Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leonel Pereira | -- | 4361 | 2023-09-06 10:38:10 | | | |
| 2 | Lindsay Dong | Meta information modification | 4361 | 2023-09-08 02:56:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pereira, L.; Valado, A. Algae-Derived Natural Products in Diabetes and Its Complications. Encyclopedia. Available online: https://encyclopedia.pub/entry/48872 (accessed on 08 February 2026).
Pereira L, Valado A. Algae-Derived Natural Products in Diabetes and Its Complications. Encyclopedia. Available at: https://encyclopedia.pub/entry/48872. Accessed February 08, 2026.
Pereira, Leonel, Ana Valado. "Algae-Derived Natural Products in Diabetes and Its Complications" Encyclopedia, https://encyclopedia.pub/entry/48872 (accessed February 08, 2026).
Pereira, L., & Valado, A. (2023, September 06). Algae-Derived Natural Products in Diabetes and Its Complications. In Encyclopedia. https://encyclopedia.pub/entry/48872
Pereira, Leonel and Ana Valado. "Algae-Derived Natural Products in Diabetes and Its Complications." Encyclopedia. Web. 06 September, 2023.
Copy Citation
Algae-derived natural products have emerged as promising candidates for the management of diabetes and its complications. The diverse chemical constituents and multifaceted pharmacological activities of algae make them attractive sources of bioactive compounds with potential antidiabetic properties.
diabetes
natural products
algae
antidiabetic
glycemic control
1. Introduction
Diabetes, a chronic metabolic disorder characterized by impaired insulin production or utilization, has reached epidemic proportions worldwide. The disease affects millions of individuals, imposing a significant burden on healthcare systems and economies. Diabetes is associated with various complications that significantly impact the quality of life and increase morbidity and mortality rates among affected individuals [1][2].
Type 2 diabetes mellitus (T2DM) is the most prevalent form, accounting for approximately 90% of all diabetes cases [3]. It arises due to a combination of insulin resistance and inadequate insulin secretion. T2DM is often associated with lifestyle factors such as sedentary behavior, unhealthy dietary habits, and obesity [4]. Conversely, type 1 diabetes (T1DM) is an autoimmune condition characterized by the destruction of insulin-producing beta cells in the pancreas, resulting in absolute insulin deficiency [5]. Other forms of diabetes, such as gestational diabetes, also pose significant health risks, particularly to pregnant women and their offspring [6].
The complications associated with diabetes are diverse and affect multiple organ systems. Chronic hyperglycemia in diabetes leads to the development of microvascular and macrovascular complications. Microvascular complications primarily affect the small blood vessels, including the eyes (diabetic retinopathy), kidneys (diabetic nephropathy), and nerves (diabetic neuropathy). Macrovascular complications involve the body’s large blood vessels and contribute to an increased risk of cardiovascular diseases such as coronary artery disease, stroke, and peripheral arterial disease [7][8].
These complications can result in severe health consequences, including visual impairment, end-stage renal disease, lower limb amputations, and cardiovascular events [9]. Moreover, diabetes is associated with a higher risk of other conditions such as hypertension, dyslipidemia, and non-alcoholic fatty liver disease, further exacerbating the overall disease burden [10].
The management of diabetes and its complications requires a comprehensive approach, including lifestyle modifications, glucose-lowering medications, and targeted interventions to address specific complications. While advancements have been made in conventional diabetes management, there is a growing interest in exploring alternative therapeutic strategies that offer improved efficacy, fewer side effects, and disease-modifying potential [11][12].
In this context, natural products and their derivatives have gained significant attention as potential agents for diabetes management. These compounds, derived from various sources including plants, fungi, marine organisms, and algae, possess diverse chemical constituents and exhibit a wide range of pharmacological activities [13][14]. Exploiting the potential of natural products in diabetes research holds promise for the development of novel therapeutics and interventions that can effectively control blood glucose levels and mitigate the complications associated with the disease [15][16].
2. Algae as a Source of Natural Products
2.1. Introduction to Algae and Their Diversity
Algae, a diverse group of photosynthetic organisms, encompass a wide range of species found in aquatic environments such as oceans, lakes, rivers, and even damp terrestrial habitats. Algae can be classified into multiple taxonomic groups, including Cyanobacteria (blue-green algae), Chlorophyta (green algae), Ochrophyta/Phaeophyceae (brown algae), Rhodophyta (red algae), and Bacillariophyta (diatoms), among others not mentioned. This immense diversity of algae offers a vast and virtually untapped resource for the discovery and exploration of natural products with potential applications in various fields, including medicine, agriculture, industry, etc. [17][18].
One of the distinguishing features of algae is their ability to undergo photosynthesis, utilizing sunlight to convert carbon dioxide and water into organic compounds. This photosynthetic capacity not only contributes to oxygen production in our atmosphere but also enables algae to synthesize a wide array of unique and complex molecules [18][19]. Algae are known to produce a diverse range of bioactive compounds, including polysaccharides, proteins, pigments, lipids, sterols, terpenes, and phenolic compounds [20].
Algae exhibit an impressive chemical diversity, which is driven by genetic variability and adaptation to different ecological niches. Each taxonomic group of algae possesses distinct metabolic pathways and biosynthetic capabilities, resulting in the synthesis of species-specific compounds [21]. For example, red algae (Rhodophyta) are well-known for their production of sulfated polysaccharides, such as carrageenan and agar, which have demonstrated anticoagulant, antiviral, and anti-inflammatory activities [22]. Brown algae (Ochrophyta and Phaeophyceae), on the other hand, are rich in unique polysaccharides called fucoidans, which have shown promising immunomodulatory and anticancer properties [23].
2.2. Bioactive Compounds Present in Algae with Potential Antidiabetic Properties
2.2.1. Polysaccharides
Algae are known to produce a variety of polysaccharides with potential antidiabetic effects. For example, sulfated polysaccharides derived from red algae, such as carrageenan and agar, have demonstrated antihyperglycemic activity by inhibiting the α-glucosidase and α-amylase enzymes involved in carbohydrate digestion, thereby reducing postprandial glucose levels. Additionally, these polysaccharides possess antioxidant properties and can enhance insulin sensitivity [24][25].
2.2.2. Polyphenols
Algae-derived polyphenols, including flavonoids and phenolic acids, have attracted attention for their potential antidiabetic effects. These compounds exhibit antioxidant and anti-inflammatory properties, which can help mitigate the oxidative stress and chronic inflammation associated with diabetes. Some brown algae-derived polyphenols, such as phlorotannins, have shown inhibitory effects on α-glucosidase and α-amylase enzymes, leading to reduced glucose absorption and improved glycemic control [26][27].
2.2.3. Pigments
Algae are rich in pigments such as chlorophylls, carotenoids, and phycobiliproteins, which possess diverse biological activities. Fucoxanthin, a carotenoid found in brown algae, has shown promise in diabetes management by stimulating glucose uptake in skeletal muscle cells and adipocytes, improving insulin sensitivity. Moreover, certain algae-derived pigments possess antioxidant and anti-inflammatory properties, contributing to their potential role in mitigating diabetes-related complications [28][29].
2.2.4. Lipids
Algae-derived lipids, particularly omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been extensively studied for their beneficial effects on diabetes. These fatty acids have demonstrated antihyperglycemic, anti-inflammatory, and insulin-sensitizing properties [30]. Additionally, lipids derived from microalgae, such as Chlorella and Spirulina, have shown potential for improving lipid profiles and reducing triglyceride levels in individuals with diabetes [31].
2.2.5. Peptides and Proteins
Algae produce a wide range of peptides and proteins with various biological activities, including antidiabetic effects. Some algae-derived peptides have demonstrated inhibitory effects on dipeptidyl peptidase-4 (DPP-4), an enzyme involved in the degradation of incretin hormones. By inhibiting DPP-4, these peptides can prolong the action of incretins, leading to enhanced insulin secretion and improved glucose control [32].
2.3. Mechanisms of Action of Algae-Derived Natural Products in Diabetes Management
The effectiveness of algae-derived natural products in diabetes management stems from their diverse array of mechanisms of action. These bioactive constituents exhibit the capacity to intervene in the crucial pathways associated with glucose homeostasis, insulin sensitivity, and the prevention or alleviation of diabetes-related complications. A comprehensive understanding of these mechanisms is pivotal in harnessing the full therapeutic potential of algae-derived natural products [28][33].
While certain algal derivatives demonstrate considerable potential in terms of ameliorating antidiabetic effects, a rigorous comparison of their relative efficacy remains a subject demanding further exploration. Algae extracts exhibit variegated profiles of bioactive components, each potentially addressing distinct aspects of diabetes. Some extracts may exhibit superior effects on glucose regulation, while others could excel in enhancing insulin sensitivity or mitigating complications. Identifying the most promising algae extracts necessitates meticulous investigation, encompassing both in vitro and in vivo studies, along with comparative assessments of their therapeutic impact [13][34].
2.3.1. Regulation of Glucose Metabolism
Algae-derived natural products have been found to modulate the enzymes involved in glucose metabolism, such as α-glucosidase and α-amylase. By inhibiting these enzymes, algae-derived compounds can slow down the breakdown and absorption of carbohydrates, leading to reduced postprandial glucose levels. This effect is particularly important in managing blood glucose fluctuations after meals [28][35].
2.3.2. Enhancement of Insulin Sensitivity
Insulin resistance, a hallmark of type 2 diabetes, is characterized by an impaired response to insulin and reduced uptake of glucose by the cells. Algae-derived natural products have shown potential for improving insulin sensitivity. They can activate the signaling pathways involved in insulin action, such as the PI3K/Akt pathway, promoting glucose uptake and its utilization by cells. By enhancing insulin sensitivity, these compounds contribute to better glycemic control and metabolic regulation [36][37].
2.3.3. Antioxidant and Anti-Inflammatory Activities
Chronic hyperglycemia in diabetes leads to the production of reactive oxygen species (ROS) and the activation of inflammatory pathways, contributing to oxidative stress and chronic inflammation. Algae-derived natural products possess antioxidant and anti-inflammatory properties, effectively neutralizing ROS and reducing inflammatory responses. This antioxidant and anti-inflammatory activity can help mitigate the cellular damage and tissue dysfunction associated with diabetes [38].
2.3.4. Modulation of Adipokine Secretion
Adipokines are signaling molecules secreted by adipose tissue that play a crucial role in glucose and lipid metabolism. Algae-derived natural products have been shown to influence adipokine secretion, including adiponectin and leptin. Adiponectin, an insulin-sensitizing hormone, promotes glucose utilization and enhances insulin sensitivity [39]. Algae-derived compounds can increase adiponectin levels, thereby improving insulin action and glucose metabolism. Conversely, the modulation of leptin levels by algae-derived compounds may help regulate appetite and body weight, which are important factors in diabetes management [40].
2.3.5. Prevention of Diabetes-Related Complications
Algae-derived natural products have demonstrated some potential in terms of mitigating diabetes-related complications such as diabetic retinopathy and nephropathy. These compounds possess antiangiogenic and anti-inflammatory properties, which can protect retinal cells and prevent the development or progression of retinopathy [41]. Additionally, algae-derived compounds have shown reno-protective effects by reducing albuminuria, attenuating renal fibrosis, and modulating the inflammatory pathways. These effects contribute to the preservation of renal function in individuals with diabetes [42].
3. Algae-Derived Natural Products and Glycemic Control
3.1. Exploration of Algae-Derived Compounds for Regulating Blood Glucose Levels
3.1.1. Inhibition of Carbohydrate-Digesting Enzymes
Algae-derived compounds have been found to inhibit enzymes involved in the digestion and absorption of carbohydrates, such as α-glucosidase and α-amylase. By inhibiting these enzymes, algae-derived compounds can delay the breakdown of complex carbohydrates into glucose and reduce the rate of glucose absorption from the intestine. This results in a slower and more controlled release of glucose into the bloodstream, preventing postprandial hyperglycemia [34][35].
3.1.2. Stimulation of Glucose Uptake
Algae-derived natural products have demonstrated the ability to enhance the uptake of glucose by cells, particularly in skeletal muscle and adipose tissue. These compounds can activate the signaling pathways, such as the PI3K/Akt pathway, which are involved in insulin-mediated glucose uptake. By promoting glucose uptake, algae-derived compounds help to lower blood glucose levels and improve insulin sensitivity [34][43].
3.1.3. Modulation of Insulin Secretion
Algae-derived compounds have been found to influence insulin secretion from pancreatic β-cells. Some compounds have shown the ability to enhance insulin secretion in response to elevated glucose levels, helping to maintain proper glucose homeostasis. This effect is particularly beneficial in individuals with impaired insulin secretion, such as those with type 2 diabetes [28][34].
3.1.4. Regulation of Hepatic Glucose Production
The liver plays a crucial role in maintaining glucose balance by producing and releasing glucose into the bloodstream. Algae-derived compounds have been shown to regulate hepatic glucose production by modulating key enzymes involved in gluconeogenesis and glycogenolysis [44]. By inhibiting these enzymes, algae-derived compounds can reduce excessive glucose production by the liver and contribute to improved glycemic control [13][28].
3.1.5. Anti-Inflammatory and Antioxidant Effects
Chronic inflammation and oxidative stress are closely associated with insulin resistance and impaired glucose metabolism. Algae-derived compounds possess anti-inflammatory and antioxidant properties, which can help mitigate these underlying factors in diabetes. By reducing inflammation and oxidative stress, these compounds contribute to improved glycemic control and overall metabolic health [45].
3.2. Evaluation of the Effects of Algae Extracts on Insulin Secretion and Sensitivity
The effects of algae extract on insulin secretion and sensitivity have been the subject of extensive investigation, shedding light on their potential for glycemic control and diabetes management. Algae-derived natural products offer a diverse array of bioactive compounds that have demonstrated promising effects in modulating insulin secretion and enhancing insulin sensitivity.
3.2.1. Insulin Secretion
Algae extracts have been found to influence insulin secretion from pancreatic β-cells. Studies have shown that certain algae-derived compounds can stimulate insulin secretion in response to glucose stimulation. These compounds act through various mechanisms, including the closure of ATP-sensitive potassium channels and the subsequent influx of calcium ions, leading to the release of insulin vesicles. By promoting insulin secretion, algae extracts can help regulate blood glucose levels and maintain glucose homeostasis [13][28].
3.2.2. Insulin Sensitivity
Insulin resistance, characterized by the reduced responsiveness of target tissues to insulin, is a key feature of type 2 diabetes. Algae extracts have demonstrated the ability to enhance insulin sensitivity in different cell types and animal models. These extracts can activate the intracellular signaling pathways, such as the PI3K/Akt pathway, which play a critical role in insulin-mediated glucose uptake. By improving insulin sensitivity, algae extracts facilitate the efficient utilization of glucose by cells and contribute to better glycemic control [46].
3.2.3. Adipocyte Function
Adipose tissue is an important regulator of insulin sensitivity and glucose metabolism. Algae extracts have been shown to modulate adipocyte function, influencing adipokine secretion and adipocyte differentiation. Adipokines, such as adiponectin, play a crucial role in glucose and lipid metabolism. Algae extracts can increase adiponectin secretion, which enhances insulin sensitivity and improves the uptake of glucose by cells. Additionally, algae extracts have been found to inhibit adipocyte hypertrophy and promote the browning of white adipose tissue, further contributing to metabolic improvements [40][47].
3.2.4. Mitochondrial Function
Impaired mitochondrial function has been implicated in insulin resistance and glucose intolerance. Algae extracts have demonstrated the ability to improve mitochondrial function, enhancing cellular energy metabolism and insulin sensitivity. These extracts can promote mitochondrial biogenesis, increase mitochondrial respiration, and enhance ATP production. By improving mitochondrial function, algae extracts optimize cellular energy utilization and promote the uptake and metabolism of glucose [48].
3.2.5. Inflammatory and Oxidative Stress Modulation
Chronic inflammation and oxidative stress contribute to insulin resistance and impaired glucose metabolism. Algae extracts possess anti-inflammatory and antioxidant properties, which can attenuate inflammation and reduce oxidative stress in insulin-responsive tissues. By mitigating these underlying factors, algae extracts improve insulin sensitivity and promote better glycemic control [49].

3.3. Algae-Based Natural Products as Inhibitors of Carbohydrate-Digesting Enzymes
3.3.1. α-Glucosidase Inhibition
Algae-based natural products have been found to inhibit α-glucosidase, an enzyme responsible for the final step in the breakdown of complex carbohydrates into glucose. By inhibiting α-glucosidase, algae-based compounds delay the digestion and absorption of carbohydrates, leading to the slower release of glucose into the bloodstream. This inhibition helps prevent rapid glucose spikes after meals and contributes to better glycemic control [35][50].
3.3.2. α-Amylase Inhibition
Algae-based natural products have also demonstrated inhibitory effects on α-amylase, an enzyme that initiates the digestion of starch into smaller carbohydrate units. The inhibition of α-amylase by algae-based compounds slows down the breakdown of starch, reducing the rate at which glucose is released into the bloodstream. By inhibiting α-amylase, algae-based natural products can help regulate postprandial blood glucose levels and prevent excessive glucose fluctuations [51][52].
3.3.3. Mechanisms of Inhibition
The inhibitory effects of algae-based natural products (for example, from Halimeda tuna or Chlorophyta; see Figure 1) on carbohydrate-digesting enzymes are attributed to their bioactive components, such as polyphenols, flavonoids, and polysaccharides. These compounds interact with the active sites of α-glucosidase and α-amylase, preventing their enzymatic activity and any substrate binding. This inhibition leads to reduced carbohydrate breakdown and glucose absorption, resulting in improved glycemic control [53][54].

Figure 1. The marine green macroalga, Halimeda tuna. Scale = 1 cm.
3.3.4. Synergistic Effects
Algae-based natural products often contain a complex mixture of bioactive compounds that can act synergistically to enhance their inhibitory effects on carbohydrate-digesting enzymes. The combined action of multiple compounds may result in more potent enzyme inhibition and better glycemic control.
3.3.5. Therapeutic Potential
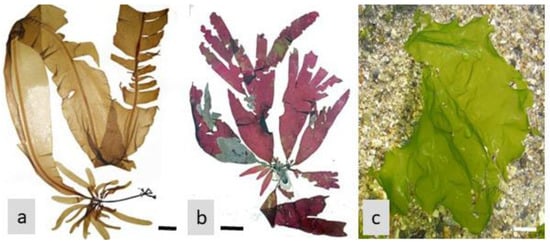
The inhibition of carbohydrate-digesting enzymes by algae-based natural products (for example, from Ascophyllum nodosum, Fucus vesiculosus, and Undaria pinnatifida, in the Phaeophyceae; see Figure 2) presents an attractive therapeutic approach for glycemic control and diabetes management. By slowing down carbohydrate digestion and reducing glucose absorption, these natural products offer a natural and potentially safer alternative to synthetic enzyme inhibitors.



Figure 2. Marine brown macroalgae: (a)—Ascophyllum nodosum; (b)—Fucus vesiculosus; (c)—Undaria pinnatifida. Scale = 1 cm.
The exploration of algae-based natural products (for example, Dictyopteris polypodioides in the Phaeophyceae; see Figure 3) as inhibitors of carbohydrate-digesting enzymes highlights their potential for glycemic control and diabetes management. The inhibitory effects of these natural products on α-glucosidase and α-amylase contribute to the regulation of postprandial blood glucose levels and the prevention of glucose spikes [55].

Figure 3. The marine brown macroalga, Dictyopteris polypodioides (Phaeophyceae). Scale = 1 cm.

4. Algae-Derived Natural Products and Diabetic Complications
4.1. Role of Algae-Derived Compounds in Preventing and Managing Diabetic Retinopathy
Algae-derived natural products (for example, from Alaria, Palmaria, and Ulva; see Figure 4) have shown potential for preventing and managing diabetic retinopathy, a common complication of diabetes that affects the eyes [56]. Diabetic retinopathy is characterized by damage to the blood vessels in the retina, leading to vision impairment and, in severe cases, blindness.

Figure 4. Alaria (Phaeophyceae) (a); Palmaria (Rhodophyta) (b); Ulva (Chlorophyta) (c); Scale = 1 cm.
4.1.1. Anti-Angiogenic Effects
Diabetic retinopathy is associated with abnormal blood vessel growth in the retina, a process known as angiogenesis [57]. Algae-derived compounds have demonstrated anti-angiogenic properties by inhibiting the proliferation and migration of endothelial cells, which are essential for blood vessel formation. These compounds target various signaling pathways that are involved in angiogenesis, such as vascular endothelial growth factor (VEGF) signaling, to suppress abnormal blood vessel growth and reduce the risk of diabetic retinopathy progression [58].
4.1.2. Antioxidant and Anti-Inflammatory Properties
Oxidative stress and chronic inflammation play significant roles in the development and progression of diabetic retinopathy [59]. Algae-derived compounds possess antioxidant and anti-inflammatory properties, which help mitigate these damaging processes. These compounds scavenge reactive oxygen species, reduce oxidative stress, and suppress inflammatory signaling pathways. By alleviating oxidative stress and inflammation, algae-derived compounds protect retinal cells from damage and promote retinal health [49].
4.1.3. Neuroprotective Effects
Diabetic retinopathy involves not only vascular changes but also neuronal damage in the retina. Algae-derived compounds (for example, from Acetabularia acetabulum in the Chlorophyta; see Figure 5) have shown neuroprotective effects by preserving retinal ganglion cells, photoreceptor cells, and other retinal neurons. These compounds can enhance neuronal survival, promote neurite outgrowth, and modulate neurotrophic factors. By protecting the retinal neurons, algae-derived compounds contribute to the prevention and management of diabetic retinopathy-related vision loss [60][61].

Figure 5. Marine green macroalga: Acetabularia acetabulum. Scale = 1 cm.
4.1.4. Modulation of Retinal Blood Flow
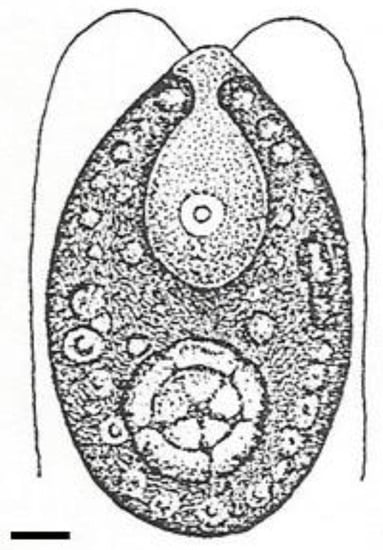
Algae-derived compounds (for example, from Dunaliella salina in the Chlorophyta; see Figure 6) have the potential to modulate retinal blood flow, which is often compromised in diabetic retinopathy. These compounds can improve blood circulation, enhance microvascular perfusion, and regulate endothelial function. By optimizing retinal blood flow, algae-derived compounds support the delivery of proper oxygen and nutrient supply to retinal cells, reducing the risk of ischemia and further damage [62].

Figure 6. Marine green microalga illustration: Dunaliella salina. Scale = 5 µm.
4.1.5. Restoration of Retinal Barrier Function
Disruption of the blood–retinal barrier is a hallmark of diabetic retinopathy, leading to increased vascular permeability and fluid leakage [63]. Algae-derived compounds have shown the ability to restore the integrity of the blood-retinal barrier by regulating tight junction proteins and reducing endothelial permeability. By preserving the barrier function, these compounds prevent the accumulation of fluid in the retina and inhibit the progression of diabetic retinopathy [64].

4.2. Algae-Based Interventions for Diabetic Nephropathy and Its Associated Complications
Algae-derived natural products, particularly those derived from specific algae species (for example, Fucus spiralis and Taonia atomaria in the Phaeophyceae; see Figure 7), hold promise as interventions for diabetic nephropathy and its associated complications [13][28]. Diabetic nephropathy, a progressive kidney disease caused by diabetes, is characterized by kidney damage and impaired renal function.

Figure 7. Marine brown macroalgae: (a)—Taonia atomaria, (b)—Fucus spiralis. Scale = 1 cm.
4.2.1. Reno-Protective Effects
Studies have shown that extracts from the brown macroalga species Fucus vesiculosus possess reno-protective effects by preserving renal function and attenuating kidney damage. The antioxidant and anti-inflammatory properties of F. vesiculosus extracts help mitigate oxidative stress and inflammation in the kidneys, key contributors to diabetic nephropathy [65].
4.2.2. Anti-Fibrotic Properties
Extracts from the macroalga species Gracilariopsis lemaneiformis (formerly Gracilaria lemaneiformis) (Rhodophyta) have demonstrated anti-fibrotic effects in diabetic nephropathy models. These extracts inhibit the production and deposition of collagen and other fibrotic markers in the kidneys, thereby preventing the progression of renal fibrosis and preserving renal function [66].
4.2.3. Blood Pressure Regulation
The green microalga species Chlorella vulgaris has been investigated for its blood pressure-regulating effects [67]. C. vulgaris supplementation has shown promising results in regulating blood pressure levels by modulating certain vasoactive factors and improving endothelial function [68].
4.2.4. Glycemic Control
Extracts from the Cyanobacteria species Arthrospira platensis (formerly Spirulina platensis) have been shown to regulate blood glucose levels and improve insulin sensitivity [69]. The supplementation of A. platensis contributes to better glycemic control, reducing the burden on the kidneys and minimizing the risk of renal complications [70].
4.2.5. Mineral and Electrolyte Balance
The brown macroalga species Ecklonia cava has been investigated for its ability to maintain proper mineral and electrolyte balance. E. cava extracts have been shown to regulate serum levels of potassium, sodium, calcium, and phosphate, supporting renal homeostasis and preventing the complications associated with electrolyte imbalance [71].
4.2.6. Anti-Inflammatory and Antioxidant Effects
Extracts from the green microalga species Dunaliella salina possess anti-inflammatory and antioxidant properties. These extracts help reduce inflammation and oxidative stress in the kidneys, alleviating renal damage and promoting renal health [72].
4.3. Algae-Derived Natural Products as Potential Therapeutics for Diabetic Neuropathy
Algae-derived natural products have shown potential as potential therapeutics for diabetic neuropathy, a common complication of diabetes characterized by nerve damage and dysfunction. The diverse chemical constituents of various algae species offer a rich source of bioactive compounds with neuroprotective properties.
Extracts from the brown macroalga Ecklonia cava have demonstrated neuroprotective effects in diabetic neuropathy models. These extracts contain phlorotannins, a class of polyphenols known for their antioxidant and anti-inflammatory properties. E. cava extracts have been shown to protect nerve cells from oxidative stress, reduce inflammation, and improve nerve function in diabetic neuropathy [73][74].
The green macroalga Ulva lactuca has been investigated for its potential neuroprotective effects. U. lactuca extracts contain bioactive compounds such as ulvan, which exhibits antioxidant and anti-inflammatory activities. These extracts have shown promise in reducing nerve damage and improving nerve conduction velocity in diabetic neuropathy models [75].
Extracts from the red seaweed Gracilaria edulis have been studied for their potential in the context of diabetic neuropathy management. G. edulis extracts contain sulfated polysaccharides, which possess antioxidant and anti-inflammatory properties. These extracts have shown promising effects in protecting nerve cells, improving nerve conduction, and reducing neuropathic pain in diabetic neuropathy models [76].
The brown algae genus Sargassum has been investigated for its potential for treating diabetic neuropathy. Sargassum spp. extracts contain various bioactive compounds such as fucoidan, fucoxanthin, and phlorotannins, which exhibit antioxidant, anti-inflammatory, and neuroprotective activities. These extracts have shown potential in terms of protecting nerve cells and improving nerve function in diabetic neuropathy models [77].
Extracts from the red macroalga Gelidium elegans have been studied for their neuroprotective effects in diabetic neuropathy. G. elegans extracts contain bioactive compounds, such as agarobiose and agar-oligosaccharides, which possess antioxidant and anti-inflammatory properties. These extracts have shown potential for improving nerve function and reducing neuropathic pain in diabetic neuropathy models [78].

5. Mechanistic Insights and Molecular Targets
5.1. Elucidating the Molecular Mechanisms Underlying the Antidiabetic Effects of Algae-Derived Compounds
5.1.1. AMP-Activated Protein Kinase (AMPK) Activation
AMPK is a key regulator of energy metabolism and glucose homeostasis. Several algae-derived compounds, such as fucoidan from the brown macroalga F. vesiculosus [79] and phycocyanin from the blue-green microalga Arthrospira platensis [80], have been found to activate AMPK. The activation of AMPK leads to increased glucose uptake, enhanced insulin sensitivity, and improved glycemic control.
5.1.2. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Modulation
PPARγ is a nuclear receptor involved in insulin sensitivity and glucose metabolism. Some algae-derived compounds, such as fucoxanthin from brown algae [81] and ulvan from the green macroalga Ulva lactuca [82], have been shown to modulate PPARγ activity. The activation of PPARγ by these compounds can improve insulin sensitivity, promote adipocyte differentiation, and regulate lipid metabolism [81].
5.1.3. Inhibition of Carbohydrate-Digesting Enzymes
Algae-derived compounds, including sulfated polysaccharides from the red macroalga Gracilaria edulis [83] and fucoidan from brown algae (F. vesiculosus and A. nodosum) [84], have demonstrated inhibitory effects on carbohydrate-digesting enzymes such as α-amylase and α-glucosidase. By inhibiting these enzymes, algae-derived compounds can reduce postprandial glucose levels and attenuate hyperglycemia.
5.1.4. Antioxidant and Anti-Inflammatory Effects
Oxidative stress and chronic inflammation play crucial roles in the development and progression of diabetes. Algae-derived compounds, such as phlorotannins from Ecklonia cava [85] and sulfated polysaccharides from Pyropia tenera (formerly Porphyra tenera) (Figure 8) [86], exhibit potent antioxidant and anti-inflammatory activities. These compounds can mitigate oxidative stress, suppress the inflammatory pathways, and protect pancreatic beta cells from damage.

Figure 8. Red macroalga: Pyropia tenera (Nori). Scale = 1 cm.
5.1.5. Modulation of the Insulin Signaling Pathway
Algae-derived compounds have been found to modulate various components of the insulin signaling pathway. For instance, phycocyanins from Arthrospira maxima and A. platensis have been shown to increase insulin receptor substrate 1 (IRS-1) phosphorylation and activate the downstream signaling cascades [28][87]. These compounds can improve insulin signaling, promote glucose uptake, and regulate insulin secretion.
5.2. Identification of Key Molecular Targets Influenced by Algae-Derived Natural Products
5.2.1. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ)
PPARγ is a nuclear receptor involved in insulin sensitivity and glucose metabolism. Several algae-derived compounds, such as fucoxanthin from brown algae [88] and ulvan from Ulva lactuca [89], have been shown to modulate PPARγ activity. The activation of PPARγ improves insulin sensitivity, promotes adipocyte differentiation, and regulates lipid metabolism.
5.2.2. AMP-Activated Protein Kinase (AMPK)
AMPK is a key regulator of energy metabolism and glucose homeostasis. Algae-derived compounds, including fucoidan from F. vesiculosus [90] and phycocyanin from A. platensis [87], have been found to activate AMPK. The activation of AMPK leads to increased glucose uptake, enhanced insulin sensitivity, and improved glycemic control.
5.2.3. Protein Tyrosine Phosphatase 1B (PTP1B)
PTP1B is a negative regulator of insulin signaling and is a therapeutic target for diabetes. Algae-derived compounds, such as phlorotannins from Ecklonia cava [91] and sulfated polysaccharide (porphyran) from Pyropia yezoensis [92], have been shown to inhibit PTP1B activity. The inhibition of PTP1B improves insulin signaling and glucose metabolism.
5.2.4. Nuclear Factor-Kappa B (NF-κB)
NF-κB is a transcription factor involved in inflammation and insulin resistance. Algae-derived compounds, including phlorotannins from Ecklonia cava [93] and fucoidan from brown algae (for example, Sargassum siliquastrum) [94], have demonstrated anti-inflammatory effects by suppressing NF-κB activation. Inhibition of the NF-κB signaling pathway alleviates inflammation and improves insulin sensitivity.
5.2.5. Alpha-Glucosidase and Alpha-Amylase
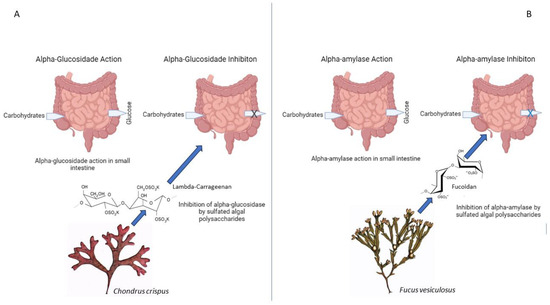
Algae-derived compounds, such as sulfated polysaccharides from Gracilaria edulis and Chondrus crispus (Rhodophyta) and fucoidan from Fucus spp. (Phaeophyceae), have shown inhibitory effects on carbohydrate-digesting enzymes, including alpha-glucosidase and alpha-amylase. The inhibition of these enzymes reduces postprandial glucose levels and attenuates hyperglycemia (Figure 9) [82].

Figure 9. Inhibition of carbohydrate-digesting enzymes by algae-derived compounds. Algae-derived compounds exhibit inhibitory effects on key carbohydrate-digesting enzymes, α-glucosidase (A) and α-amylase (B). This inhibition results in decreased carbohydrate breakdown, leading to the improved regulation of postprandial glucose levels and reduced hyperglycemia. “X” and “X” = reduction of glucose levels.
References
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200.
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539.
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275.
- Kyrou, I.; Tsigos, C.; Mavrogianni, C.; Cardon, G.; Van Stappen, V.; Latomme, J.; Tsochev, K.; Nanasi, A.; Semanova, C.; Lamiquiz-Moneo, I.; et al. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: A narrative review with emphasis on data from Europe. BMC Endocr. Disord. 2020, 20, 134.
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422.
- Choudhury, A.A.; Devi Rajeswari, V. Gestational diabetes mellitus—A metabolic and reproductive disorder. Biomed. Pharmacother. 2021, 143, 112183.
- Cade, W.T. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys. Ther. 2008, 88, 1322–1335.
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551.
- Li, K.; Ferguson, T.; Embil, J.; Rigatto, C.; Komenda, P.; Tangri, N. Risk of Kidney Failure, Death, and Cardiovascular Events After Lower Limb Complications in Patients With CKD. Kidney Int. Rep. 2020, 6, 381–388.
- Kosmalski, M.; Śliwińska, A.; Drzewoski, J. Non-Alcoholic Fatty Liver Disease or Type 2 Diabetes Mellitus—The Chicken or the Egg Dilemma. Biomedicines 2023, 11, 1097.
- Williams, D.M.; Jones, H.; Stephens, J.W. Personalized Type 2 Diabetes Management: An Update on Recent Advances and Recommendations. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 281–295.
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786.
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460.
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100.
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551.
- Liu, Y.; Zeng, S.; Ji, W.; Yao, H.; Lin, L.; Cui, H.; Santos, H.A.; Pan, G. Emerging Theranostic Nanomaterials in Diabetes and Its Complications. Adv. Sci. 2022, 9, 2102466.
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29.
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188.
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153.
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877.
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and Industrial Implications of Dynamic Seaweed-Associated Microbiota Interactions. Mar. Drugs 2020, 18, 641.
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202.
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620.
- Lu, L.W.; Chen, J.-H. Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective. Foods 2022, 11, 714.
- Bhuyan, P.P.; Nayak, R.; Patra, S.; Abdulabbas, H.S.; Jena, M.; Pradhan, B. Seaweed-Derived Sulfated Polysaccharides; The New Age Chemopreventives: A Comprehensive Review. Cancers 2023, 15, 715.
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470.
- Bocanegra, A.; Macho-González, A.; Garcimartín, A.; Benedí, J.; Sánchez-Muniz, F.J. Whole Alga, Algal Extracts, and Compounds as Ingredients of Functional Foods: Composition and Action Mechanism Relationships in the Prevention and Treatment of Type-2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3816.
- Figueiredo, F.; Encarnação, T.; Campos, M. Algae as Functional Foods for the Elderly. Food Nutr. Sci. 2016, 7, 1122–1148.
- Sinha, S.; Haque, M.; Lugova, H.; Kumar, S. The Effect of Omega-3 Fatty Acids on Insulin Resistance. Life 2023, 13, 1322.
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825.
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176.
- Sharifuddin, Y.; Chin, Y.-X.; Lim, P.-E.; Phang, S.-M. Potential Bioactive Compounds from Seaweed for Diabetes Management. Mar. Drugs 2015, 13, 5447–5491.
- Popović-Djordjević, B.J.; Katanić Stanković, S.J.; Mihailović, V.; Pereira, G.A.; Garcia-Oliveira, P.; Prieto, A.M.; Simal-Gandara, J. Algae as a Source of Bioactive Compounds to Prevent the Development of Type 2 Diabetes Mellitus. Curr. Med. Chem. 2021, 28, 4592–4615.
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goñi, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of edible brown algae extracts for the inhibition of intestinal carbohydrate digestive enzymes involved in glucose release from the diet. J. Nutr. Sci. 2021, 10, e5.
- Melloni, M.; Sergi, D.; Simioni, C.; Passaro, A.; Neri, L.M. Microalgae as a Nutraceutical Tool to Antagonize the Impairment of Redox Status Induced by SNPs: Implications on Insulin Resistance. Biology 2023, 12, 449.
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996.
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162.
- Pheiffer, C.; Dias, S.; Jack, B.; Malaza, N.; Adam, S. Adiponectin as a Potential Biomarker for Pregnancy Disorders. Int. J. Mol. Sci. 2021, 22, 1326.
- Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Effects of Microalgae on Metabolic Syndrome. Antioxidants 2023, 12, 449.
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799.
- Khan, M.A.; Kassianos, A.J.; Hoy, W.E.; Alam, A.K.; Healy, H.G.; Gobe, G.C. Promoting Plant-Based Therapies for Chronic Kidney Disease. J. Evid. Based. Integr. Med. 2022, 27, 2515690X221079688.
- Nyakundi, B.B.; Yang, J. Uses of Papaya Leaf and Seaweed Supplementations for Controlling Glucose Homeostasis in Diabetes. Int. J. Mol. Sci. 2023, 24, 6846.
- Shehadeh, M.B.; Suaifan, G.A.R.Y.; Abu-Odeh, A.M. Plants Secondary Metabolites as Blood Glucose-Lowering Molecules. Molecules 2021, 26, 4333.
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035.
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797.
- de Sousa Neto, I.V.; Durigan, J.L.Q.; da Silva, A.S.R.; de Cássia Marqueti, R. Adipose Tissue Extracellular Matrix Remodeling in Response to Dietary Patterns and Exercise: Molecular Landscape, Mechanistic Insights, and Therapeutic Approaches. Biology 2022, 11, 765.
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications: Potential Role of Alterations in Mitochondrial Function in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Endocrinology 2020, 161, bqaa017.
- Vignaud, J.; Loiseau, C.; Hérault, J.; Mayer, C.; Côme, M.; Martin, I.; Ulmann, L. Microalgae Produce Antioxidant Molecules with Potential Preventive Effects on Mitochondrial Functions and Skeletal Muscular Oxidative Stress. Antioxidants 2023, 12, 1050.
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722.
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Alpha-amylase Inhibition and Antioxidant Activity of Marine Green Algae and its Possible Role in Diabetes Management. Pharmacogn. Mag. 2015, 11, S511–S515.
- Yin, S.; Siahaan, E.A.; Niu, L.; Shibata, M.; Liu, Y.; Hagiwara, T. Real time monitoring and evaluation of the inhibition effect of fucoxanthin against α-amylase activity by using QCM-A. Front. Nutr. 2023, 9, 1110615.
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692.
- Gazali, M.; Jolanda, O.; Husni, A.; Nurjanah; Majid, F.A.A.; Zuriat; Syafitri, R. In Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Green Seaweed Halimeda tuna Extract from the Coast of Lhok Bubon, Aceh. Plants 2023, 12, 393.
- Truong, T.P.T.; Tran, T.M.; Dai, T.X.T.; Tran, C.L. Antihyperglycemic and anti-type 2 diabetic activity of marine hydroquinone isolated from brown algae (Dictyopteris polypodioides). J. Tradit. Complement. Med. 2023, 13, 408–416.
- Lauritano, C.; Ianora, A. Marine Organisms with Anti-Diabetes Properties. Mar. Drugs 2016, 14, 220.
- Rezzola, S.; Loda, A.; Corsini, M.; Semeraro, F.; Annese, T.; Presta, M.; Ribatti, D. Angiogenesis-Inflammation Cross Talk in Diabetic Retinopathy: Novel Insights from the Chick Embryo Chorioallantoic Membrane/Human Vitreous Platform. Front. Immunol. 2020, 11, 581288.
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273.
- Kowluru, R.A.; Chan, P.-S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 043603.
- Brillante, S.; Galasso, C.; Lauritano, C.; Carrella, S. From the Sea for the Sight: Marine Derived Products for Human Vision. Front. Aging Neurosci. 2022, 14, 892764.
- Fathalipour, M.; Fathalipour, H.; Safa, O.; Nowrouzi-Sohrabi, P.; Mirkhani, H.; Hassanipour, S. The Therapeutic Role of Carotenoids in Diabetic Retinopathy: A Systematic Review. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2347–2358.
- El-Baz, F.K.; Salama, A.; Salama, R.A.A. Dunaliella salina Attenuates Diabetic Neuropathy Induced by STZ in Rats: Involvement of Thioredoxin. BioMed Research International 2020, 2020, 1295492.
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol. Res. 2020, 161, 105115.
- Chiang, Y.-F.; Chen, H.-Y.; Chang, Y.-J.; Shih, Y.-H.; Shieh, T.-M.; Wang, K.-L.; Hsia, S.-M. Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells. Antioxidants 2020, 9, 1176.
- Catarino, M.D.; Marçal, C.; Bonifácio-Lopes, T.; Campos, D.; Mateus, N.; Silva, A.M.S.; Pintado, M.M.; Cardoso, S.M. Impact of Phlorotannin Extracts from Fucus vesiculosus on Human Gut Microbiota. Mar. Drugs 2021, 19, 375.
- Guo, D.; Yu, K.; Sun, X.-Y.; Ouyang, J.-M. Structural Characterization and Repair Mechanism of Gracilaria lemaneiformis Sulfated Polysaccharides of Different Molecular Weights on Damaged Renal Epithelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 7410389.
- Sanayei, M.; Kalejahi, P.; Mahinkazemi, M.; Fathifar, Z.; Barzegar, A. The effect of Chlorella vulgaris on obesity related metabolic disorders: A systematic review of randomized controlled trials. J. Complement. Integr. Med. 2022, 19, 833–842.
- Fallah, A.A.; Sarmast, E.; Habibian Dehkordi, S.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901.
- Hannan, J.M.A.; Ansari, P.; Azam, S.; Flatt, P.R.; Abdel Wahab, Y.H.A. Effects of Spirulina platensis on insulin secretion, dipeptidyl peptidase IV activity and both carbohydrate digestion and absorption indicate potential as an adjunctive therapy for diabetes. Br. J. Nutr. 2020, 124, 1021–1034.
- Hatami, E.; Ghalishourani, S.-S.; Najafgholizadeh, A.; Pourmasoumi, M.; Hadi, A.; Clark, C.C.T.; Assaroudi, M.; Salehi-sahlabadi, A.; Joukar, F.; Mansour-Ghanaei, F. The effect of spirulina on type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2021, 20, 883–892.
- Koirala, P.; Jung, H.A.; Choi, J.S. Recent advances in pharmacological research on Ecklonia species: A review. Arch. Pharm. Res. 2017, 40, 981–1005.
- Mawed, S.A.; Centoducati, G.; Farag, M.R.; Alagawany, M.; Abou-Zeid, S.M.; Elhady, W.M.; El-Saadony, M.T.; Di Cerbo, A.; Al-Zahaby, S.A. Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish. Biology 2022, 11, 1447.
- Cho, C.-H.; Yoo, G.; Kim, M.; Kurniawati, U.D.; Choi, I.-W.; Lee, S.-H. Dieckol, derived from the Edible Brown Algae Ecklonia cava, Attenuates Methylglyoxal-Associated Diabetic Nephropathy by Suppressing AGE–RAGE Interaction. Antioxidants 2023, 12, 593.
- Jo, S.-L.; Yang, H.; Jeong, K.-J.; Lee, H.-W.; Hong, E.-J. Neuroprotective Effects of Ecklonia cava in a Chronic Neuroinflammatory Disease Model. Nutrients 2023, 15, 2007.
- Shibasaki, S.; Ueda, M. Utilization of Macroalgae for the Production of Bioactive Compounds and Bioprocesses Using Microbial Biotechnology. Microorganisms 2023, 11, 1499.
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In Vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilaria Gracilaria edulis and robust sea moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411.
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362.
- Choi, J.; Kim, K.-J.; Koh, E.-J.; Lee, B.-Y. Gelidium elegans Extract Ameliorates Type 2 Diabetes via Regulation of MAPK and PI3K/Akt Signaling. Nutrients 2018, 10, 51.
- Gabbia, D.; De Martin, S. Brown Seaweeds for the Management of Metabolic Syndrome and Associated Diseases. Molecules 2020, 25, 4182.
- Hao, S.; Li, F.; Li, Q.; Yang, Q.; Zhang, W. Phycocyanin Protects against High Glucose High Fat Diet Induced Diabetes in Mice and Participates in AKT and AMPK Signaling. Foods 2022, 11, 3183.
- Din, N.A.S.; Mohd Alayudin, ‘A.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235.
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484.
- Sugii, S.; Olson, P.; Sears, D.D.; Saberi, M.; Atkins, A.R.; Barish, G.D.; Hong, S.-H.; Castro, G.L.; Yin, Y.-Q.; Nelson, M.C.; et al. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 2009, 106, 22504–22509.
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33.
- Zheng, H.; Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742.
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-Inflammatory Properties and Gut Microbiota Modulation of Porphyra tenera Extracts in Dextran Sodium Sulfate-Induced Colitis in Mice. Antioxidants 2020, 9, 988.
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845.
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Karim, M.M.; Das, R.; Park, M.N.; Chandran, D.; et al. Fucoxanthin: A Promising Phytochemical on Diverse Pharmacological Targets. Front. Pharmacol. 2022, 13, 929442.
- Wu, D.; Chen, Y.; Wan, X.; Liu, D.; Wen, Y.; Chen, X.; Zhao, C. Structural characterization and hypoglycemic effect of green alga Ulva lactuca oligosaccharide by regulating microRNAs in Caenorhabditis elegans. Algal Res. 2020, 51, 102083.
- Wang, X.; Shan, X.; Dun, Y.; Cai, C.; Hao, J.; Li, G.; Cui, K.; Yu, G. Anti-Metabolic Syndrome Effects of Fucoidan from Fucus vesiculosus via Reactive Oxygen Species-Mediated Regulation of JNK, Akt, and AMPK Signaling. Molecules 2019, 24, 3319.
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2017, 22, 56.
- Tsuge, K.; Okabe, M.; Yoshimura, T.; Sumi, T.; Tachibana, H.; Yamada, K. Dietary Effects of Porphyran from Porphyra yezoensis on Growth and Lipid Metabolism of Sprague-Dawley Rats. Food Sci. Technol. Res. 2004, 10, 147–151.
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kuznetsova, T.A.; Kryzhanovsky, S.P.; Ermakova, S.P.; Galkina, I.V.; Shchelkanov, M.Y. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Mar. Drugs 2022, 20, 243.
- Jayasinghe, A.M.K.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Kim, K.-N.; Oh, J.-Y.; Ahn, G. The Anti-Inflammatory Effect of Low Molecular Weight Fucoidan from Sargassum siliquastrum in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via Inhibiting NF-κB/MAPK Signaling Pathways. Mar. Drugs 2023, 21, 347.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
556
Revisions:
2 times
(View History)
Update Date:
08 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




