You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abdul Wahab | -- | 2743 | 2023-09-06 08:35:52 | | | |
| 2 | Sirius Huang | Meta information modification | 2743 | 2023-09-06 11:26:22 | | | | |
| 3 | Abdul Wahab | Meta information modification | 2743 | 2023-09-07 07:53:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in the Ecosystem. Encyclopedia. Available online: https://encyclopedia.pub/entry/48867 (accessed on 02 January 2026).
Wahab A, Muhammad M, Munir A, Abdi G, Zaman W, Ayaz A, et al. Role of Arbuscular Mycorrhizal Fungi in the Ecosystem. Encyclopedia. Available at: https://encyclopedia.pub/entry/48867. Accessed January 02, 2026.
Wahab, Abdul, Murad Muhammad, Asma Munir, Gholamreza Abdi, Wajid Zaman, Asma Ayaz, Chandni Khizar, Sneha Priya Pappula Reddy. "Role of Arbuscular Mycorrhizal Fungi in the Ecosystem" Encyclopedia, https://encyclopedia.pub/entry/48867 (accessed January 02, 2026).
Wahab, A., Muhammad, M., Munir, A., Abdi, G., Zaman, W., Ayaz, A., Khizar, C., & Reddy, S.P.P. (2023, September 06). Role of Arbuscular Mycorrhizal Fungi in the Ecosystem. In Encyclopedia. https://encyclopedia.pub/entry/48867
Wahab, Abdul, et al. "Role of Arbuscular Mycorrhizal Fungi in the Ecosystem." Encyclopedia. Web. 06 September, 2023.
Copy Citation
Arbuscular mycorrhizal fungi (AMF) form symbiotic relationships with the roots of nearly all land-dwelling plants, increasing growth and productivity, especially during abiotic stress. AMF improves plant development by improving nutrient acquisition, such as phosphorus, water, and mineral uptake. AMF improves plant tolerance and resilience to abiotic stressors such as drought, salt, and heavy metal toxicity.
arbuscular mycorrhiza fungi
crop productivity
ecosystem
sustainable
soil
1. Introduction
It is believed that many of the fungi in the Chromista group, such as slime molds and oomycetes, have the same ability to survive in extreme environments as fungi. This means that they are likely to be present in areas not usually explored by humans. Thus, their study is essential for understanding how it works in different environments and how diseases can be spread. Furthermore, since many fungi are parasites or symbiotes, previewing the literature can help us better understand the complex relationships between animals and fungi. It is also important to note that fungi do not possess animals’ digestive, nervous, or circulatory systems [1][2][3].
Furthermore, fungi reproduce by releasing spores or other reproductive cells, whereas animals reproduce sexually. In typical fungi, filamentous cytoplasms are surrounded by plasma membranes and cell walls called hyphae, similar to insect exoskeletons. Chitin is a flexible polysaccharide found in the fungi’s cell walls. In hyphae, several auxiliary cell walls are called “cross walls” or “septa” and are typically perforated by large pores, allowing ribosomes, mitochondria, and nuclei to pass through [4]. Mycelium has a multicellular structure that forms hyphae when it matures; it digests organic food externally before absorption by secreting enzymes that help it. The fungal mycelium can spread over large areas, allowing the plant root system to obtain phosphate and other minerals far away from nutrient-depleted walls known as “zones”. In contrast, the plant provides sugars to the fungus [5].
Among the many benefits of endomycorrhizal systems, plants can obtain more water and nutrients and increase protection against pathogens and droughts [6]. The mycelium breaks down organic matter in the soil and transfers nutrients to the plant, whereas the plant provides the mycelium with carbohydrates for energy; this relationship between the mycelium and the root tissue is mutually beneficial [7]. In addition to increasing plant nutrient absorption, AM fungus can make plants more resilient to abiotic stressors [8]. According to Spatafora et al. (2016), most AMF species are members of the Mucoromycota phylum’s Glomeromycotina subphylum [9]. This subphylum has 25 taxa and many orders, including the Glomerales, Archaeosporales, Paraglomerales, and Diversisporales [10]. To complete their life cycle, they consume lipids [11][12] and products from photosynthetic plants [13]. AMF shields plants against fungal diseases and enhances water and mineral nutrient intake from surrounding soils [14]. As a result, AMFs are essential for the ecosystem and plant production. It is impossible to overestimate the significance of these crops for sustainable agricultural development [15].
2. Sustainability of Agriculture and AM Fungi
By harnessing the natural abilities of the soil, it is possible to reduce the need for chemical inputs, which are costly and harmful to the environment. Additionally, reducing input overheads such as water and fuel can help reduce costs and increase efficiency [16]. Finally, preventing ecological contamination helps to protect the ecosystems in which agribusiness operates and can help to ensure sustainability in the end. An effective management system must be implemented and monitored to ensure the soil environment is suitable for crop growth while promoting beneficial soil microbes. This includes maintaining adequate moisture and nutrient levels and controlling nutrient leaching and compaction [17].
Other edaphic factors must be accounted for, such as the presence of nematodes and the application of organic or inorganic fertilizers. Because plants and microbes work together interestingly, mycorrhizal fungi significantly ensure sustainable agriculture [18]. A link between these symbiotic fungi in sustainable farming systems appears imperative, especially when certain ingredients are in short supply. AM is invaluable in mobilizing nutrients into usable forms under these conditions [19]. The extra-radical mycelium within this culture can be invaluable because AM fungal proliferation has enhanced soil quality and structural stability [20]. This has been shown to encourage plant growth. AM fungal proliferation is becoming an increasingly significant part of sustainable agricultural practices as it contributes to soil quality and structural stability. Utilizing these mycorrhizal associations would efficiently boost productivity by reducing chemical inputs such as insecticides and fertilizers [21]. The use of inorganic fertilizers on soils with low fertility has been spread widely, organic matter has been added, and practices such as fallow tilling and incorporation of leguminous crops have all been employed to improve soil ecosystems, boost soil microbial growth, and increase nutrient reuse to minimize external inputs while maximizing the effectiveness of those inputs [22].
This is why earthworms and microsymbionts are so helpful for managing soil ecosystems—they act as a form of natural fertilizer, helping to promote nutrient cycling in the soil and cultivating beneficial microbial associations [23]. Additionally, research has indicated that soil fungi have a variety of functions, such as aiding in decomposition and providing essential nutrients for plants. However, more research is needed to understand the full extent of their role in the soil ecosystem. It should be noted that mycorrhizae play a significant role in ecology [24]. It is because of their widespread distribution and potential contribution to nutrient cycling and biomass production by soil microbes. As a result, a crop’s germplasm must be tailored specifically to the environment where it will be grown [25]. In this way, the plant will receive the exact number of essential elements and minor nutrients needed for optimal growth if it grows in an optimal environment. This also allows the plant to cope with biotic and abiotic stresses like heat and drought, which can negatively affect crop yields. Because of this study, the symbiosis between plants and AMF existed long before plants colonized the land [26][27]. The fossil record indicates that there have probably been AMF on the land for at least 480 million years. This indicates that they likely influenced their success before plants were introduced to the land. As a result, they likely had a significant impact on terrestrial ecosystems during that period. This would suggest that they played a significant role in shaping them during that period [28][29][30][31][32][33][34].
3. AM Fungi in Crops Defense Mechanisms
AMF strengthens the plant’s immune system, giving it a more extraordinary ability to resist pests and pathogens more effectively [35]. In addition to providing nutrition to the plant, fungi also improve its growth and development, making it more likely for the plant to grow and develop more rapidly and effectively. Furthermore, fungi also produce compounds that act as antibiotics, which helps to protect the plant from specific pathogens [36]. This suggests that the colonization of plants by these beneficial microbes may be due to their ability to modify the plant’s defense pathways, allowing the microbes to colonize the plant without triggering an immune response. Additionally, the microbes may be able to access nutrients inaccessible to the plant or produce compounds that make the plant more resilient to environmental stress [37]. If mycorrhizal fungi colonize plants, a systemic priming effect can result from reassigning defense molecules/signals to the plants. This leads to a significantly higher crop defense DNA production when the plants are not colonized [38]. As well as improving plant nutrient uptake and growth, mycorrhizal colonization can also increase the attractiveness and quantity of plants and improve plant nutrient uptake and growth. Herbivores are also encouraged to consume plants that are abundant in nutrients, as this increases their availability of nutrients [39]. It has been demonstrated that crops colonized by AMF are more resistant to phloem-feeding insects than crops without AMF. Any farming management strategy involving AMF should include the notion that AMF symbionts may assist plants in mounting unspecific defenses against phloem-feeding herbicides. These mycelial networks can communicate between plants infested with aphids and plants not infested with aphids [40][41]. Thus, plants produce a rapid flow of aphid-repellent volatile compounds before they are attacked, thus preventing the spread of the disease and reducing productivity. Essentially, the mycorrhizal fungi form a symbiotic relationship with the roots of a crop, exchanging nutrients, water, and carbon for the crop’s nutrients. In addition, the fungi produce numerous secondary metabolites that are antibacterial and antifungal, which can assist in protecting the plant from microorganism-related diseases. The fungi can strengthen the plant’s ability to resist certain diseases and stimulate the plant’s immune system [39].
When pathogens are present in the root zone, this resistance mechanism can be triggered, causing the plant to release phenolic compounds that inhibit these pathogens’ growth. Furthermore, plant cells may develop physical barriers that inhibit the spread of pathogens and reduce their ability to cause damage [42][43]. For example, a study found Fusarium solani f. sp. After Glomus intraradical was inoculated, phaseoli genomic DNA, quantified using quantitative RT-PCR, significantly decreased in the mycorrhizosphere, microsphere, and bulk soil [44]. To increase crop yield, reduce soil erosion, maintain soil fertility, and limit pesticide use, scientists can develop better agronomic practices by understanding the interactions between mycorrhizae, other microorganisms, and plants [45]. Further, understanding the role of mycorrhizae in disease resistance can assist farmers in protecting their crops from soil-borne pathogens and reduce chemical treatment use [46]. A mycorrhiza is a beneficial fungus that forms a symbiotic relationship with plants’ roots to help the plants get more nutrients from the soil and resist pathogens. By understanding how mycorrhizae work, farmers can use them to protect their crops from diseases without relying on potentially harmful chemical treatments. Additionally, certain mycorrhizae can produce safer biocides for humans and the environment [47].
4. AM Fungi Play an Essential Role in Increasing Crops’ Productivity Maintenance of Soil Health
By doing so, the soil is better able to sustain its structure and fertility over time, providing a stable foundation for various plants and animals [48]. These accumulations are critical for developing and conserving a microporous, water-permeable soil shape, essential for disintegration obstruction and practical supplement cycling [49]. Chemicals and phosphate fertilizers are widely used, which results in pollution problems and health dangers. Therefore, utilizing AMF is energized in horticulture. Taking advantage of Mycorrhiza parasites is not generally simple since it is still impossible to produce a lot of AMF on a large scale in the field [50]. Aside from the results of compost utility on arbuscular mycorrhiza fungi, different techniques such as yield pivot, negligible development, monoculture, culturing, regular changes, and alertness of biocides impact the AMF [51]. Mycorrhiza’s advantageous interaction plays a vital function in the tropics’ horticultural vegetation because, in the tropical locale, there is a phosphorous deficiency in the soil [52]. They do not consume 75% of the phosphorus but get converted to forms inaccessible to plants.
Crop plants’ reliance on AMF for nutrient uptake is influenced by root factors, surface area, the quantity and length of root hairs, the rate of growth, and their reactions to soil circumstances and exudations. Smith and Read explained that yields like corn (Zea mays L.) and flax (Linum usitatissiumum) depend incredibly on arbuscular mycorrhiza fungus to fulfil their advanced demands of phosphorous [53][54]. Mycorrhizal fungi establish a symbiotic relationship with plants, enabling them to enhance their nutrient uptake from the soil. This mutually beneficial association allows plants to access more essential nutrients, including phosphorus and nitrogen. Arbuscular mycorrhizal fungi (AMF) employ various mechanisms to enhance stress tolerance and promote the growth of medicinal and aromatic plants. Arbuscular mycorrhizal fungi (AMF) are crucial in triggering plant responses to abiotic stress. These responses occur at various morphological, physiological, and molecular levels. The purpose of these responses is to help plants cope with the harmful effects of abiotic stress. The symbiosis between arbuscular mycorrhizal fungi and plants has enhanced water and nutrient acquisition, improving plant growth and increasing tolerance to abiotic stress. The symbol “+” is used to indicate a positive effect. EOs refers to essential oils, SOD stands for superoxide dismutase, POD stands for peroxidase, APX stands for ascorbate peroxidase, H2O2 represents hydrogen peroxide, MDA stands for malondialdehyde, and P represents phosphorus. The relationship depicted in Figure 1 plays a crucial role in promoting the growth and general health of the plant [38].
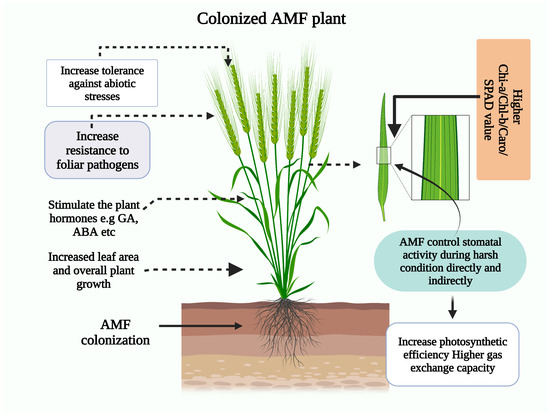
Figure 1. Role of AM fungi colonization in the productivity maintenance of soil health.
5. Role of AMF in the Ecosystem and Mitigation of Environmental Stresses
It is instructive, for instance, to rotate corn and soybean crops to improve soil fertility. Since corn takes up more nitrogen from the soil than soybeans, planting soybeans after corn helps replenish the soil’s nitrogen supply by adding nitrogen [55]. The ‘rotation impact’ situation cannot be defined through nutritional outcomes. Other features containing arbuscular mycorrhiza fungi might also be crucial in fulfilling yield turns, as shown in Figure 2 [56]. Non-mycorrhizal fungi are a reservoir for the soil inoculum, storing and preserving the beneficial microbes. When mycorrhizal plants colonize the soil, they can help to increase the rate at which the beneficial microbes are spread through the soil, thereby increasing the productivity of the soil [57]. A blast in the colonization of AMF also developed in maize came about after sunflower (Helianthus annuus, mycorrhizal) when contrasted with corn after mustard (non-mycorrhiza) [58]. This pivot shows the importance of non-ectomycorrhizal vegetation in lowering the rate of AMF colonization among the plants around it. Unlike the colonization of corn after the species alfalfa (Medicago sativa L.) and brome grass (Bromus spp.), which are mycorrhizal hosts, corn colonization is not an ectomycorrhizal process. Instead, the colonization of corn (Zea mays L.) after canola (Brassica napus L.) is a non-mycorrhizal host. There was a significant reduction in corn colonization after canola planting for 62 days; however, there was a significant increase in corn colonization similar to that of an AMF host species. In light of these findings, it is possible to increase AMF populations and reverse the inhibitory effects of non-mycorrhizal crops by following up with mycorrhizal crops [59].
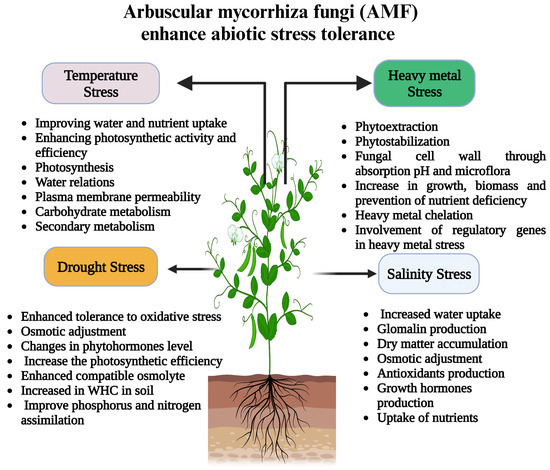
Figure 2. Schematic representation of the different mechanisms imparting abiotic stress tolerance in plants by arbuscular mycorrhiza fungi (AMF).
The advantages of arbuscular mycorrhiza fungi are significant in frameworks wherein phosphorous inside the dirt is economical [60][61]. Plant tissue phosphorus will rise along with the amount of available phosphorous, and the amount of carbon the plant invests in mycorrhiza is not necessarily profitable for the plant [62]. Support of beneficial mycorrhizal interaction can enhance phosphorous uptake, further developing yield ability without starter P fertilizer spray [63]. AMF additionally plays a critical function in hit reforestation, and numerous reviews of the multiplied foundation of many woodland seedlings inside the field, similar to Quercus rubra [64]. A review plan on the foundation of Desmoncus orthacanthos and vaccination of AM parasites brought about a triple blast in the endurance of seedlings in the field. Although it is conceivable that different networks of arbuscular mycorrhizal fungus could provide a range of environmental benefits, more research on range/characteristic correlations is necessary to respond to this inquiry [65]. AMF parasitic species/confines can exhibit an unmistakable physiological range.
In contrast, choice or reproduction for plant assortments beneath excessive mineral situations that disregard harmonious movement may result in the era, i.e., kinds of plants that might be less or non-responsive to mycorrhizal [66]. The earliest mycorrhizal morphology combination of the plant and contagious tissues will probably be considered in both companions via simple genetic and ametabolous programs endured over the ages [67]. However, plants have evolved significantly, reaching about 260,000 species that can often be attributed to biological niches or environments that have been described [68]. In evaluation, Glomeromycotan seems to have persisted fantastically unaltered for a considerable number of years, which is a state of affairs that has been explained due to morphological balance [69]; also, something like two hundred transform types of these parasites are known to present daily [70]. A comparable reduction of the cooperative movement because of choice has been located for soya beans [71]. Under low-entry conditions, such adverse effects on the symbiotic feature likely to be neglected in high-input agriculture will be significant. In this context, comparing types conventionally bred with those acclimated to low-input environments will help to light the potential negative influence of breeding on AM characteristics [72].
In any case, it must be noted that no possibility exists where the capacity of a host plant to shape AM via standard choice or rearing exercises has vanished [73]. As such, it is essential to monitor the phosphate levels in the surrounding environment to determine the impact of the fungus on the host plant [74]. Based totally on the finding that plant range impacts the AM fungal range, [75] indicates that long-term monocropping may additionally hurt the AM contagious variety even though it might very well be challenging for each situation to split direct results from the components of complete agrarian control, for example, excessive-supplement, pesticide input, and soil aggravation [76].
References
- Panstruga, R.; Antonin, W.; Lichius, A. Looking outside the box: A comparative cross-kingdom view on the cell biology of the three major lineages of eukaryotic multicellular life. Cell. Mol. Life Sci. 2023, 80, 198.
- Tang, S.-K.; Zhi, X.-Y.; Zhang, Y.; Makarova, K.S.; Liu, B.-B.; Zheng, G.-S.; Zhang, Z.-P.; Zheng, H.-J.; Wolf, Y.I.; Zhao, Y.-R. Cellular differentiation into hyphae and spores in halophilic archaea. Nat. Commun. 2023, 14, 1827.
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal symbiosis in plant growth and stress adaptation: From genes to ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607.
- Tajuddin, R.Y.; Asfar, M.; Dirpan, A. An overview-is mycelium composite foam can be used as a food packaging. AIP Conf. Proc. 2023, 2596, 040010.
- Sun, X.; Shi, J.; Ding, G. Combined effects of arbuscular mycorrhiza and drought stress on plant growth and mortality of forage sorghum. Appl. Soil Ecol. 2017, 119, 384–391.
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046.
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531.
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175.
- Bago, B.; Pfeffer, P.; Shachar-Hill, Y. Carbon transport and metabolism in arbuscular mycorrhiza. Plant Physiol 2000, 124, 949–957.
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008.
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F. A critical review of mineral–microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, nwac128.
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530.
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088.
- Astapati, A.D.; Nath, S. The complex interplay between plant-microbe and virus interactions in sustainable agriculture: Harnessing phytomicrobiomes for enhanced soil health, designer plants, resource use efficiency, and food security. Crop Des. 2023, 2, 100028.
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326.
- Li, W.; Yao, J.; He, C.; Ren, Y.; Zhao, L.; He, X. The synergy of dark septate endophytes and organic residue on Isatis indigotica growth and active ingredients accumulation under drought stress. Ind. Crops Prod. 2023, 203, 117147.
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The multifaceted roles of Arbuscular Mycorrhizal Fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36.
- Díaz-Urbano, M.; Goicoechea, N.; Velasco, P.; Poveda, J. Development of agricultural bio-inoculants based on mycorrhizal fungi and endophytic filamentous fungi: Co-inoculants for improve plant-physiological responses in sustainable agriculture. Biol. Control 2023, 182, 105223.
- Li, G.; Tang, X.; Hou, Q.; Li, T.; Xie, H.; Lu, Z.; Zhang, T.; Liao, Y.; Wen, X. Response of soil organic carbon fractions to legume incorporation into cropping system and the factors affecting it: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 342, 108231.
- Medina-Sauza, R.M.; Álvarez-Jiménez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdán, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms building up soil microbiota, a review. Front. Environ. Sci. 2019, 7, 81.
- Janowski, D.; Leski, T. Factors in the distribution of mycorrhizal and soil fungi. Diversity 2022, 14, 1122.
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to microbiome: A paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 622926.
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; Behera, C. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665.
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934.
- Etikala, B.; Adimalla, N.; Madhav, S.; Somagouni, S.G.; Keshava Kiran Kumar, P. Salinity Problems in Groundwater and Management Strategies in Arid and Semiarid Regions. In Groundwater Geochemistry: Pollution and Remediation Methods; Wiley: Toronto, ON, Canada, 2021; pp. 42–56.
- Lin, C.; Wang, Y.; Liu, M.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci. Rep. 2020, 10, 12260.
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664.
- Kastner, T.; Chaudhary, A.; Gingrich, S.; Marques, A.; Persson, U.M.; Bidoglio, G.; Le Provost, G.; Schwarzmüller, F. Global agricultural trade and land system sustainability: Implications for ecosystem carbon storage, biodiversity, and human nutrition. One Earth 2021, 4, 1425–1443.
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640.
- Mbodj, D.; Effa-Effa, B.; Kane, A.; Manneh, B.; Gantet, P.; Laplaze, L.; Diedhiou, A.; Grondin, A. Arbuscular mycorrhizal symbiosis in rice: Establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizosphere 2018, 8, 12–26.
- Dong, S.; Shang, Z.; Gao, J.; Boone, R.B. Enhancing sustainability of grassland ecosystems through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2020, 287, 106684.
- Luiz, J.M. Economics, transitions, and traps in emerging markets. In The Oxford Handbook of Management in Emerging Markets; Oxford University Press: Oxford UK, 2019; p. 77.
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55.
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487.
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068.
- Edlinger, A.; Garland, G.; Hartman, K.; Banerjee, S.; Degrune, F.; García-Palacios, P.; Hallin, S.; Valzano-Held, A.; Herzog, C.; Jansa, J. Agricultural management and pesticide use reduce the functioning of beneficial plant symbionts. Nat. Ecol. Evol. 2022, 6, 1145–1154.
- Nie, M.; Bell, C.; Wallenstein, M.D.; Pendall, E. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci. Rep. 2015, 5, 9212.
- Babikova, Z.; Gilbert, L.; Bruce, T.; Dewhirst, S.Y.; Pickett, J.A.; Johnson, D. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct. Ecol. 2014, 28, 375–385.
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320.
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front. Plant Sci. 2022, 13, 1044896.
- Filion, M.; St-Arnaud, M.; Jabaji-Hare, S. Quantification of Fusarium solani f. sp. phaseoli in mycorrhizal bean plants and surrounding mycorrhizosphere soil using real-time polymerase chain reaction and direct isolations on selective media. Phytopathology 2003, 93, 229–235.
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019, 6, 23.
- George, N.P.; Ray, J.G. The inevitability of arbuscular mycorrhiza for sustainability in organic agriculture—A critical review. Front. Sustain. Food Syst. 2023, 7, 1124688.
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front. Plant Sci. 2018, 9, 1270.
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction. Sustainability 2022, 14, 7840.
- Loo, W.T.; Chua, K.-O.; Mazumdar, P.; Cheng, A.; Osman, N.; Harikrishna, J.A. Arbuscular Mycorrhizal Symbiosis: A Strategy for Mitigating the Impacts of Climate Change on Tropical Legume Crops. Plants 2022, 11, 2875.
- Liang, T.; Chen, G.; Zhao, J.; Sinha, S.; Zhang, W. AMF-Placer 2.0: Open Source Timing-driven Analytical Mixed-size Placer for Large-scale Heterogeneous FPGA. arXiv 2022, arXiv:2210.08682.
- Islam, M.A. Effects of Arbuscular Mycorrhizal Fungi on Plant Growth, Yield, and Plant-Parasitic Nematode Control in Organic Tomato Production: A Field Study and a Systematic Review; Hochschule Rhein-Waal: Westfalen, Germany, 2022.
- Dhiman, M.; Sharma, L.; Kaushik, P.; Singh, A.; Sharma, M.M. Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability 2022, 14, 10220.
- Watts-Williams, S.J. Track and trace: How soil labelling techniques have revealed the secrets of resource transport in the arbuscular mycorrhizal symbiosis. Mycorrhiza 2022, 32, 257–267.
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084.
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030.
- Reicosky, D.; Calegari, A.; dos Santos, D.R.; Tiecher, T. Cover Crop Mixes for Diversity, Carbon and Conservation Agriculture. In Cover Crops and Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2021; pp. 169–208.
- Kaur, J. Effects of Arbuscular Mycorrhizal Fungi (AMF) on Growth and Herbivore Defenses in Sorghum Sudangrass (Sorghum X drummondii). Master’s Thesis, The University of Texas Rio Grande Valley, Rio Grande Valley, TX, USA, 2020.
- Salomon, M.; Watts-Williams, S.; McLaughlin, M.; Brien, C.; Jewell, N.; Berger, B.; Cavagnaro, T. Evaluation of commercial composts and potting mixes and their ability to support arbuscular mycorrhizal fungi with maize (Zea mays) as host plant. Waste Manag. 2021, 134, 187–196.
- Li, W.; Li, W.-B.; Xing, L.-J.; Guo, S.-X. Effect of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) on microorganism of phenanthrene and pyrene contaminated soils. Int. J. Phytoremediation 2022, 25, 240–251.
- Konvalinková, T.; Püschel, D.; Řezáčová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333.
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313.
- Van’t Padje, A.; Werner, G.D.; Kiers, E.T. Mycorrhizal fungi control phosphorus value in trade symbiosis with host roots when exposed to abrupt ‘crashes’ and ‘booms’ of resource availability. New Phytol. 2021, 229, 2933–2944.
- Akhtar, M.; Yousaf, S.; Sarwar, N.; Hussain, S. Zinc biofortification of cereals—Role of phosphorus and other impediments in alkaline calcareous soils. Environ. Geochem. Health 2019, 41, 2365–2379.
- Wall, C.B.; Egan, C.P.; Swift, S.I.; Hynson, N.A. Three decades post-reforestation has not led to the reassembly of arbuscular mycorrhizal fungal communities associated with remnant primary forests. Mol. Ecol. 2020, 29, 4234–4247.
- Pérez-Tienda, J.; Valderas, A.; Camañes, G.; García-Agustín, P.; Ferrol, N. Kinetics of NH 4+ uptake by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mycorrhiza 2012, 22, 485–491.
- Jenks, G.K. Restoring the natural functional capacity of coastal dune ecosystems: Utilising research records for New Zealand littoral refurbishment as a proxy for analogous global responses. J. Coast. Conserv. 2018, 22, 623–665.
- Xie, L.; Bi, Y.; Ma, S.; Shang, J.; Hu, Q.; Christie, P. Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: Synergistic or competitive growth effects on maize? BMC Plant Biol. 2021, 21, 498.
- Schüßler, A.; Martin, H.; Cohen, D.; Fitz, M.; Wipf, D. Arbuscular mycorrhiza: Studies on the geosiphon symbiosis lead to the characterization of the first glomeromycotan sugar transporter. Plant Signal. Behav. 2007, 2, 431–434.
- Brundrett, M.C.; Ashwath, N. Glomeromycotan mycorrhizal fungi from tropical Australia III. Measuring diversity in natural and disturbed habitats. Plant Soil 2013, 370, 419–433.
- Paranavithana, T.; Marasinghe, S.; Perera, G.; Ratnayake, R. Effects of crop rotation on enhanced occurrence of arbuscular mycorrhizal fungi and soil carbon stocks of lowland paddy fields in seasonaly dry tropics. Paddy Water Environ. 2021, 19, 217–226.
- Messerli, P.; Murniningtyas, E.; Eloundou-Enyegue, P.; Foli, E.G.; Furman, E.; Glassman, A.; Hernández Licona, G.; Kim, E.M.; Lutz, W.; Moatti, J.-P. Global Sustainable Development Report 2019: The Future Is Now–Science for Achieving Sustainable Development; United Nations: New York, NY, USA, 2019.
- Burridge, J.D.; Findeis, J.L.; Jochua, C.N.; Miguel, M.A.; Mubichi-Kut, F.M.; Quinhentos, M.L.; Xerinda, S.A.; Lynch, J.P. A case study on the efficacy of root phenotypic selection for edaphic stress tolerance in low-input agriculture: Common bean breeding in Mozambique. Field Crops Res. 2019, 244, 107612.
- An, J.; Zeng, T.; Ji, C.; de Graaf, S.; Zheng, Z.; Xiao, T.T.; Deng, X.; Xiao, S.; Bisseling, T.; Limpens, E. A Medicago truncatula SWEET transporter implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis. New Phytol. 2019, 224, 396–408.
- Meena, R.S.; Vijayakumar, V.; Yadav, G.S.; Mitran, T. Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant Growth Regul. 2018, 84, 207–223.
- Borowicz, V.A. Do arbuscular mycorrhizal fungi alter plant–pathogen relations? Ecology 2001, 82, 3057–3068.
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 2020, 101, e02978.
- Kazerooni, E.A.; Maharachchikumbura, S.S.; Al-Sadi, A.M.; Rashid, U.; Kang, S.-M.; Lee, I.-J. Actinomucor elegans and Podospora bulbillosa Positively Improves Endurance to Water Deficit and Salinity Stresses in Tomato Plants. J. Fungi 2022, 8, 785.
- Liu, M.; Shen, Y.; Li, Q.; Xiao, W.; Song, X. Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C: N: P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant Soil 2021, 461, 421–440.
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
3 times
(View History)
Update Date:
07 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




