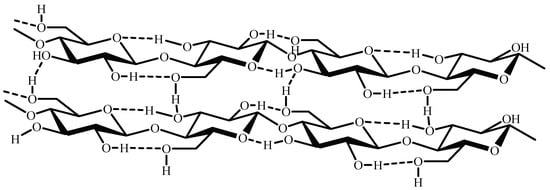
Figure 2. Rearrangement of cellulose chains.
In fact, He et al. illustrated the enhanced adsorption capacity when native cellulose was modified. In this case, native cellulose achieved maximum capacity values of 9.8 and 14.3 mg g−1 for Cr (III) and Fe (III), while 377.2 and 84.0 mg g−1 were attained by preparing a porous spherical polyethyleneimine-cellulose (PEI-cell) absorbent [49].
In that context, intensive work has been carried out on the design of efficient adsorbents from cellulose. The strategies are based either on the preparation of composite materials or on chemical modifications to this polymer.
Approaches to composite materials reported in literature include the preparation of cellulose-based materials using alginate [50][51][52][53][54], chitosan [55][56][57][58][59], hydroxyapatite [60][61][62], crown ethers [63], graphene oxide (GO) [64][65], magnetite (Fe3O4) [66][67], montmorillonite [68][69][70][71][72][73], titanium dioxide [74], collagen [75], bentonite [76][77], and activated carbon [78][79], among others.
2.2. Alginate
Alginate is a polysaccharide which naturally exists as its sodium salt and is constituted of (1→4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues (Figure 3). Alginate does not have regular repetitive units, since it is a block copolymer composed of homopolymeric regions of M or G and interspersed with regions with MG blocks [80].
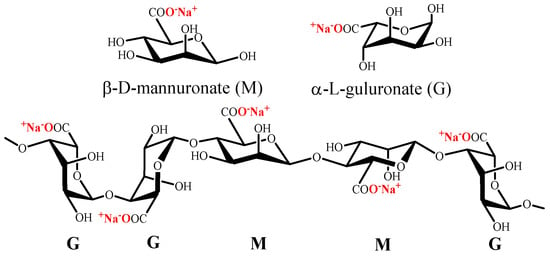
Figure 3. Chemical structure of mannuronate (M), guluronate (G) and alginate.
Its abundance is mainly due to its presence in marine brown algae Phaeophyceae, Ascophyllum nodosum, Laminaria hyperborea, and Macrocystis pyrifera as a structure-forming component and in soil bacteria as a capsular polysaccharide. Several bacteria can produce alginate, such as Azotobacter sp. and Pseudomonas sp. However, commercial alginates are mainly obtained from algal sources, since industrial bacterial production is not economically feasible at present despite being technically possible [81].
The distribution and sequence of M and G blocks of alginate varies according to its source and affects the polysaccharides’ properties, such as viscosity. In fact, for example, alginate obtained from brown algae of the specie Sargassum is considered to be of “borderline” quality due to its low G content [82]. It is important to note that both the environmental conditions during the growth of algae and the extraction techniques used are also two variables that have a high impact on the final structure of the alginate [83].
The interaction between sodium alginate and divalent or trivalent metal cations produces ionic gelation [84], which is related to the length of G blocks in alginate. The formation of coordinate sites is generated by G chains looping, known as the egg-box model (Figure 4).
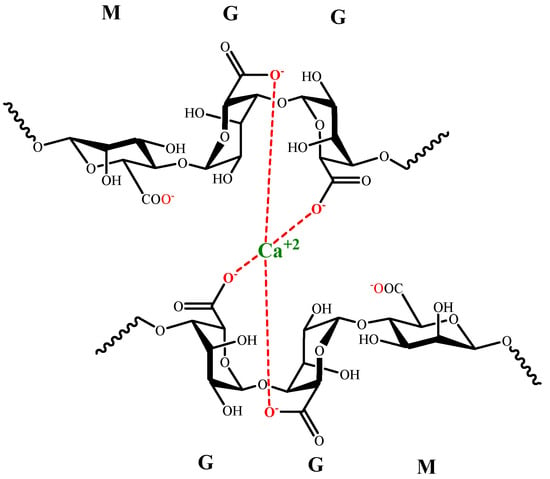
Figure 4. Egg-box model for G alginate residues and Ca (II).
In the presence of monovalent metal cations such as sodium, alginate acts as semiflexible chains instead of the stiff aggregated rigid chains obtained via egg-box formation with Ca (II) [85].
Calcium alginate beads are well known for their ability to adsorb cationic pollutants. In particular, there is some evidence that relates metal cation size to the adsorption efficacy of alginate beads. Both He et al. and Papageorgiou et al. observed higher adsorption capacity values when cation size increases: Pb (II) > Cu (II)> Cd (II), suggesting that larger ions fit better in binding sites [86][87].
Alginate capsules have been proven to have a high lead-binding capacity, with maximum adsorption capacity values of 1540.0 mg g−1 reported by Gyu Park et al. In this case, capsules were prepared by dropping CaCl2 solution and xanthan gum into sodium alginate [88].
Vijaya et al. also obtained insoluble calcium alginate beads which were assayed for Ni (II) adsorption, obtaining 310.4 mg g−1 for calcium alginate. Ni (II) uptake was attributed to both pore volume and large surface area [89].
Nevertheless, the mentioned alginate adsorbents still face two main drawbacks: alginate calcium complex instability and low porosity. Additionally, many of the alginate beads tried in packed column operation exhibited pressure drops that were too high to flow and were not rigid enough. Therefore, intensive work has been done to improve their adsorption performance. To this end, Jeon et al. reported an alginic acid immobilized with PVA-boric acid and crosslinked with glutaraldehyde, designed to reduce the hydration of the bead, obtaining a mechanically strong adsorbent, stable at pH levels under 1.0 and temperatures above 170 °C, with a maximum adsorption capacity value of 390 mg g−1 for Pb (II) [90].
Considering the issues mentioned before, another alternative to the use of calcium alginate on its own is the preparation of composite materials as reported in the literature.
2.3. Chitosan
Chitosan is a polysaccharide obtained via partial deacetylation of naturally existing chitin, the second most abundant polysaccharide on Earth after cellulose, which can be found in the shells of insects and crustaceans [91][92]. Industrialization and commercialization of shrimps and prawns are relevant to the economy of many countries but, at the same time, crustacean shells accumulate when discarded, with a significant negative environmental impact. Therefore, the utilization of this waste contributes to solving environmental problems associated with its accumulation [37][93].
Chemical structure of chitosan (poly (β (1→4)-2-amino-2-deoxy-D-glucopyranose) is shown in Figure 5 [94].
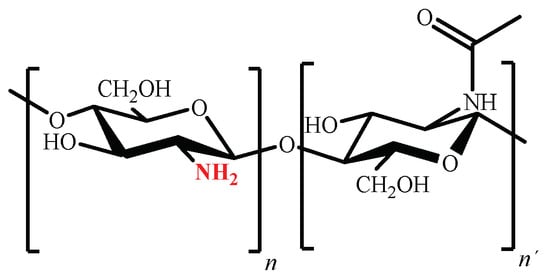
Figure 5. Chemical structure of partially acetylated chitosan.
Considering that the amino groups of the D-glucosamine residues of chitosan have a pKa value of 6.5 [34], at pH values below 6.5 they are predominantly positively charged, making chitosan a polycation [37]. Nevertheless, despite the polycation’s capacity to form ionic complexes with a wide variety of anionic pollutants [95], its complete solubility in aqueous media at pH values lower than 5 limits its use as adsorbent [34][37].
On the other hand, regarding cationic pollutants such as lead, mercury, copper, cobalt, and cadmium, among others, chitosan chains must be predominantly deprotonated to avoid electrostatic repulsion and, therefore, pH values above 6.5 are required. In this case, the main mechanism involved in metal retention is chelation, in which the amino groups play a key role, serving as coordination sites for metal-binding [95][96]. Additionally, it is important to highlight that cation diffusion into chitosan’s structure could affect its adsorption capacity, depending on the physical nature of chitosan (i.e., flakes, beads, or membranes) and its crystallinity [95][96].
From an economic point of view, the use of an adsorbent on an industrial scale is only feasible if it is reusable, so regeneration of the materials is critical. Hence, the fact that chitosan is soluble in the acidic aqueous mediums required for heavy metal desorption prevents it from regenerating and consequently from being used on a large scale.
Therefore, efforts are focused towards producing insoluble chitosan materials in an acidic environment and enhancing the materials’ adsorption capacity. In this context, the main strategies reported include: substitutions involving either the C-6 hydroxyl group, the C-2 amino group, or both; crosslinking reactions; and grafting of polymeric chains.
2.4. Starch
Researchers have been also focused on other abundant polysaccharide: starch. It is constituted by two homopolysaccharides, amylose and amylopectin [97]. The former is a water-soluble linear polymer, consisting in D-glucopyranose units linked by α-(1→4) bonds. Amylopectin has a branched structure with mostly short chains of α-(1→4) linked D-glucopyranose units with 5–6% branches linked by α-(1→6) glycosidic bonds to the main chain (Figure 6) [98].
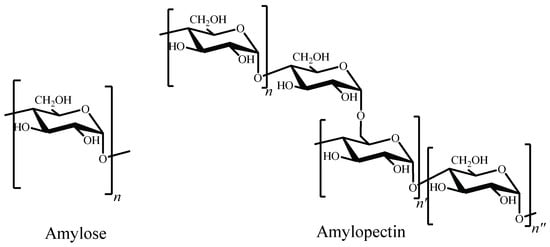
Figure 6. Chemical structures of amylose and amylopectin.
Depending on the source from which starch is obtained, the proportion of amylose and amylopectin varies, and so does its average molecular weight [99]. Some of the most common sources include maize, potato, wheat and rice. Nevertheless, there are promising unconventional sources under study, such as fruits and vegetables waste. Kringel et al. conducted studies on amylose and amylopectin content of waste from fruits and vegetables with the aim of analysing its suitability as valorised residues from natural sources. The reported starch content of outer pericarp and core tissues of kiwi, mango kernels, pineapple stems, apple pulp, banana peels and avocado seeds was in a 20–50% range [100].
Regarding water treatment, native starch has been reported to have a poor adsorption capacity [101], which can mainly be attributed to its low surface area, limited thermal stability and absence of highly-absorptive functional groups, such as carboxyl, xanthate, acrylate, acetyl, hydroxypropyl, amine or amide [102][103]. Analogously to cellulose, alginate and chitosan, there are reports describing starch’s chemical modifications as well as the preparation of starch composites.
For instance, Guo et al. compared the efficiency for MB removal attained by native starch and a modified porous derivative, prepared by crosslinking native starch with epichlorohydrin and further hydrolysis with α-amylase, obtaining a maximum adsorption capacity value for MB almost three times higher than native starch [104]. In accordance with the results reported by Guo et al., Alvarado et al. also observed an enhancement of the adsorption capacity for the MB of starch after being modified by esterification with malonic, glutaric and valeric acids [105].
Soto et al. also compared modified and native starch. In this case, maleic acid and itaconic acids were used for esterification of starch, achieving maximum adsorption capacity values for Pb (II) and Zn (II) of 25.2 and 7.9 mg g−1, respectively for itaconate starch, while 5.2 and 3.2 mg g−1 were the corresponding values for native starch [106]. Soto et al. also reported oxidized starch adsorbents that achieved higher heavy metal removal capacity values compared to native starch; for Cd (II): 11.1 and 8.0 mg g−1; for Ni (II): 22.6 and 8.3 mg g−1; for Pb (II): 18.0 and 5.2 mg g−1; and for Zn (II): 10.3 and 3.2 mg g−1, respectively [107].
Sancey et al. reported the crosslinking of starch with 1,4-butanediol diglycidylether in presence of ammonium hydroxide and 2,3-epoxy-propyltrimethylammonium chloride, with further carboxymethylation. Herein, metal adsorption studies were conducted, achieving almost complete removal of Cu (II) and Fe (III) [108]. Copper removal was also assayed by Zheng et al. using starch hydrogels prepared with poly(acrylic acid), achieving an uptake of 179.9 mg g−1 Cu(II) in aqueous medium [109].
Chaudhari et al. prepared a starch xanthate that achieved almost complete removal of Hg (II), Cu (II), Cd (II) and Ni (II), and attributed the heavy metals uptake to the complexation of metals with xanthate groups [110].
As far as starch composites are concerned, composites with layer double hydroxide for methyl orange and fluoride removal from wastewater were reported. These materials were prepared by co-precipitation and different metals were used for the hydroxide layers, such as Mg (II), Al (III), Zn (II), Fe (III) and Ni (II). In particular, Zubair et al. reported a Starch-Ni/Fe-layered double hydroxide composite that achieved a maximum methyl orange (MO) adsorption capacity value of 387.6 mg g−1 [111]. However, Tao et al. achieved a higher maximum adsorption capacity value towards MO of 1555.0 mg g−1 [112] for the Zn/Mg/Al- Layered double hydroxide starch. On the other hand, a Mg/Al layered double hydroxides reported by Liu et al. achieved almost complete removal of fluoride [113].
Besides, iron nanoparticles are well-known to contribute to arsenic removal due to electrostatic attraction, ion exchange, and surface complexation [114]. It is therefore interesting to analyze iron nanoparticles’ effect when used in materials prepared with starch, such as composite materials with magnetite, maghemite and ferromanganese binary oxide. Next, some of the reported materials are described, in which starch acted only as a support or stabilizer of the mentioned inorganic particles.
Adsorption studies towards As (III) on iron-starch materials were carried out in aqueous solutions, achieving a maximum adsorption capacity value of 161.3 mg g−1 with the material reported by Xu et al. [115], while Siddiqui et al., obtained a maximum capacity value of 8.9 mg g−1 for a functionalized maghemite [116] and Robinson et al., 55.9 mg g−1 for a starch/maghemite nano-adsorbent [117].
On the other hand, as in the case of the other polysaccharides mentioned in previous sections, the preparation of ion exchangers from starch is a widely used strategy for ionic pollutants removal. For instance, Ma et al. [118] chemically modified starch attaching either xanthate or citrate groups to their structure (Figure 7I,II). In this work, adsorption assays towards Pb (II) were carried out, and the maximum adsorption capacity values were 109.1 and 57.6 mg g−1 for starch xanthate and starch citrate, respectively.
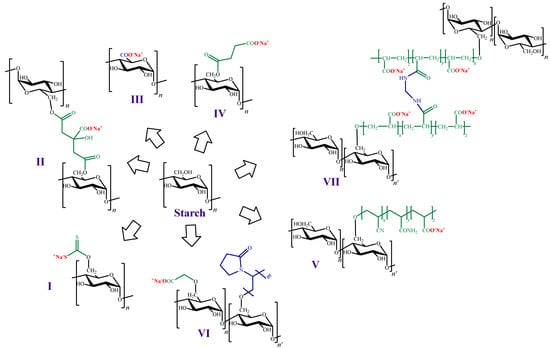
Figure 7. Chemical structures of cation exchangers derived from starch.
Another strategy for introducing negative charges in the starch structure is the oxidation of C-6 to a carboxyl group (Figure 7III). In this context, Liu et al. [119] modified starch nanoparticles through oxidization, using sodium hypochlorite. The new materials were preliminarily explored as adsorbents of heavy metals, using Pb (II) and Cu (II) as adsorbate models. Compared to the unmodified nanoparticles, the modified ones had remarkably higher adsorption capacity values (94.5 and 81.0 mg g−1 for Pb (II) and Cu (II), respectively).
Chen et al. [120] also used starch nanoparticles, but in this case the incorporation of negative charges in their surface was achieved by esterification with succinyl anhydride (Figure 7IV). The maximum degree of substitution was 0.1, which resulted in an increase on the surface Z-potential. The modified nanoparticles were assayed for the adsorption of Cu (II) and MB, showing that the adsorption process was greatly influenced by the pH value, as expected in presence of carboxyl groups. At low pH values, carboxyl groups are highly protonated, and the adsorption could be neglected (less than 3.0 mg g−1). At pH values above 4.0, the degree of protonation decreases and the surface charge of the adsorbents becomes negative, exerting electrostatic attraction to the adsorbate [34][38], which enhances the adsorption capacity of Cu (II) and MB (8.4 and 24.4 mg g−1, respectively).
Another example of modifications to obtain negatively charged starch was performed by Hashem et al. [121], who reported starch hydrogels prepared via graft polymerization of acrylonitrile, followed by nitrile groups hydrolysis to generate carboxylic acids and amides (Figure 7V). In this work, adsorption of Hg (II) on the starch hydrogels were carried out and a maximum adsorption capacity value of 1250 mg g−1 was reported by the authors. Subsequently, the same author reported adsorption studies against Pb (II) carried out on a similar material. In this last case, the value of the maximum adsorption capacity value reported was 264.4 mg g−1 [122].
Haroon et al. [123] also obtained cation exchangers by grafting carboxymethylated starch with N-vinylpyrrolidone (Figure 7VI). The new material was assayed as adsorbent towards rhodamine 6G from wastewater with a maximum adsorption capacity value of 363.9 mg g−1.
There are also reports of materials prepared using acrylic acid as the grafting monomer. For instance, Bahadoran et al. [124] reported a starch-g-poly(acrylic acid) hydrogel, achieving 736.0 mg g−1 of Cu (II) removal. Additionally, cellulose nanofibers were incorporated into the hydrogel, which enhanced its adsorption capacity, obtaining a value of 957.0 mg g−1 of Cu (II).
Ma et al., also prepared a composite material from starch, obtaining a starch-graft-poly(acrylic acid)/organo-modified zeolite, which was assayed for Cr (III) adsorption. The adsorbent’s removal of the mentioned metal was 651.4 mg g−1 [125].
Moreover, Chen et al. [126] prepared a starch-based high-performance adsorptive hydrogel by grafting polyacrylic acid onto starch, and then crosslinking it with N,N’-methylene-bisacrylamide (Figure 7VII). This adsorbent was employed to remove the organic cationic dye MB from effluents in which its concentration was high, yielding a maximum adsorption capacity value of 2967.7 mg g−1.
Additionally, Shoaib et al. prepared a composite material using starch, acrylic acid, and activated carbon from the red alga Pterocladia capillacea with N,N′-methylenebisacrylamide as a crosslinker and ammonium persulphate as an initiator. The adsorbent was assayed for MB dye removal, achieving 1428.6 mg g−1 as the maximum adsorption capacity value [127].
On the other hand, anion exchangers have been obtained by incorporating positive charges into the starch structure by introducing amino groups.
References
- Khilchevskyi, V.K.; Oliinyk, Y.B.; Zatserkovnyi, V.I. Global problems of water resources scarcity. In Proceedings of the XIV International Scientific Conference “Monitoring of Geological Processes and Ecological Condition of the Environment”, Kyiv, Ukraine, 10 November 2020; pp. 1–5.
- UNESCO World Water Assessment Programme. The United Nations World Water Development Report 2021: Valuing Water. Available online: https://www.unesco.org/reports/wwdr/2021/en/download-report (accessed on 30 June 2023).
- Simonovic, S.P. World water dynamics: Global modeling of water resources. J. Environ. Manag. 2002, 66, 249–267.
- Kumar, V.; Kumar, S.; Américo-Pinheiro, J.H.P.; Vinthange, M.; Sher, F. Editorial: Emerging approaches for sustainable management for wastewater. Front. Environ. Sci. 2023, 10, 1122659.
- Skouteris, G.; Rodriguez-Garcia, G.; Reinecke, S.F.; Hampel, U. The use of pure oxygen for aeration in aerobic wastewater treatment: A review of its potential and limitations. Bioresour. Technol. 2020, 312, 123595.
- Cescon, A.; Jiang, J.-Q. Filtration process and alternative filter media material in water treatment. Water 2020, 12, 3377.
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375.
- Ding, H.; Zhang, J.; He, H.; Zhu, Y.; Dionysiou, D.D.; Liu, Z.; Zhao, C. Do membrane filtration systems in drinking water treatment plants release nano/microplastics? Sci. Total Environ. 2021, 755, 142658.
- Chang, L.; Cao, Y.; Fan, G.; Li, C.; Peng, W. A review of the applications of ion floatation: Wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv. 2019, 9, 20226–20239.
- Roy, A.; Thakur, B.; Debsarkar, A. Water Pollution and Treatment Technologies. In Environmental Management: Issues and Concerns in Developing Countries; Sikdar, P.K., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 79–106.
- Kyzas, G.Z.; Matis, K.A. Flotation in Water and Wastewater Treatment. Processes 2018, 6, 116.
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020, 10, 20231–20244.
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795.
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71.
- Jumadi, J.; Kamari, A.; Hargreaves, J.S.J.; Yusof, N. A review of nano-based materials used as flocculants for water treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3571–3594.
- Sookhak Lari, K.; Rayner, J.L.; Davis, G.B. Towards characterizing LNAPL remediation endpoints. J. Environ. Manag. 2018, 224, 97–105.
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable polysulfides for oil spill remediation: Repurposing industrial waste for environmental benefit. Adv. Sustain. Syst. 2018, 2, 1800024.
- Rachmadi, A.T.; Kitajima, M.; Kato, T.; Kato, H.; Okabe, S.; Sano, D. Required Chlorination Doses to Fulfill the Credit Value for Disinfection of Enteric Viruses in Water: A Critical Review. Environ. Sci. Technol. 2020, 54, 2068–2077.
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Rahisuddin; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination disinfection by-products in municipal drinking water—A review. J. Clean. Prod. 2020, 273, 123159.
- Cimadoro, J.; Ribba, L.; Ledesma, S.; Goyanes, S. Electrospun mats: From white to transparent with a drop. Macromol. Mater. Eng. 2018, 303, 1800237.
- Pereira, P.P.; Fernandez, M.; Cimadoro, J.; González, P.S.; Morales, G.M.; Goyanes, S.; Agostini, E. Biohybrid membranes for effective bacterial vehiculation and simultaneous removal of hexavalent chromium (Cr VI) and phenol. Appl. Microbiol. Biotechnol. 2021, 105, 827–838.
- Cimadoro, J.; Goyanes, S. Reversible swelling as a strategy in the development of smart membranes from electrospun polyvinyl alcohol nanofiber mats. J. Polym. Sci. 2020, 58, 737–746.
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89.
- Giuliani, L.; De Angelis, L.; Diaz Bukvic, G.; Zanini, M.; Minotti, F.; Errea, M.I.; Grondona, D. Trielectrode plasma reactor for water treatment. J. Appl. Phys. 2020, 127, 223303.
- Bermúdez, L.A.; Pascual, J.M.; Martínez, M.d.M.M.; Poyatos Capilla, J.M. Effectiveness of advanced oxidation processes in wastewater treatment: State of the art. Water 2021, 13, 2094.
- Abdullah, N.H.S.; Karsiti, M.N.; Ibrahim, R. A review of pH neutralization process control. In Proceedings of the 2012 4th International Conference on Intelligent and Advanced Systems (ICIAS2012), Kuala Lumpur, Malaysia, 12–14 June 2012; pp. 594–598.
- Picón, D.; Torasso, N.; Baudrit, J.R.V.; Cerveny, S.; Goyanes, S. Bio-inspired membranes for adsorption of arsenic via immobilized L-Cysteine in highly hydrophilic electrospun nanofibers. Chem. Eng. Res. Des. 2022, 185, 108–118.
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617.
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res. 2022, 222, 118888.
- Tran, T.V.; Nguyen, D.T.C.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Jalil, A.A.; Nabgan, W.; Lee, T. A critical review on pineapple (Ananas comosus) wastes for water treatment, challenges and future prospects towards circular economy. Sci. Total Environ. 2023, 856, 158817.
- Huang, X.; Hadi, P.; Joshi, R.; Alhamzani, A.G.; Hsiao, B.S. A Comparative Study of Mechanism and Performance of Anionic and Cationic Dialdehyde Nanocelluloses for Dye Adsorption and Separation. ACS Omega 2023, 8, 8634–8649.
- Kennedy, K.K.; Maseka, K.J.; Mbulo, M. Selected adsorbents for removal of contaminants from wastewater: Towards engineering clay minerals. Open J. Appl. Sci. 2018, 8, 355–369.
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water Treatment and Purification; Springer: Cham, Switzerland, 2017; Volume 256.
- Rossi, E.; Ramírez, J.A.Á.; Errea, M.I. Preparation of an environmentally friendly lead adsorbent. A contribution to the rational design of heavy metal adsorbents. J. Environ. Chem. Eng. 2020, 8, 104210.
- Atkins, P.W.; De Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2014.
- Dinh, V.-P.; Le, N.-C.; Tuyen, L.A.; Hung, N.Q.; Nguyen, V.-D.; Nguyen, N.-T. Insight into adsorption mechanism of lead(II) from aqueous solution by chitosan loaded MnO2 nanoparticles. Mater. Chem. Phys. 2018, 207, 294–302.
- Errea, M.I.; Rossi, E.; Goyanes, S.N.; D’Accorso, N.B. Chitosan: From organic pollutants to high-value polymeric materials. In Industrial Applications of Renewable Biomass Products; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–264.
- Rossi, E.; Montoya Rojo, Ú.; Cerrutti, P.; Foresti, M.L.; Errea, M.I. Carboxymethylated bacterial cellulose: An environmentally friendly adsorbent for lead removal from water. J. Environ. Chem. Eng. 2018, 6, 6844–6852.
- Dambies, L.; Salinaro, R.; Alexandratos, S.D. Immobilized N-Methyl-d-glucamine as an Arsenate-Selective Resin. Environ. Sci. Technol. 2004, 38, 6139–6146.
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213.
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593.
- Heinze, T.; El Seoud, O.A.; Koschella, A. Production and Characteristics of Cellulose from Different Sources. In Cellulose Derivatives: Synthesis, Structure, and Properties; Heinze, T., El Seoud, O.A., Koschella, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–38.
- Rossi, E.; Salvay, A.G.; Errea, M.I.; Foresti, M.L. Dried water-redispersible bacterial nanocellulose with sorbitol as capping agent. Food Hydrocoll. 2023, 143, 108916.
- Bovi, J.; Butto, M.; Arroyo, S.; Bernal, C.; Foresti, M.L. Bacterial Cellulose nanofibrils from a By-Product of the Growing Kombucha Business: Obtention and Use in the Development of Poly (Lactic Acid) Composites. Lat. Am. Appl. Res. 2023; in press.
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374.
- Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076.
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173.
- Marimuthu, T.; Chee, C.Y.; Sulaiman, N.M.N. A review on the use of cellulose nanomaterials for wastewater remediation of heavy metal ions. Int. J. Environ. Sci. Technol. 2023, 20, 3421–3436.
- He, Z.; Song, H.; Cui, Y.; Zhu, W.; Du, K.; Yao, S. Porous Spherical Cellulose Carrier Modified with Polyethyleneimine and Its Adsorption for Cr(III) and Fe(III) from Aqueous Solutions. Chin. J. Chem. Eng. 2014, 22, 984–990.
- Aichour, A.; Zaghouane-Boudiaf, H. Single and competitive adsorption studies of two cationic dyes from aqueous mediums onto cellulose-based modified citrus peels/calcium alginate composite. Int. J. Biol. Macromol. 2020, 154, 1227–1236.
- Shen, J.; Xu, X.; Ouyang, X.; Jin, M. Adsorption of Pb(II) from Aqueous Solutions Using Nanocrystalline Cellulose/Sodium Alginate/K-Carrageenan Composite Hydrogel Beads. J. Polym. Environ. 2022, 30, 1995–2006.
- Iravani Mohammadabadi, S.; Javanbakht, V. Fabrication of dual cross-linked spherical treated waste biomass/alginate adsorbent and its potential for efficient removal of lead ions from aqueous solutions. Ind. Crop. Prod. 2021, 168, 113575.
- Soleimani, S.; Heydari, A.; Fattahi, M.; Motamedisade, A. Calcium alginate hydrogels reinforced with cellulose nanocrystals for methylene blue adsorption: Synthesis, characterization, and modelling. Ind. Crop. Prod. 2023, 192, 115999.
- Kasbaji, M.; Mennani, M.; Grimi, N.; Oubenali, M.; Mbarki, M.; El Zakhem, H.; Moubarik, A. Adsorption of cationic and anionic dyes onto coffee grounds cellulose/sodium alginate double-network hydrogel beads: Isotherm analysis and recyclability performance. Int. J. Biol. Macromol. 2023, 239, 124288.
- Wu, J.; Dong, Z.; Li, X.; Li, P.; Wei, J.; Hu, M.; Geng, L.; Peng, X. Constructing acid-resistant chitosan/cellulose nanofibrils composite membrane for the adsorption of methylene blue. J. Environ. Chem. Eng. 2022, 10, 107754.
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127.
- Wang, Y.; Wang, H.; Peng, H.; Wang, Z.; Wu, J.; Liu, Z. Dye Adsorption from Aqueous Solution by Cellulose/Chitosan Composite: Equilibrium, Kinetics, and Thermodynamics. Fibers Polym. 2018, 19, 340–349.
- Liu, K.; Chen, L.; Huang, L.; Lai, Y. Evaluation of ethylenediamine-modified nanofibrillated cellulose/chitosan composites on adsorption of cationic and anionic dyes from aqueous solution. Carbohydr. Polym. 2016, 151, 1115–1119.
- Liu, C.; Yu, J.; You, J.; Wang, Z.; Zhang, M.; Shi, L.; Zhuang, X. Cellulose/Chitosan Composite Sponge for Efficient Protein Adsorption. Ind. Eng. Chem. Res. 2021, 60, 9159–9166.
- Marrane, S.E.; Dänoun, K.; Allouss, D.; Sair, S.; Channab, B.-E.; Rhihil, A.; Zahouily, M. A Novel Approach to Prepare Cellulose-g-Hydroxyapatite Originated from Natural Sources as an Efficient Adsorbent for Heavy Metals: Batch Adsorption Optimization via Response Surface Methodology. ACS Omega 2022, 7, 28076–28092.
- Saber-Samandari, S.; Saber-Samandari, S.; Gazi, M. Cellulose-graft-polyacrylamide/hydroxyapatite composite hydrogel with possible application in removal of Cu (II) ions. React. Funct. Polym. 2013, 73, 1523–1530.
- Jin, Y.; Ni, Y.; Pudukudy, M.; Zhang, H.; Wang, H.; Jia, Q.; Shan, S. Synthesis of nanocrystalline cellulose/hydroxyapatite nanocomposites for the efficient removal of chlortetracycline hydrochloride in aqueous medium. Mater. Chem. Phys. 2022, 275, 125135.
- Mututuvari, T.M.; Tran, C.D. Synergistic adsorption of heavy metal ions and organic pollutants by supramolecular polysaccharide composite materials from cellulose, chitosan and crown ether. J. Hazard. Mater. 2014, 264, 449–459.
- Eltaweil, A.S.; Abd El-Monaem, E.M.; El-Subruiti, G.M.; Ali, B.M.; Abd El-Latif, M.M.; Omer, A.M. Graphene oxide incorporated cellulose acetate beads for efficient removal of methylene blue dye; isotherms, kinetic, mechanism and co-existing ions studies. J. Porous Mater. 2023, 30, 607–618.
- Ogunleye, D.T.; Akpotu, S.O.; Moodley, B. Crystalline Nanocellulose Anchored on Reduced Graphene Oxide for the Removal of Pharmaceuticals from Aqueous Systems: Adsorbent Characterization and Adsorption Performance. ChemistrySelect 2023, 8, e202202533.
- Abou-Zeid, R.E.; Kamal, K.H.; Abd El-Aziz, M.E.; Morsi, S.M.; Kamel, S. Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int. J. Biol. Macromol. 2021, 167, 1091–1101.
- Anugrahwati, M.; Sari, A.W.; Munawwaroh, L.A.; Musawwa, M.M. Synthesis of microcrystalline cellulose (MCC)-Fe3O4 for malachite green adsorption; kinetics, isotherms, thermodynamics, and regeneration studies. J. Eng. Sci. Technol. 2022, 17, 91–101.
- Pan, Y.; Xie, H.; Liu, H.; Cai, P.; Xiao, H. Novel cellulose/montmorillonite mesoporous composite beads for dye removal in single and binary systems. Bioresour. Technol. 2019, 286, 121366.
- Rong, N.; Chen, C.; Ouyang, K.; Zhang, K.; Wang, X.; Xu, Z. Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep. Purif. Technol. 2021, 274, 119120.
- Luo, J.; Ma, X.; Zhou, X.; Xu, Y. Construction of physically crosslinked cellulose nanofibrils/alkali lignin/montmorillonoite/polyvinyl alcohol network hydrogel and its application in methylene blue removal. Cellulose 2021, 28, 5531–5543.
- Zhang, X.; Li, F.; Zhao, X.; Cao, J.; Liu, S.; Zhang, Y.; Yuan, Z.; Huang, X.; De Hoop, C.F.; Peng, X.; et al. Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions. Gels 2023, 9, 40.
- Kumar, A.S.K.; Kalidhasan, S.; Rajesh, V.; Rajesh, N. Application of Cellulose-Clay Composite Biosorbent toward the Effective Adsorption and Removal of Chromium from Industrial Wastewater. Ind. Eng. Chem. Res. 2012, 51, 58–69.
- Cai, J.; Lei, M.; Zhang, Q.; He, J.-R.; Chen, T.; Liu, S.; Fu, S.-H.; Li, T.-T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@ Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A Appl. Sci. Manuf. 2017, 92, 10–16.
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971.
- Jing, L.; Yang, S.; Li, X.; Jiang, Y.; Lou, J.; Liu, Z.; Ding, Q.; Han, W. Effective adsorption and sensitive detection of Cr6+ by degradable collagen-based porous fluorescent aerogel. Ind. Crop. Prod. 2022, 182, 114882.
- Shamsudin, M.S.; Azha, S.F.; Shahadat, M.; Ismail, S. Cellulose/bentonite-zeolite composite adsorbent material coating for treatment of N-based antiseptic cationic dye from water. J. Water Process Eng. 2019, 29, 100764.
- Ding, W.; Liang, H.; Zhang, H.; Sun, H.; Geng, Z.; Xu, C. A cellulose/bentonite grafted polyacrylic acid hydrogel for highly-efficient removal of Cd(II). J. Water Process Eng. 2023, 51, 103414.
- Xu, D.; Huang, Y.; Ma, Q.; Qiao, J.; Guo, X.; Wu, Y. A 3D porous structured cellulose nanofibrils-based hydrogel with carbon dots-enhanced synergetic effects of adsorption and photocatalysis for effective Cr(VI) removal. Chem. Eng. J. 2023, 456, 141104.
- Mubarak, M.F.; Zayed, A.M.; Ahmed, H.A. Activated Carbon/Carborundum@Microcrystalline Cellulose core shell nano-composite: Synthesis, characterization and application for heavy metals adsorption from aqueous solutions. Ind. Crop. Prod. 2022, 182, 114896.
- Draget, K.I. 29–Alginates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 807–828.
- Hasnain, M.S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Chapter 1–Alginates: Sources, structure, and properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–17.
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181.
- Andriamanantoanina, H.; Rinaudo, M. Characterization of the alginates from five madagascan brown algae. Carbohydr. Polym. 2010, 82, 555–560.
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389.
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167.
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78.
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772.
- Park, H.G.; Chae, M.Y. Novel type of alginate gel-based adsorbents for heavy metal removal. J. Chem. Technol. Biotechnol. 2004, 79, 1080–1083.
- Vijaya, Y.; Popuri, S.R.; Boddu, V.M.; Krishnaiah, A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008, 72, 261–271.
- Jeon, C.; Park, J.Y.; Yoo, Y.J. Novel immobilization of alginic acid for heavy metal removal. Biochem. Eng. J. 2002, 11, 159–166.
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8.
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14.
- Gerente, C.; Lee, V.K.C.; Cloirec, P.L.; McKay, G. Application of Chitosan for the Removal of Metals from Wastewaters by Adsorption—Mechanisms and Models Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127.
- Carvalho, A.J.F. Chapter 15–Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342.
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Traisnel, M. Remediation of Heavy Metals by Biomolecules: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1644–1704.
- Robyt, J.F. Starch: Structure, Properties, Chemistry, and Enzymology. In Glycoscience: Chemistry and Chemical Biology; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472.
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.D.R.; Gandra, E.A. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch 2020, 72, 1900200.
- Awokoya, K.N.; Oninla, V.O.; Bello, D.J. Synthesis of oxidized Dioscorea dumentorum starch nanoparticles for the adsorption of lead(II) and cadmium(II) ions from wastewater. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100440.
- Abd El-Ghany, N.A.; Elella, M.H.A.; Abdallah, H.M.; Mostafa, M.S.; Samy, M. Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review. J. Polym. Environ. 2023, 31, 2792–2825.
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Zain-ul-Abdin; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical modification of starch and its application as an adsorbent material. RSC Adv. 2016, 6, 78264–78285.
- Guo, L.; Li, G.; Liu, J.; Meng, Y.; Tang, Y. Adsorptive decolorization of methylene blue by crosslinked porous starch. Carbohydr. Polym. 2013, 93, 374–379.
- Alvarado, N.; Abarca, R.L.; Urdaneta, J.; Romero, J.; Galotto, M.J.; Guarda, A. Cassava starch: Structural modification for development of a bio-adsorber for aqueous pollutants. Characterization and adsorption studies on methylene blue. Polym. Bull. 2021, 78, 1087–1107.
- Soto, D.; Urdaneta, J.; Pernía, K.; León, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Removal of heavy metal ions in water by starch esters. Starch 2016, 68, 37–46.
- Soto, D.; Urdaneta, J.; Pernía, K.; León, O.; Muñoz-Bonilla, A.; Fernández-García, M. Heavy metal (Cd2+, Ni2+, Pb2+ and Ni2+) adsorption in aqueous solutions by oxidized starches. Polym. Adv. Technol. 2015, 26, 147–152.
- Sancey, B.; Trunfio, G.; Charles, J.; Minary, J.-F.; Gavoille, S.; Badot, P.-M.; Crini, G. Heavy metal removal from industrial effluents by sorption on cross-linked starch: Chemical study and impact on water toxicity. J. Environ. Manag. 2011, 92, 765–772.
- Zheng, Y.; Hua, S.; Wang, A. Adsorption behavior of Cu2+ from aqueous solutions onto starch-g-poly(acrylic acid)/sodium humate hydrogels. Desalination 2010, 263, 170–175.
- Chaudhari, S.; Tare, V. Analysis and evaluation of heavy metal uptake and release by insoluble starch xanthate in aqueous environment. Water Sci. Technol. 1996, 34, 161–168.
- Zubair, M.; Jarrah, N.; Ihsanullah; Khalid, A.; Manzar, M.S.; Kazeem, T.S.; Al-Harthi, M.A. Starch-NiFe-layered double hydroxide composites: Efficient removal of methyl orange from aqueous phase. J. Mol. Liq. 2018, 249, 254–264.
- Tao, X.; Liu, D.; Cong, W.; Huang, L. Controllable synthesis of starch-modified ZnMgAl-LDHs for adsorption property improvement. Appl. Surf. Sci. 2018, 457, 572–579.
- Liu, J.; Yue, X.; Lu, X.; Guo, Y. Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides. Water 2018, 10, 745.
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560.
- Xu, F.; Chen, H.; Dai, Y.; Wu, S.; Tang, X. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water. J. Environ. Manag. 2019, 245, 160–167.
- Siddiqui, S.I.; Singh, P.N.; Tara, N.; Pal, S.; Chaudhry, S.A.; Sinha, I. Arsenic removal from water by starch functionalized maghemite nano-adsorbents: Thermodynamics and kinetics investigations. Colloids Interface Sci. Commun. 2020, 36, 100263.
- Robinson, M.R.; Coustel, R.; Abdelmoula, M.; Mallet, M. As (V) and As (III) sequestration by starch functionalized magnetite nanoparticles: Influence of the synthesis route onto the trapping efficiency. Sci. Technol. Adv. Mater. 2020, 21, 524–539.
- Ma, X.; Liu, X.; Anderson, D.P.; Chang, P.R. Modification of porous starch for the adsorption of heavy metal ions from aqueous solution. Food Chem. 2015, 181, 133–139.
- Liu, Q.; Li, F.; Lu, H.; Li, M.; Liu, J.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced dispersion stability and heavy metal ion adsorption capability of oxidized starch nanoparticles. Food Chem. 2018, 242, 256–263.
- Chen, Q.-J.; Zheng, X.-M.; Zhou, L.-L.; Zhang, Y.-F. Adsorption of Cu (II) and Methylene Blue by Succinylated Starch Nanocrystals. Starch 2019, 71, 1800266.
- Hashem, A.; Ahmad, F.; Fahad, R. Application of some starch hydrogels for the removal of mercury (II) ions from aqueous solutions. Adsorpt. Sci. Technol. 2008, 26, 563–579.
- Aniagor, C.O.; Afifi, M.A.; Hashem, A. Rapid and efficient uptake of aqueous lead pollutant using starch-based superabsorbent hydrogel. Polym. Bull. 2022, 79, 6373–6388.
- Haroon, M.; Wang, L.; Yu, H.; Ullah, R.S.; Zain-ul-Abdin; Khan, R.U.; Chen, Q.; Liu, J. Synthesis of carboxymethyl starch-g-polyvinylpyrolidones and their properties for the adsorption of Rhodamine 6G and ammonia. Carbohydr. Polym. 2018, 186, 150–158.
- Bahadoran Baghbadorani, N.; Behzad, T.; Etesami, N.; Heidarian, P. Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly(acrylic acid) superadsorbent hydrogels. Compos. Part B Eng. 2019, 176, 107084.
- Ma, K.; Zhao, L.; Jiang, Z.; Huang, Y.; Sun, X. Adsorption characteristics of Cr (III) onto starch-graft-poly(acrylic acid)/organo-modifed zeolite 4A composite: A novel path to the adsorption mechanisms. Polym. Compos. 2018, 39, 1223–1233.
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953.
- Shoaib, A.G.M.; Sikaily, A.E.; Ragab, S.; Masoud, M.S.; Ramadan, M.S.; El Nemr, A. Starch-grafted-poly(acrylic acid)/Pterocladia capillacea–derived activated carbon composite for removal of methylene blue dye from water. Biomass Convers. Biorefinery 2022.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
497
Revisions:
2 times
(View History)
Update Date:
07 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




