Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Adsorption processes, due to their technical simplicity and cost-effectiveness, have arisen as one of the most well-known, straightforward solutions to water pollution. In this context, polysaccharides, due to their abundance, biodegradability, and biocompatibility, are appealing raw materials for the design of adsorbents.
- adsorption
- polysaccharides’ chemical modification
- water pollutants
- water treatment
1. Introduction
Water is a scarce natural resource globally, not only due to its limited physical access but also to the progressive deterioration in its quality caused by pollution [1]. Over the last century, freshwater use has increased by a factor of six and has been growing at a rate of one per cent per year since 1980, which is a result of population growth, economic development, and consumption habits. Some concerning facts reported by the United Nations are that eight out of ten people lack drinking water access in rural areas, and by 2030, only 81% of the need for clean water will be met, which means that 1.6 billion people would not manage to have safe water by then [2]. Thus, one of the greatest challenges of the XXI century is guaranteeing access to safe drinking water. Hence, technologies aiming to pursue this goal intend to make water affordable and accessible for all by developing efficient systems for polluted water remediation [3,4].
Depending on the target pollutant and its concentration, contaminated water can be treated via biological, physical, and chemical processes. These approaches include several technologies such as aeration [5], filtration [6,7,8], flotation [9,10,11], coagulation [12,13,14], flocculation [15], skimming [16,17], chlorination [18,19], membrane technologies [20,21,22], ozonation [23], oxidation and advanced oxidation processes [24,25], neutralization [26], and adsorption [27,28,29], among others.
Moreover, adsorption processes have famously provoked interest among the scientific community since they provide a simple, feasible, cost-effective, and straightforward solution to water pollution [30,31].
Adsorption is a superficial phenomenon in which one or more fluid phase components interact with a solid surface, adhering to it. Based on interaction forces between the adsorbate (the substance attached to the surface) and the adsorbent (which provides the surface where adsorbate adheres), adsorption can be either physical (physisorption) or chemical (chemisorption) [32].
One of the main differences between physisorption and chemisorption lies in enthalpy values of 40 kJ mol−1 or lower for physical adsorption and higher values for chemically governed adsorption processes [33,34]. Adsorbents’ capacity, selectivity, and adsorption rate is determined by their structure and properties [34,35,36,37,38,39]. It is therefore crucial to study the materials to select the most adequate material according to the pollutants found.
There is a wide variety of adsorbents, which include conventional materials such as inorganic materials (e.g., zeolites, aluminas, silica gel, iron oxides, and clays), activated carbons, ion-exchange resins; and non-conventional adsorbents such as polysaccharides and their derivatives (e.g., chitosan, cellulose, starch, alginate, or cyclodextrin), biosorbents (e.g., algae, fungi, or yeasts) and either by-products or waste from agricultural and industrial activities (e.g., sawdust, corn cob, sugar bagasse, wheat straw, or sewage sludges) [40].
In this context, polysaccharides are materials that are remarkable for their low cost and environmentally friendliness. Unfortunately, as it will be discussed throughout this manuscript, due to either their low adsorption capacities, physicochemical properties or both, polysaccharides are not suitable for water treatment in their native form. However, their polyhydroxylated structure provides them enough versatility to be subjected to chemical modifications in order to impart specific physicochemical properties depending on the products’ final application.
2. Polysaccharides Involved in Water Remediation
Among naturally existing polysaccharides, cellulose, alginate, chitosan, and starch are the most studied in this field because of their remarkable relative abundance and availability.
2.1. Cellulose
It is well known that cellulose, one of the most abundant organic compounds on Earth, is composed of D-glucopyranose units linked by β-(1→4) bonds (Figure 1).
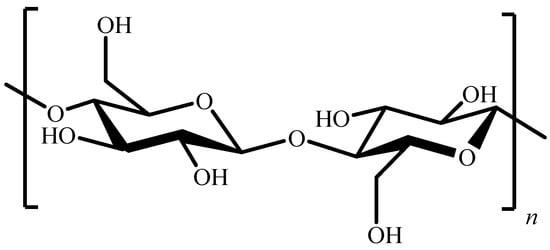
Figure 1. Chemical structure of cellulose.
Although traditionally cellulose has been mainly obtained from wood, over the past few decades, alternative vegetable sources have been increasingly researched to relieve the demand on wood-based biomass and improve process sustainability [41]. In this context, grasses such as bamboo as well as agricultural residues and agro-industrial crop by-products are among the most explored sources due to their low initial cost and fast regeneration. In the case of waste, their use also contributes to their valorisation [41], but they have as a drawback a lower content of cellulose than wood due to the higher content of lignin and hemicellulose [42].
The search for alternatives beyond vegetable sources has led to the study of bacterial cellulose. Bacterial cellulose is produced via fermentation with specific bacteria under suitable conditions and is obtained as a highly hydrated gelatinous pellicle that grows on the aerated surface of the fermentation vessel [43]. Bacterial cellulose is recognized for its high crystallinity, polymerization degree, and chemical purity, as well as for its unique intrinsic nanofibrillar structure. Furthermore, while the isolation of cellulose from plant sources implies the use of reagents that can have a negative environmental impact, bacterial cellulose is produced free of lignin, hemicelluloses, and pectin, thus, avoiding the need for aggressive chemical treatments for the removal of these compounds [38].
The production of bacterial cellulose has the disadvantage of being more expensive than plant cellulose since until now, it has mainly been produced using specific bacteria, typically Komagataeibacter xylinus (formerly called Gluconacetobacter xylinus and Acetobacter xylinum), which require axenic conditions and strict control of fermentation variables [44]. However, for certain applications, it could be alternatively obtained from the floating pellicle developed during kombucha tea production, in a much simpler process which does not require laboratory conditions, complex growth media, or sophisticated cultivation and processing equipment [44,45]. Given the proliferation in recent years of not only classical home-scale breweries, but also larger-scale kombucha enterprises, this production method offers an attractive alternative to obtain pure cellulose by valorising this by-product of kombucha tea.
As far as cellulose tridimensional structure is concerned, the D-glucopyranose units of its chains have a linear arrangement that allows for the formation of numerous intercatenary hydrogen bonds, which result in the polymer’s water insolubility [46] (Figure 2). Additionally, this cellulose arrangement is responsible for its low surface area and consequent low adsorption efficiency of pollutants when employed unmodified, necessitating the functionalization of cellulose [47,48].
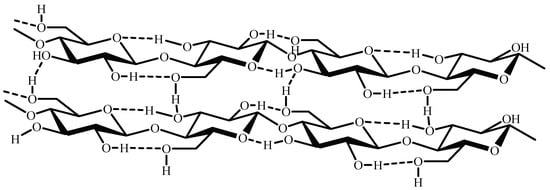
Figure 2. Rearrangement of cellulose chains.
In fact, He et al. illustrated the enhanced adsorption capacity when native cellulose was modified. In this case, native cellulose achieved maximum capacity values of 9.8 and 14.3 mg g−1 for Cr (III) and Fe (III), while 377.2 and 84.0 mg g−1 were attained by preparing a porous spherical polyethyleneimine-cellulose (PEI-cell) absorbent [49].
In that context, intensive work has been carried out on the design of efficient adsorbents from cellulose. The strategies are based either on the preparation of composite materials or on chemical modifications to this polymer.
Approaches to composite materials reported in literature include the preparation of cellulose-based materials using alginate [50,51,52,53,54], chitosan [55,56,57,58,59], hydroxyapatite [60,61,62], crown ethers [63], graphene oxide (GO) [64,65], magnetite (Fe3O4) [66,67], montmorillonite [68,69,70,71,72,73], titanium dioxide [74], collagen [75], bentonite [76,77], and activated carbon [78,79], among others.
2.2. Alginate
Alginate is a polysaccharide which naturally exists as its sodium salt and is constituted of (1→4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues (Figure 3). Alginate does not have regular repetitive units, since it is a block copolymer composed of homopolymeric regions of M or G and interspersed with regions with MG blocks [120].
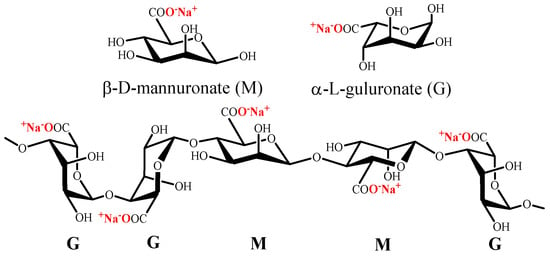
Figure 3. Chemical structure of mannuronate (M), guluronate (G) and alginate.
Its abundance is mainly due to its presence in marine brown algae Phaeophyceae, Ascophyllum nodosum, Laminaria hyperborea, and Macrocystis pyrifera as a structure-forming component and in soil bacteria as a capsular polysaccharide. Several bacteria can produce alginate, such as Azotobacter sp. and Pseudomonas sp. However, commercial alginates are mainly obtained from algal sources, since industrial bacterial production is not economically feasible at present despite being technically possible [121].
The distribution and sequence of M and G blocks of alginate varies according to its source and affects the polysaccharides’ properties, such as viscosity. In fact, for example, alginate obtained from brown algae of the specie Sargassum is considered to be of “borderline” quality due to its low G content [122]. It is important to note that both the environmental conditions during the growth of algae and the extraction techniques used are also two variables that have a high impact on the final structure of the alginate [123].
The interaction between sodium alginate and divalent or trivalent metal cations produces ionic gelation [124], which is related to the length of G blocks in alginate. The formation of coordinate sites is generated by G chains looping, known as the egg-box model (Figure 4).
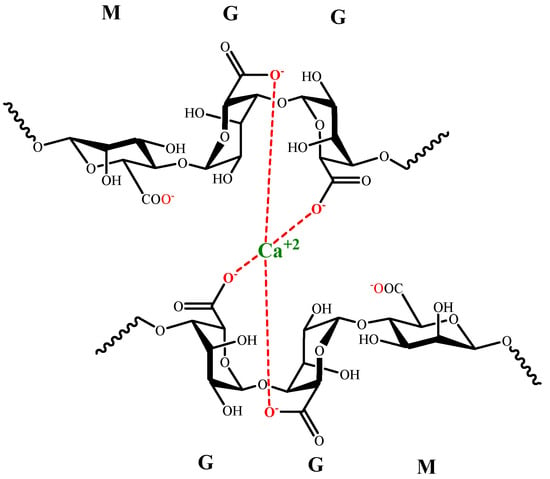
Figure 4. Egg-box model for G alginate residues and Ca (II).
In the presence of monovalent metal cations such as sodium, alginate acts as semiflexible chains instead of the stiff aggregated rigid chains obtained via egg-box formation with Ca (II) [125].
Calcium alginate beads are well known for their ability to adsorb cationic pollutants. In particular, there is some evidence that relates metal cation size to the adsorption efficacy of alginate beads. Both He et al. and Papageorgiou et al. observed higher adsorption capacity values when cation size increases: Pb (II) > Cu (II)> Cd (II), suggesting that larger ions fit better in binding sites [126,127].
Alginate capsules have been proven to have a high lead-binding capacity, with maximum adsorption capacity values of 1540.0 mg g−1 reported by Gyu Park et al. In this case, capsules were prepared by dropping CaCl2 solution and xanthan gum into sodium alginate [128].
Vijaya et al. also obtained insoluble calcium alginate beads which were assayed for Ni (II) adsorption, obtaining 310.4 mg g−1 for calcium alginate. Ni (II) uptake was attributed to both pore volume and large surface area [129].
Nevertheless, the mentioned alginate adsorbents still face two main drawbacks: alginate calcium complex instability and low porosity. Additionally, many of the alginate beads tried in packed column operation exhibited pressure drops that were too high to flow and were not rigid enough. Therefore, intensive work has been done to improve their adsorption performance. To this end, Jeon et al. reported an alginic acid immobilized with PVA-boric acid and crosslinked with glutaraldehyde, designed to reduce the hydration of the bead, obtaining a mechanically strong adsorbent, stable at pH levels under 1.0 and temperatures above 170 °C, with a maximum adsorption capacity value of 390 mg g−1 for Pb (II) [130].
Considering the issues mentioned before, another alternative to the use of calcium alginate on its own is the preparation of composite materials as reported in the literature.
2.3. Chitosan
Chitosan is a polysaccharide obtained via partial deacetylation of naturally existing chitin, the second most abundant polysaccharide on Earth after cellulose, which can be found in the shells of insects and crustaceans [156,157]. Industrialization and commercialization of shrimps and prawns are relevant to the economy of many countries but, at the same time, crustacean shells accumulate when discarded, with a significant negative environmental impact. Therefore, the utilization of this waste contributes to solving environmental problems associated with its accumulation [37,158].
Chemical structure of chitosan (poly (β (1→4)-2-amino-2-deoxy-D-glucopyranose) is shown in Figure 5 [159].
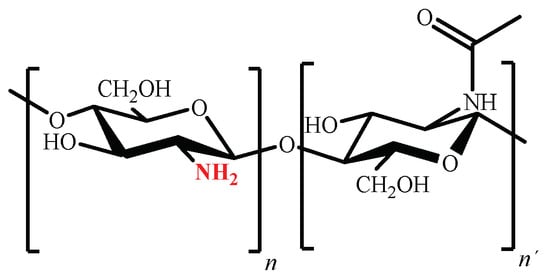
Figure 5. Chemical structure of partially acetylated chitosan.
Considering that the amino groups of the D-glucosamine residues of chitosan have a pKa value of 6.5 [34], at pH values below 6.5 they are predominantly positively charged, making chitosan a polycation [37]. Nevertheless, despite the polycation’s capacity to form ionic complexes with a wide variety of anionic pollutants [160], its complete solubility in aqueous media at pH values lower than 5 limits its use as adsorbent [34,37].
On the other hand, regarding cationic pollutants such as lead, mercury, copper, cobalt, and cadmium, among others, chitosan chains must be predominantly deprotonated to avoid electrostatic repulsion and, therefore, pH values above 6.5 are required. In this case, the main mechanism involved in metal retention is chelation, in which the amino groups play a key role, serving as coordination sites for metal-binding [160,161]. Additionally, it is important to highlight that cation diffusion into chitosan’s structure could affect its adsorption capacity, depending on the physical nature of chitosan (i.e., flakes, beads, or membranes) and its crystallinity [160,161].
From an economic point of view, the use of an adsorbent on an industrial scale is only feasible if it is reusable, so regeneration of the materials is critical. Hence, the fact that chitosan is soluble in the acidic aqueous mediums required for heavy metal desorption prevents it from regenerating and consequently from being used on a large scale.
Therefore, efforts are focused towards producing insoluble chitosan materials in an acidic environment and enhancing the materials’ adsorption capacity. In this context, the main strategies reported include: substitutions involving either the C-6 hydroxyl group, the C-2 amino group, or both; crosslinking reactions; and grafting of polymeric chains.
2.4. Starch
Researchers have been also focused on other abundant polysaccharide: starch. It is constituted by two homopolysaccharides, amylose and amylopectin [176]. The former is a water-soluble linear polymer, consisting in D-glucopyranose units linked by α-(1→4) bonds. Amylopectin has a branched structure with mostly short chains of α-(1→4) linked D-glucopyranose units with 5–6% branches linked by α-(1→6) glycosidic bonds to the main chain (Figure 6) [177].
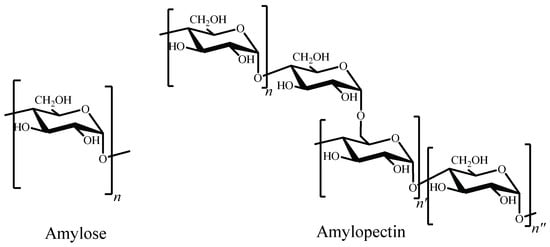
Figure 6. Chemical structures of amylose and amylopectin.
Depending on the source from which starch is obtained, the proportion of amylose and amylopectin varies, and so does its average molecular weight [178]. Some of the most common sources include maize, potato, wheat and rice. Nevertheless, there are promising unconventional sources under study, such as fruits and vegetables waste. Kringel et al. conducted studies on amylose and amylopectin content of waste from fruits and vegetables with the aim of analysing its suitability as valorised residues from natural sources. The reported starch content of outer pericarp and core tissues of kiwi, mango kernels, pineapple stems, apple pulp, banana peels and avocado seeds was in a 20–50% range [179].
Regarding water treatment, native starch has been reported to have a poor adsorption capacity [180], which can mainly be attributed to its low surface area, limited thermal stability and absence of highly-absorptive functional groups, such as carboxyl, xanthate, acrylate, acetyl, hydroxypropyl, amine or amide [181,182]. Analogously to cellulose, alginate and chitosan, there are reports describing starch’s chemical modifications as well as the preparation of starch composites.
For instance, Guo et al. compared the efficiency for MB removal attained by native starch and a modified porous derivative, prepared by crosslinking native starch with epichlorohydrin and further hydrolysis with α-amylase, obtaining a maximum adsorption capacity value for MB almost three times higher than native starch [183]. In accordance with the results reported by Guo et al., Alvarado et al. also observed an enhancement of the adsorption capacity for the MB of starch after being modified by esterification with malonic, glutaric and valeric acids [184].
Soto et al. also compared modified and native starch. In this case, maleic acid and itaconic acids were used for esterification of starch, achieving maximum adsorption capacity values for Pb (II) and Zn (II) of 25.2 and 7.9 mg g−1, respectively for itaconate starch, while 5.2 and 3.2 mg g−1 were the corresponding values for native starch [185]. Soto et al. also reported oxidized starch adsorbents that achieved higher heavy metal removal capacity values compared to native starch; for Cd (II): 11.1 and 8.0 mg g−1; for Ni (II): 22.6 and 8.3 mg g−1; for Pb (II): 18.0 and 5.2 mg g−1; and for Zn (II): 10.3 and 3.2 mg g−1, respectively [186].
Sancey et al. reported the crosslinking of starch with 1,4-butanediol diglycidylether in presence of ammonium hydroxide and 2,3-epoxy-propyltrimethylammonium chloride, with further carboxymethylation. Herein, metal adsorption studies were conducted, achieving almost complete removal of Cu (II) and Fe (III) [187]. Copper removal was also assayed by Zheng et al. using starch hydrogels prepared with poly(acrylic acid), achieving an uptake of 179.9 mg g−1 Cu(II) in aqueous medium [188].
Chaudhari et al. prepared a starch xanthate that achieved almost complete removal of Hg (II), Cu (II), Cd (II) and Ni (II), and attributed the heavy metals uptake to the complexation of metals with xanthate groups [189].
As far as starch composites are concerned, composites with layer double hydroxide for methyl orange and fluoride removal from wastewater were reported. These materials were prepared by co-precipitation and different metals were used for the hydroxide layers, such as Mg (II), Al (III), Zn (II), Fe (III) and Ni (II). In particular, Zubair et al. reported a Starch-Ni/Fe-layered double hydroxide composite that achieved a maximum methyl orange (MO) adsorption capacity value of 387.6 mg g−1 [190]. However, Tao et al. achieved a higher maximum adsorption capacity value towards MO of 1555.0 mg g−1 [191] for the Zn/Mg/Al- Layered double hydroxide starch. On the other hand, a Mg/Al layered double hydroxides reported by Liu et al. achieved almost complete removal of fluoride [192].
Besides, iron nanoparticles are well-known to contribute to arsenic removal due to electrostatic attraction, ion exchange, and surface complexation [193]. It is therefore interesting to analyze iron nanoparticles’ effect when used in materials prepared with starch, such as composite materials with magnetite, maghemite and ferromanganese binary oxide. Next, some of the reported materials are described, in which starch acted only as a support or stabilizer of the mentioned inorganic particles.
Adsorption studies towards As (III) on iron-starch materials were carried out in aqueous solutions, achieving a maximum adsorption capacity value of 161.3 mg g−1 with the material reported by Xu et al. [194], while Siddiqui et al., obtained a maximum capacity value of 8.9 mg g−1 for a functionalized maghemite [195] and Robinson et al., 55.9 mg g−1 for a starch/maghemite nano-adsorbent [196].
On the other hand, as in the case of the other polysaccharides mentioned in previous sections, the preparation of ion exchangers from starch is a widely used strategy for ionic pollutants removal. For instance, Ma et al. [197] chemically modified starch attaching either xanthate or citrate groups to their structure (Figure 7I,II). In this work, adsorption assays towards Pb (II) were carried out, and the maximum adsorption capacity values were 109.1 and 57.6 mg g−1 for starch xanthate and starch citrate, respectively.
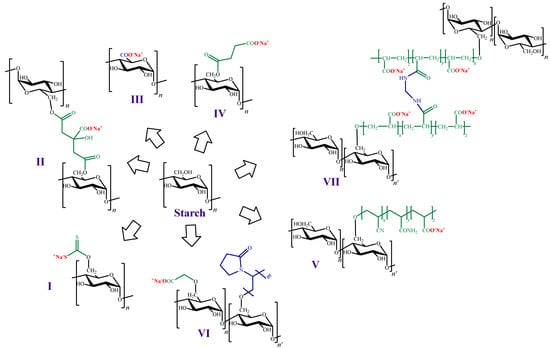
Figure 7. Chemical structures of cation exchangers derived from starch.
Another strategy for introducing negative charges in the starch structure is the oxidation of C-6 to a carboxyl group (Figure 7III). In this context, Liu et al. [198] modified starch nanoparticles through oxidization, using sodium hypochlorite. The new materials were preliminarily explored as adsorbents of heavy metals, using Pb (II) and Cu (II) as adsorbate models. Compared to the unmodified nanoparticles, the modified ones had remarkably higher adsorption capacity values (94.5 and 81.0 mg g−1 for Pb (II) and Cu (II), respectively).
Chen et al. [199] also used starch nanoparticles, but in this case the incorporation of negative charges in their surface was achieved by esterification with succinyl anhydride (Figure 7IV). The maximum degree of substitution was 0.1, which resulted in an increase on the surface Z-potential. The modified nanoparticles were assayed for the adsorption of Cu (II) and MB, showing that the adsorption process was greatly influenced by the pH value, as expected in presence of carboxyl groups. At low pH values, carboxyl groups are highly protonated, and the adsorption could be neglected (less than 3.0 mg g−1). At pH values above 4.0, the degree of protonation decreases and the surface charge of the adsorbents becomes negative, exerting electrostatic attraction to the adsorbate [34,38], which enhances the adsorption capacity of Cu (II) and MB (8.4 and 24.4 mg g−1, respectively).
Another example of modifications to obtain negatively charged starch was performed by Hashem et al. [200], who reported starch hydrogels prepared via graft polymerization of acrylonitrile, followed by nitrile groups hydrolysis to generate carboxylic acids and amides (Figure 7V). In this work, adsorption of Hg (II) on the starch hydrogels were carried out and a maximum adsorption capacity value of 1250 mg g−1 was reported by the authors. Subsequently, the same author reported adsorption studies against Pb (II) carried out on a similar material. In this last case, the value of the maximum adsorption capacity value reported was 264.4 mg g−1 [201].
Haroon et al. [202] also obtained cation exchangers by grafting carboxymethylated starch with N-vinylpyrrolidone (Figure 7VI). The new material was assayed as adsorbent towards rhodamine 6G from wastewater with a maximum adsorption capacity value of 363.9 mg g−1.
There are also reports of materials prepared using acrylic acid as the grafting monomer. For instance, Bahadoran et al. [82] reported a starch-g-poly(acrylic acid) hydrogel, achieving 736.0 mg g−1 of Cu (II) removal. Additionally, cellulose nanofibers were incorporated into the hydrogel, which enhanced its adsorption capacity, obtaining a value of 957.0 mg g−1 of Cu (II).
Ma et al., also prepared a composite material from starch, obtaining a starch-graft-poly(acrylic acid)/organo-modified zeolite, which was assayed for Cr (III) adsorption. The adsorbent’s removal of the mentioned metal was 651.4 mg g−1 [203].
Moreover, Chen et al. [204] prepared a starch-based high-performance adsorptive hydrogel by grafting polyacrylic acid onto starch, and then crosslinking it with N,N’-methylene-bisacrylamide (Figure 7VII). This adsorbent was employed to remove the organic cationic dye MB from effluents in which its concentration was high, yielding a maximum adsorption capacity value of 2967.7 mg g−1.
Additionally, Shoaib et al. prepared a composite material using starch, acrylic acid, and activated carbon from the red alga Pterocladia capillacea with N,N′-methylenebisacrylamide as a crosslinker and ammonium persulphate as an initiator. The adsorbent was assayed for MB dye removal, achieving 1428.6 mg g−1 as the maximum adsorption capacity value [205].
On the other hand, anion exchangers have been obtained by incorporating positive charges into the starch structure by introducing amino groups.
This entry is adapted from the peer-reviewed paper 10.3390/polysaccharides4030016
This entry is offline, you can click here to edit this entry!
