Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikolay K Shakhpazyan | -- | 2572 | 2023-09-05 07:23:44 | | | |
| 2 | Camila Xu | Meta information modification | 2572 | 2023-09-05 07:28:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shakhpazyan, N.; Mikhaleva, L.; Bedzhanyan, A.; Gioeva, Z.; Sadykhov, N.; Mikhalev, A.; Atiakshin, D.; Buchwalow, I.; Tiemann, M.; Orekhov, A. The Gut Microbiome and Colorectal Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/48803 (accessed on 08 February 2026).
Shakhpazyan N, Mikhaleva L, Bedzhanyan A, Gioeva Z, Sadykhov N, Mikhalev A, et al. The Gut Microbiome and Colorectal Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/48803. Accessed February 08, 2026.
Shakhpazyan, Nikolay, Liudmila Mikhaleva, Arkady Bedzhanyan, Zarina Gioeva, Nikolay Sadykhov, Alexander Mikhalev, Dmitri Atiakshin, Igor Buchwalow, Markus Tiemann, Alexander Orekhov. "The Gut Microbiome and Colorectal Cancer" Encyclopedia, https://encyclopedia.pub/entry/48803 (accessed February 08, 2026).
Shakhpazyan, N., Mikhaleva, L., Bedzhanyan, A., Gioeva, Z., Sadykhov, N., Mikhalev, A., Atiakshin, D., Buchwalow, I., Tiemann, M., & Orekhov, A. (2023, September 05). The Gut Microbiome and Colorectal Cancer. In Encyclopedia. https://encyclopedia.pub/entry/48803
Shakhpazyan, Nikolay, et al. "The Gut Microbiome and Colorectal Cancer." Encyclopedia. Web. 05 September, 2023.
Copy Citation
Colorectal cancer (CRC) represents a significant global health burden, ranking as the third most common cancer and the second leading cause of cancer-related deaths worldwide. The gut microbiome, composed of trillions of commensal microorganisms, plays a vital role in maintaining homeostasis and overall health. Mounting evidence suggests that alterations in the gut microbiome, referred to as dysbiosis, may contribute to the initiation and progression of CRC by modulating the tumor microenvironment (TME), including the tumor stroma.
colorectal cancer
Gut Microbiome
Tumor Stroma
1. Introduction
Colorectal cancer (CRC) represents a significant global health burden, ranking as the third most common cancer and the second leading cause of cancer-related deaths worldwide. According to the World Health Organization, approximately 1.8 million new cases of CRC were diagnosed, and nearly 900,000 deaths were reported, in 2020 [1]. The high morbidity and mortality associated with CRC can be attributed to several factors, including late-stage diagnosis, limited treatment options, and therapy resistance.
The early detection and diagnosis of CRC are crucial for improving patient outcomes, as the 5-year survival rate for patients diagnosed at an early stage is significantly higher than for those diagnosed at advanced stages [2]. Current screening methods for CRC include fecal occult blood tests (FOBT), fecal immunochemical tests (FIT), flexible sigmoidoscopy, and colonoscopies [3]. While these methods have been effective in reducing CRC incidence and mortality, they are not without limitations. Barriers to CRC screening include patient discomfort, invasiveness, financial constraints, and a low adherence to screening guidelines [4].
Treatment options for CRC primarily depend on the stage of the disease and may include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. Despite advances in surgical techniques and the development of novel therapeutic agents, the prognosis for patients with advanced or metastatic CRC remains poor, with a 5-year survival rate of less than 20% [5]. Furthermore, the emergence of therapy resistance and the occurrence of tumor recurrence after initial treatment contribute to the challenges associated with CRC management.
Shared characteristics with the stroma of other solid tumors, including cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), extracellular matrix (ECM) components, and immune cells, mark the tumor stroma in CRC [6]. Despite these commonalities, specific features distinguish the colorectal tumor stroma from other cancer types.
One of the key distinguishing factors is the influence of the microbiome, where bacteria can modulate the tumor stroma by influencing immune cell recruitment and activation, and promote a pro-inflammatory environment [7]. In addition to this, the colorectal mucosa harbors a unique immune system known as the gut-associated lymphoid tissue (GALT) [8]. Disruptions to the balance between immune tolerance and anti-tumor immune responses can lead to alterations in the composition and function of immune cells within the tumor stroma.
A crucial feature that sets CRC apart is the aberrant activation of the Wnt signaling pathway, a hallmark of this disease, especially with mutations in the adenomatous polyposis coli (APC) gene [9]. This pathway can also influence the tumor stroma by promoting the activation of CAFs and the secretion of factors that support cancer cell growth and invasion.
Furthermore, CRC is characterized by tumor budding, identified by the presence of small clusters or single cancer cells at the invasive front of the tumor. This distinctive feature has been associated with a more aggressive phenotype and a worse prognosis [10]. Budding cancer cells can interact with stromal cells, such as CAFs and immune cells, to promote invasion and metastasis [11].
Finally, the CRC stroma displays unique gene expression signatures known as Conserved Oncogenic Signatures (COS). These are specific to the stromal compartment of colorectal tumors and include signatures that reflect an immunosuppressive environment, thereby contributing to the complexity of the tumor’s immune landscape [12][13].
The complexity and heterogeneity of CRC, underscored by the dynamic interplay between cancer cells and the TME, highlight the necessity for a more profound understanding of the disease’s underlying mechanisms. An important aspect, indicative of the tumor stroma’s relevance in CRC evaluation, is the tumor–stroma ratio (TSR). This straightforward marker has emerged as a significant factor in determining CRC prognosis, with a high TSR correlating with an increased risk of cancer recurrence and potential resistance to chemotherapy [14]. The TSR is established by analyzing histological slides, typically from the tumor’s most invasive part, and is categorized into stroma-high (>50% stromal area) and stroma-low (≤50% stromal area) [15]. Recent technological advances, specifically in artificial intelligence, have facilitated automated TSR quantification. This development has proven to be prognostically valid, assisting in clinical decision-making by offering a more objective, standardized analysis, and reducing the workload of pathologists [16].
Nevertheless, the TSR’s exclusive use for patient prognosis remains a matter of de-bate. Various studies suggest that other markers, such as tumor budding, tumor infiltrating pattern, and lymphocyte-to-monocyte ratio—the latter being an independent factor influencing both relapse-free survival and overall survival outcomes—are equally, if not more, critical [17]. Moreover, the reliability of TSR assessment has been questioned due to the poor-to-moderate inter-pathologist agreement [18]. The inclusion of additional markers, such as CAFs or tumor-infiltrating lymphocytes, may result in a more comprehensive patient stratification tool [19][20]. Thus, it is evident that more comprehensive studies are needed to enhance biomarker assessment consistency and validate these findings [18][21]. This intricate scenario involving even a seemingly straightforward and universally accessible marker like the TSR hints at the extreme complexity of the stroma’s cellular and molecular organization when examined at the histological level.
2. The Gut Microbiome and CRC: Dysbiosis, Tumor Stroma Modulation, and Emerging Therapeutic Strategies
The gut microbiome, composed of trillions of commensal microorganisms, plays a vital role in maintaining homeostasis and overall health. Mounting evidence suggests that alterations in the gut microbiome, referred to as dysbiosis, may contribute to the initiation and progression of CRC by modulating the tumor microenvironment (TME), including the tumor stroma [22].
One crucial aspect of this modulation is the direct interaction of specific bacterial species with the tumor stroma. For instance, Fusobacterium nucleatum, Bacteroides fragilis, and Escherichia coli have been closely associated with CRC development, while some species exhibit antitumor activity (Table 1) [23][24][25][26][27]. These bacteria directly interact with stromal cells, including CAFs and immune cells, influencing their activation and function. F. nucleatum, for example, adheres to and invades CAFs, leading to the production of pro-inflammatory cytokines like IL-6 and IL-8, which in turn promote cancer cell proliferation, survival, and migration [28][29][30] (Figure 1).
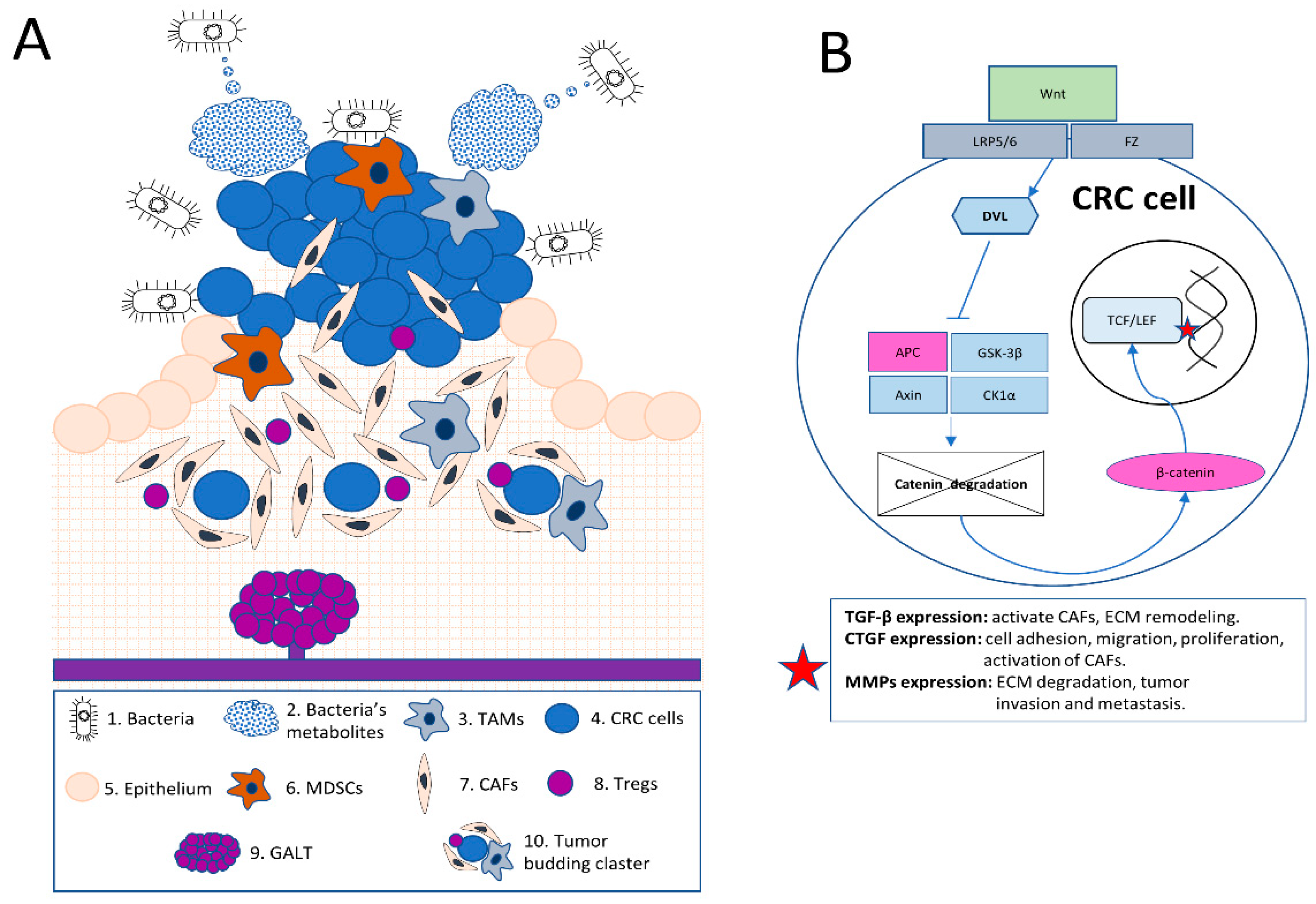
Figure 1. Unique Features and Complexity of the Colorectal Tumor Microenvironment (TME).
Table 1. Bacteria of gut microbiome with pro- and anticancer activities.
| Bacteria | Mechanism of Action | References |
|---|---|---|
| Preliminary Pro-Cancer | ||
| Fusobacterium nucleatum | Induces DNA damage and genetic changes, promotes cytokine production, influences immune regulation, possibly enhancing CRC progression. | [23][24][31][32] |
| Bacteroides fragilis | Produces cytotoxic BFT, alters cellular structures, induces inflammation and activates signaling pathways, triggers changes in host defense mechanisms contributing to CRC. | [33][34] |
| Enterococcus faecalis | Utilizes biliverdin to promote CRC cell proliferation and angiogenesis, induces immunomodulation, causes genomic instability and disrupts intestinal barrier, contributing to CRC progression. | [35][36][37] |
| Escherichia coli (phylotype B2, genotoxic pks + E. coli) | Overrepresented cytotoxic phenotype contributes to DNA damage, promotes carcinogenic effects via the production of colibactin mediated by the pks gene. | [38][39][40] |
| Peptostreptococcus anaerobius | Adheres to CRC cells via PCWBR2 protein, activates PI3K–Akt–FAK pathway, promotes cell proliferation and triggers pro-inflammatory responses, enhancing CRC progression. | [41][42] |
| Streptococcus gallolyticus | Adheres to host cells via Type VII secretion system, stimulates cell proliferation and promotes CRC via upregulation of β-catenin, c-Myc, and PCNA. | [43][44][45] |
| Clostridium septicum | Exacerbates CRC through α-toxin production, induces necrosis and mucosal ulceration, impairs immune response, fostering a conducive environment for CRC. | [46][47][48] |
| Preliminary Anti-Cancer | ||
| Ruminococcus gnavus | Reduces tumor growth and degrades inhibitory compounds like lyso-glycerophospholipids, enhancing the activity of CD8+ T cells, potentially mitigating CRC progression. | [49][50] |
| Bifidobacterium longum | Modulates oncogenic and tumor suppressor miRNAs, suppresses pro-inflammatory cytokines, enhances adhesion to the intestinal tract, increases short-chain fatty acids production, and improves intestinal barrier function, potentially mitigating CRC progression. | [51][52][53][54] |
| Lactobacillus acidophilus | Induces apoptosis in CRC cells, mitigates ulcerative colitis via increased acetate production and control of inflammation, potentially reducing CRC progression. | [51][52][55][56][57] |
| Lactobacillus rhamnosus | Triggers apoptosis in cancer cells, boosts immune responses, increases carcinoembryonic antigen secretion from cancer cells, modulates gut immune landscape by increasing CD8 T-cell responses, potentially mitigating CRC progression. | [52][58][59][60][61] |
| Faecalibacterium prausnitzii | Reduces formation of aberrant crypt foci, suppresses lipid peroxidation levels, inhibits CRC cell proliferation, enhances gut microbiota diversity, produces butyrate to augment tumor-suppressing effects, potentially mitigating CRC progression. | [62][63] |
| Bifidobacterium breve | Stimulates immune response by increasing cytotoxic CD8+ T cells, promotes production of anti-tumor cytokines, potentially reducing CRC progression. | [49][64][65][66] |
| Lactobacillus reuteri | Provokes caspase-9-dependent apoptosis in tumor cells, inhibits cell invasion and proliferation, reduces proliferation and survival in colon cancer cells with its metabolite, reuterin, potentially mitigating CRC progression. | [52][67][68][69] |
| Bifidobacterium adolescentis | Suppresses colorectal carcinogenesis, inhibits harmful bacterial enzymes such as β-glucuronidase, β-glucosidase, tryptophanase, and urease, differentially regulates Treg/Th17 immune responses, potentially reducing CRC progression. | [52][70][71][72] |
| Lactobacillus plantarum | Strengthens the intestinal mucosal barrier by regulating occludin and claudin-1 proteins, inhibits harmful bacterial enzymatic activity, regulates CRC cell proliferation and apoptosis, potentially mitigating CRC progression. | [52][73][74][75] |
Note: The presented table effectively illustrates the dual role of gut microbiota in the progression and mitigation of colorectal cancer (CRC). The ‘Preliminary Pro-cancer’ section identifies bacteria such as Fusobacterium nucleatum, Bacteroides fragilis, among others, that may contribute to CRC progression through various mechanisms including DNA damage, inflammation, and immune response impairment. On the other hand, the ‘Preliminary Anti-cancer’ section lists beneficial bacteria, including multiple Bifidobacterium and Lactobacillus species, which exhibit potential anti-carcinogenic activities, thereby possibly mitigating CRC progression. It is important to note, however, that the roles of these bacteria are not exclusive to CRC. They have multifaceted implications across a broad spectrum of health and disease states beyond CRC, underlining the complex and integral relationship between the gut microbiome and overall human health.
(A): In this illustration, a comprehensive schematic overview of the complex colorectal tumor microenvironment (TME) is provided. The image demonstrates the interaction between bacteria and their metabolites (1 and 2) with the TME, emphasizing the influence of the microbiome on the tumor stroma and inflammatory modulation, including tumor-associated macrophages or TAMs (3). Colorectal cancer (CRC) cells (4) grow at the gut epithelium (5) within the tumor and its stroma. These CRC cells are surrounded by key immune cells, such as TAMs (3) and myeloid-derived suppressor cells (MDSCs) (6). The image also features cancer-associated fibroblasts (CAFs) (7), which support tumor growth and invasion.
The illustration further showcases regulatory T cells (Tregs) (8), which modulate immune responses. Also depicted is the unique mucosal immune system in the colorectal mucosa, represented by the gut-associated lymphoid tissue (GALT) (9) and its interactions with immune cells. Tumor budding, characterized by the presence of small clusters or single cancer cells at the invasive front of the tumor (10), is also portrayed. This visual representation effectively captures the distinctive features and interactions within the colorectal TME.
(B): CRC frequently exhibits driver mutations in Wnt pathway genes, such as APC and β-catenin (CTNNB1). The canonical Wnt/β-catenin pathway is depicted, beginning with extracellular Wnt proteins binding to the cell surface receptors Frizzled (Fz) and the low-density lipoprotein receptor-related protein 5/6 (LRP5/6). Upon Wnt binding, the Fz receptor recruits and activates the intracellular protein Dishevelled (Dvl), leading to the inhibition of the β-catenin destruction complex, which consists of Axin, Adenomatous Polyposis Coli (APC), Glycogen Synthase Kinase-3β (GSK-3β), and Casein Kinase 1α (CK1α). This inhibition prevents the phosphorylation and subsequent degradation of β-catenin, allowing it to accumulate in the cytoplasm and translocate into the nucleus.
Once in the nucleus, β-catenin interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, activating the transcription of target genes (red asterisk figure in the picture) such as the Transforming growth factor-beta (TGF-β), Connective tissue growth factor (CTGF), and Matrix metalloproteinases (MMPs). These factors influence the tumor microenvironment through the activation of cancer-associated fibroblasts (CAFs), promotion of extracellular matrix (ECM) remodeling, enhancement of cell adhesion, migration, and proliferation, and facilitation of tumor invasion and metastasis.
Another aspect of gut microbiome influence on the CRC stroma is through the production of bacterial metabolites, such as short-chain fatty acids (SCFAs), secondary bile acids, and polyamines [76][77][78][79][80]. SCFAs, like butyrate, exhibit anti-inflammatory and anti-tumorigenic properties by modulating the activation of immune cells and CAFs [76][77][78]. In contrast, secondary bile acids and polyamines promote a pro-inflammatory environment and stimulate reactive oxygen species (ROS) production, leading to DNA damage and the activation of oncogenic pathways in both cancer and stromal cells [79][80][81][82][83].
Dysbiosis can also result in chronic inflammation, a major risk factor for CRC. Pro-inflammatory bacteria stimulate the production of cytokines and chemokines, such as IL-6, IL-8, IL-1b, and TNF-α, which recruit and activate various immune cells, including tumor-associated macrophages (TAMs), T cells, and myeloid-derived suppressor cells (MDSCs) [29][84][85][86]. The complex interplay among bacteria, immune cells, and the tumor stroma creates a self-perpetuating pro-inflammatory and pro-tumorigenic environment, facilitating CRC development and progression.
The gut microbiome can also impact the composition and remodeling of the extracellular matrix (ECM) in the CRC stroma. Bacteria and their metabolites modulate the expression and activity of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which are crucial for ECM remodeling [87][88]. Changes in ECM composition and stiffness influence cancer cell invasion, metastasis, angiogenesis, and immune cell infiltration.
Given the role of the gut microbiome in colorectal tumor stroma development and progression, researchers are actively exploring strategies to manipulate it. Promising approaches include probiotics and prebiotics, fecal microbiota transplantation (FMT), dietary interventions, targeted antimicrobial therapy, and combination therapies.
Probiotics, live microorganisms conferring health benefits when administered in adequate amounts, may help restore immune homeostasis and reduce pro-inflammatory and pro-tumorigenic stimuli contributing to CRC progression [69][89][90]. Prebiotics, non-digestible food components that selectively stimulate the growth and activity of beneficial gut bacteria, promote the production of beneficial bacterial metabolites, such as SCFAs, potentially counteracting dysbiosis’ adverse effects on colorectal tumor stroma development [91][92].
FMT, involving the transfer of fecal material containing a healthy donor’s gut microbiota into a recipient’s gastrointestinal tract, aims to restore the recipient’s gut microbial balance. While FMT has been primarily used for treating recurrent Clostridioides difficile infection, emerging evidence suggests the potential for modulating the gut microbiome in CRC patients, thereby affecting tumor stroma development and disease progression [93][94][95].
Dietary interventions offer another means to influence gut microbial composition and function. Adopting a diet rich in fruits, vegetables, whole grains, and lean proteins, while limiting the intake of processed and red meats, high-fat dairy products, and added sugars, can promote a healthy gut microbiome [96][97][98]. Such dietary changes may potentially reduce inflammation and the risk of CRC by modulating the gut microbiome and its interactions with the tumor stroma.
Targeted antimicrobial therapy, selectively targeting specific pathogenic bacteria implicated in CRC progression such as Fusobacterium nucleatum and Bacteroides fragilis, could be a potential approach to mitigate their influence on the tumor stroma [99][100][101]. However, developing targeted antimicrobial therapies requires a thorough understanding of the complex interactions between these bacteria and the colorectal tumor stroma, as well as the identification of specific molecular targets.
Combination therapies, which involve combining microbiome-targeting interventions with conventional cancer therapies, such as chemotherapy, radiotherapy, or immunotherapy, may enhance treatment efficacy by modulating the tumor stroma and improving the overall tumor microenvironment [102]. These combination strategies could help overcome therapy resistance and improve patient outcomes.
In summary, the gut microbiome plays a crucial role in CRC development and progression by modulating the tumor stroma through direct bacterial interactions, the production of bacterial metabolites, and bacteria-induced inflammation. Dysbiosis can lead to a pro-inflammatory and pro-tumorigenic environment, further promoting CRC. Strategies such as probiotics, prebiotics, fecal microbiota transplantation, dietary interventions, targeted antimicrobial therapy, and combination therapies hold promise for mitigating the gut microbiome’s influence on colorectal tumor stroma development and progression. Further research is needed to optimize these approaches and improve patient outcomes.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789.
- Araghi, M.; Arnold, M.; Rutherford, M.J.; Guren, M.G.; Cabasag, C.J.; Bardot, A.; Ferlay, J.; Tervonen, H.; Shack, L.; Woods, R.R.; et al. Colon and rectal cancer survival in seven high-income countries 2010-2014: Variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut 2021, 70, 114–126.
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432.
- Hultcrantz, R. Aspects of colorectal cancer screening, methods, age and gender. J. Intern. Med. 2021, 289, 493–507.
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685.
- Li, H.; Fan, X.; Houghton, J. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell Biochem. 2007, 101, 805–815.
- Dai, Z.; Zhang, J.; Wu, Q.; Chen, J.; Liu, J.; Wang, L.; Chen, C.; Xu, J.; Zhang, H.; Shi, C.; et al. The role of microbiota in the development of colorectal cancer. Int. J. Cancer 2019, 145, 2032–2041.
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802.
- He, K.; Gan, W.J. Wnt/beta-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer. Cancer Manag. Res. 2023, 15, 435–448.
- Mitrovic, B.; Handley, K.; Assarzadegan, N.; Chang, H.L.; Dawson, H.A.E.; Grin, A.; Hutchins, G.G.A.; Magill, L.; Quirke, P.; Riddell, R.H.; et al. Prognostic and Predictive Value of Tumor Budding in Colorectal Cancer. Clin. Color. Cancer 2021, 20, 256–264.
- Hatthakarnkul, P.; Quinn, J.A.; Ammar, A.; Lynch, G.; Van Wyk, H.; McMillan, D.C.; Thuwajit, C.; Edwards, J. Molecular mechanisms of tumour budding and its association with microenvironment in colorectal cancer. Clin. Sci. 2022, 136, 521–535.
- Torres, S.; Bartolome, R.A.; Mendes, M.; Barderas, R.; Fernandez-Acenero, M.J.; Pelaez-Garcia, A.; Pena, C.; Lopez-Lucendo, M.; Villar-Vazquez, R.; de Herreros, A.G.; et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013, 19, 6006–6019.
- Leonard, N.A.; Reidy, E.; Thompson, K.; McDermott, E.; Peerani, E.; Tomas Bort, E.; Balkwill, F.R.; Loessner, D.; Ryan, A.E. Stromal Cells Promote Matrix Deposition, Remodelling and an Immunosuppressive Tumour Microenvironment in a 3D Model of Colon Cancer. Cancers 2021, 13, 5998.
- Strous, M.T.A.; Faes, T.K.E.; Gubbels, A.; van der Linden, R.L.A.; Mesker, W.E.; Bosscha, K.; Bronkhorst, C.M.; Janssen-Heijnen, M.L.G.; Vogelaar, F.J.; de Bruine, A.P. A high tumour-stroma ratio (TSR) in colon tumours and its metastatic lymph nodes predicts poor cancer-free survival and chemo resistance. Clin. Transl. Oncol. 2022, 24, 1047–1058.
- van Pelt, G.W.; Kjaer-Frifeldt, S.; van Krieken, J.; Al Dieri, R.; Morreau, H.; Tollenaar, R.; Sorensen, F.B.; Mesker, W.E. Scoring the tumor-stroma ratio in colon cancer: Procedure and recommendations. Virchows Arch. 2018, 473, 405–412.
- Zhao, K.; Li, Z.; Yao, S.; Wang, Y.; Wu, X.; Xu, Z.; Wu, L.; Huang, Y.; Liang, C.; Liu, Z. Artificial intelligence quantified tumour-stroma ratio is an independent predictor for overall survival in resectable colorectal cancer. EBioMedicine 2020, 61, 103054.
- Zhang, Y.; Liu, Y.; Qiu, X.; Yan, B. Concurrent Comparison of the Prognostic Values of Tumor Budding, Tumor Stroma Ratio, Tumor Infiltrating Pattern and Lymphocyte-to-Monocyte Ratio in Colorectal Cancer Patients. Technol. Cancer Res. Treat. 2021, 20, 15330338211045826.
- Kazemi, A.; Gharib, M.; Mohamadian Roshan, N.; Taraz Jamshidi, S.; Stogbauer, F.; Eslami, S.; Schuffler, P.J. Assessment of the Tumor-Stroma Ratio and Tumor-Infiltrating Lymphocytes in Colorectal Cancer: Inter-Observer Agreement Evaluation. Diagnostics 2023, 13, 2339.
- Sandberg, T.P.; Stuart, M.; Oosting, J.; Tollenaar, R.; Sier, C.F.M.; Mesker, W.E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer 2019, 19, 284.
- van de Weerd, S.; Smit, M.A.; Roelands, J.; Mesker, W.E.; Bedognetti, D.; Kuppen, P.J.K.; Putter, H.; Tollenaar, R.; Roodhart, J.M.L.; Hendrickx, W.; et al. Correlation of Immunological and Histopathological Features with Gene Expression-Based Classifiers in Colon Cancer Patients. Int. J. Mol. Sci. 2022, 23, 2707.
- Karjula, T.; Kemi, N.; Niskakangas, A.; Mustonen, O.; Puro, I.; Pohjanen, V.M.; Kuopio, T.; Elomaa, H.; Ahtiainen, M.; Mecklin, J.P.; et al. The prognostic role of tumor budding and tumor-stroma ratio in pulmonary metastasis of colorectal carcinoma. Eur. J. Surg. Oncol. 2023, 49, 1298–1306.
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340.
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638.
- Brennan, C.A.; Clay, S.L.; Lavoie, S.L.; Bae, S.; Lang, J.K.; Fonseca-Pereira, D.; Rosinski, K.G.; Ou, N.; Glickman, J.N.; Garrett, W.S. Fusobacterium nucleatum drives a pro-inflammatory intestinal microenvironment through metabolite receptor-dependent modulation of IL-17 expression. Gut Microbes 2021, 13, 1987780.
- Sui, H.; Zhang, L.; Gu, K.; Chai, N.; Ji, Q.; Zhou, L.; Wang, Y.; Ren, J.; Yang, L.; Zhang, B.; et al. YYFZBJS ameliorates colorectal cancer progression in Apc(Min/+) mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun. Signal 2020, 18, 113.
- Knippel, R.J.; Drewes, J.L.; Sears, C.L. The Cancer Microbiome: Recent Highlights and Knowledge Gaps. Cancer Discov. 2021, 11, 2378–2395.
- Chattopadhyay, I.; Dhar, R.; Pethusamy, K.; Seethy, A.; Srivastava, T.; Sah, R.; Sharma, J.; Karmakar, S. Exploring the Role of Gut Microbiome in Colon Cancer. Appl. Biochem. Biotechnol. 2021, 193, 1780–1799.
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448.
- Engevik, M.A.; Danhof, H.A.; Ruan, W.; Engevik, A.C.; Chang-Graham, A.L.; Engevik, K.A.; Shi, Z.; Zhao, Y.; Brand, C.K.; Krystofiak, E.S.; et al. Fusobacterium nucleatum Secretes Outer Membrane Vesicles and Promotes Intestinal Inflammation. mBio 2021, 12, e10-1128.
- Casasanta, M.A.; Yoo, C.C.; Udayasuryan, B.; Sanders, B.E.; Umana, A.; Zhang, Y.; Peng, H.; Duncan, A.J.; Wang, Y.; Li, L.; et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal 2020, 13, eaba9157.
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172.
- Wang, S.; Liu, Y.; Li, J.; Zhao, L.; Yan, W.; Lin, B.; Guo, X.; Wei, Y. Fusobacterium nucleatum Acts as a Pro-carcinogenic Bacterium in Colorectal Cancer: From Association to Causality. Front. Cell Dev. Biol. 2021, 9, 710165.
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. 2020, 27, 9–21.
- Scott, N.; Whittle, E.; Jeraldo, P.; Chia, N. A systemic review of the role of enterotoxic Bacteroides fragilis in colorectal cancer. Neoplasia 2022, 29, 100797.
- Williamson, A.J.; Jacobson, R.; van Praagh, J.B.; Gaines, S.; Koo, H.Y.; Lee, B.; Chan, W.C.; Weichselbaum, R.; Alverdy, J.C.; Zaborina, O.; et al. Enterococcus faecalis promotes a migratory and invasive phenotype in colon cancer cells. Neoplasia 2022, 27, 100787.
- de Almeida, C.V.; Taddei, A.; Amedei, A. The controversial role of Enterococcus faecalis in colorectal cancer. Ther. Adv. Gastroenterol. 2018, 11, 1756284818783606.
- Zhang, L.; Liu, J.; Deng, M.; Chen, X.; Jiang, L.; Zhang, J.; Tao, L.; Yu, W.; Qiu, Y. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: Biliverdin. J. Transl. Med. 2023, 21, 72.
- Nouri, R.; Hasani, A.; Shirazi, K.M.; Alivand, M.R.; Sepehri, B.; Sotoodeh, S.; Hemmati, F.; Rezaee, M.A. Escherichia coli and Colorectal Cancer: Unfolding the Enigmatic Relationship. Curr. Pharm. Biotechnol. 2022, 23, 1257–1268.
- Wassenaar, T.M. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit. Rev. Microbiol. 2018, 44, 619–632.
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 2020, 580, 269–273.
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Ho Szeto, C.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330.
- Karpinski, T.M.; Ozarowski, M.; Stasiewicz, M. Carcinogenic microbiota and its role in colorectal cancer development. Semin. Cancer Biol. 2022, 86, 420–430.
- Pasquereau-Kotula, E.; Martins, M.; Aymeric, L.; Dramsi, S. Significance of Streptococcus gallolyticus subsp. gallolyticus Association With Colorectal Cancer. Front. Microbiol. 2018, 9, 614.
- Taylor, J.C.; Gao, X.; Xu, J.; Holder, M.; Petrosino, J.; Kumar, R.; Liu, W.; Hook, M.; Mackenzie, C.; Hillhouse, A.; et al. A type VII secretion system of Streptococcus gallolyticus subsp. gallolyticus contributes to gut colonization and the development of colon tumors. PLoS Pathog. 2021, 17, e1009182.
- Kumar, R.; Herold, J.L.; Schady, D.; Davis, J.; Kopetz, S.; Martinez-Moczygemba, M.; Murray, B.E.; Han, F.; Li, Y.; Callaway, E.; et al. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PLoS Pathog. 2017, 13, e1006440.
- Slezak, M.; Smolar, M.; Drobna Saniova, B.; Hosala, M.; Miklusica, J. Clostridium septicum foot gangrene associated with colorectal cancer. Neuro Endocrinol. Lett. 2022, 43, 57–64.
- Li, J.; Chen, D.; Shen, M. Tumor Microenvironment Shapes Colorectal Cancer Progression, Metastasis, and Treatment Responses. Front. Med. 2022, 9, 869010.
- Xu, S.; Yin, W.; Zhang, Y.; Lv, Q.; Yang, Y.; He, J. Foes or Friends? Bacteria Enriched in the Tumor Microenvironment of Colorectal Cancer. Cancers 2020, 12, 372.
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613.
- Zhang, X.; Yu, D.; Wu, D.; Gao, X.; Shao, F.; Zhao, M.; Wang, J.; Ma, J.; Wang, W.; Qin, X.; et al. Tissue-resident Lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell Host Microbe 2023, 31, 418–432.e8.
- Lee, C.W.; Chen, H.J.; Chien, Y.H.; Hsia, S.M.; Chen, J.H.; Shih, C.K. Synbiotic Combination of Djulis (Chenopodium formosanum) and Lactobacillus acidophilus Inhibits Colon Carcinogenesis in Rats. Nutrients 2019, 12, 103.
- Faghfoori, Z.; Pourghassem Gargari, B.; Saber, A.; Seyyedi, M.; Fazelian, S.; Khosroushahi, A.Y. Prophylactic effects of secretion metabolites of dairy lactobacilli through downregulation of ErbB-2 and ErbB-3 genes on colon cancer cells. Eur. J. Cancer Prev. 2020, 29, 201–209.
- Fahmy, C.A.; Gamal-Eldeen, A.M.; El-Hussieny, E.A.; Raafat, B.M.; Mehanna, N.S.; Talaat, R.M.; Shaaban, M.T. Bifidobacterium longum Suppresses Murine Colorectal Cancer through the Modulation of oncomiRs and Tumor Suppressor miRNAs. Nutr. Cancer 2019, 71, 688–700.
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J. Immunol. Res. 2021, 2021, 8030297.
- Isazadeh, A.; Hajazimian, S.; Shadman, B.; Safaei, S.; Bedoustani, A.B.; Chavoshi, R.; Shanehbandi, D.; Mashayekhi, M.; Nahaei, M.; Baradaran, B. Anti-Cancer Effects of Probiotic Lactobacillus acidophilus for Colorectal Cancer Cell Line Caco-2 through Apoptosis Induction. Pharm. Sci. 2021, 27, 262–267.
- Guo, Y.; Zhang, T.; Gao, J.; Jiang, X.; Tao, M.; Zeng, X.; Wu, Z.; Pan, D. Lactobacillus acidophilus CICC 6074 inhibits growth and induces apoptosis in colorectal cancer cells in vitro and in HT-29 cells induced-mouse model. J. Funct. Foods 2020, 75, 104290.
- Wang, M.X.; Lin, L.; Chen, Y.D.; Zhong, Y.P.; Lin, Y.X.; Li, P.; Tian, X.; Han, B.; Xie, Z.Y.; Liao, Q.F. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 2020, 159, 104978.
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferrau, F.; Libra, M. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Front. Pharmacol. 2017, 8, 603.
- Aziz Mousavi, S.M.A.; Mirhosseini, S.A.; Rastegar Shariat Panahi, M.; Mahmoodzadeh Hosseini, H. Characterization of Biosynthesized Silver Nanoparticles Using Lactobacillus rhamnosus GG and its In Vitro Assessment against Colorectal Cancer Cells. Probiotics Antimicrob. Proteins 2020, 12, 740–746.
- Amin, M.; Navidifar, T.; Saeb, S.; Barzegari, E.; Jamalan, M. Tumor-targeted induction of intrinsic apoptosis in colon cancer cells by Lactobacillus plantarum and Lactobacillus rhamnosus strains. Mol. Biol. Rep. 2023, 50, 5345–5354.
- Owens, J.A.; Saeedi, B.J.; Naudin, C.R.; Hunter-Chang, S.; Barbian, M.E.; Eboka, R.U.; Askew, L.; Darby, T.M.; Robinson, B.S.; Jones, R.M. Lactobacillus rhamnosus GG Orchestrates an Antitumor Immune Response. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1311–1327.
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128.
- Danne, C.; Sokol, H. Butyrate, a new microbiota-dependent player in CD8+ T cells immunity and cancer therapy? Cell Rep. Med. 2021, 2, 100328.
- Yoon, Y.; Kim, G.; Jeon, B.N.; Fang, S.; Park, H. Bifidobacterium Strain-Specific Enhances the Efficacy of Cancer Therapeutics in Tumor-Bearing Mice. Cancers 2021, 13, 957.
- Li, Q.; Li, Y.; Wang, Y.; Xu, L.; Guo, Y.; Wang, Y.; Wang, L.; Guo, C. Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12. Oncoimmunology 2021, 10, 1868122.
- Xia, C.; Cai, Y.; Ren, S.; Xia, C. Role of microbes in colorectal cancer therapy: Cross-talk between the microbiome and tumor microenvironment. Front. Pharmacol. 2022, 13, 1051330.
- Kim, S.J.; Kang, C.H.; Kim, G.H.; Cho, H. Anti-Tumor Effects of Heat-Killed L. reuteri MG5346 and L. casei MG4584 against Human Colorectal Carcinoma through Caspase-9-Dependent Apoptosis in Xenograft Model. Microorganisms 2022, 10, 533.
- Maghsood, F.; Johari, B.; Rohani, M.; Madanchi, H.; Saltanatpour, Z.; Kadivar, M. Anti-proliferative and Anti-metastatic Potential of High Molecular Weight Secretory Molecules from Probiotic Lactobacillus Reuteri Cell-Free Supernatant Against Human Colon Cancer Stem-Like Cells (HT29-ShE). Int. J. Pept. Res. Ther. 2020, 26, 2619–2631.
- Bell, H.N.; Rebernick, R.J.; Goyert, J.; Singhal, R.; Kuljanin, M.; Kerk, S.A.; Huang, W.; Das, N.K.; Andren, A.; Solanki, S.; et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022, 40, 185–200.e6.
- Lin, Y.; Fan, L.; Qi, Y.; Xu, C.; Jia, D.; Jiang, Y.; Chen, S.; Wang, L. Bifidobacterium adolescentis induces Decorin(+) macrophages via TLR2 to suppress colorectal carcinogenesis. J. Exp. Clin. Cancer Res. 2023, 42, 172.
- Kim, Y.; Lee, D.; Kim, D.; Cho, J.; Yang, J.; Chung, M.; Kim, K.; Ha, N. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch. Pharm. Res. 2008, 31, 468–473.
- Yu, R.; Zuo, F.; Ma, H.; Chen, S. Exopolysaccharide-Producing Bifidobacterium adolescentis Strains with Similar Adhesion Property Induce Differential Regulation of Inflammatory Immune Response in Treg/Th17 Axis of DSS-Colitis Mice. Nutrients 2019, 11, 782.
- Ding, S.; Hu, C.; Fang, J.; Liu, G. The Protective Role of Probiotics against Colorectal Cancer. Oxidative Med. Cell Longev. 2020, 2020, 8884583.
- Ghorbani, E.; Avan, A.; Ryzhikov, M.; Ferns, G.; Khazaei, M.; Soleimanpour, S. Role of lactobacillus strains in the management of colorectal cancer: An overview of recent advances. Nutrition 2022, 103–104, 111828.
- Chuah, L.O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 114.
- Ebert, M.N.; Klinder, A.; Peters, W.H.; Schaferhenrich, A.; Sendt, W.; Scheele, J.; Pool-Zobel, B.L. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis 2003, 24, 1637–1644.
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272.
- Park, C.H.; Eun, C.S.; Han, D.S. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest. Res. 2018, 16, 338–345.
- Ryu, T.Y.; Kim, K.; Han, T.S.; Lee, M.O.; Lee, J.; Choi, J.; Jung, K.B.; Jeong, E.J.; An, D.M.; Jung, C.R.; et al. Human gut-microbiome-derived propionate coordinates proteasomal degradation via HECTD2 upregulation to target EHMT2 in colorectal cancer. ISME J. 2022, 16, 1205–1221.
- Liu, Y.; Zhang, S.; Zhou, W.; Hu, D.; Xu, H.; Ji, G. Secondary Bile Acids and Tumorigenesis in Colorectal Cancer. Front. Oncol. 2022, 12, 813745.
- Fang, Y.; Yan, C.; Zhao, Q.; Xu, J.; Liu, Z.; Gao, J.; Zhu, H.; Dai, Z.; Wang, D.; Tang, D. The roles of microbial products in the development of colorectal cancer: A review. Bioengineered 2021, 12, 720–735.
- Caliceti, C.; Punzo, A.; Silla, A.; Simoni, P.; Roda, G.; Hrelia, S. New Insights into Bile Acids Related Signaling Pathways in the Onset of Colorectal Cancer. Nutrients 2022, 14, 2964.
- Huang, C.Y.; Fang, Y.J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.Y.; Zhang, X.; Feng, X.L.; Chen, Y.M.; Zhang, C.X. Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrients 2020, 12, 3575.
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298.
- Yang, Y.; Li, L.; Xu, C.; Wang, Y.; Wang, Z.; Chen, M.; Jiang, Z.; Pan, J.; Yang, C.; Li, X.; et al. Cross-talk between the gut microbiota and monocyte-like macrophages mediates an inflammatory response to promote colitis-associated tumourigenesis. Gut 2020, 70, 1495–1506.
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432.
- Rodrigues, D.M.; Sousa, A.J.; Hawley, S.P.; Vong, L.; Gareau, M.G.; Kumar, S.A.; Johnson-Henry, K.C.; Sherman, P.M. Matrix metalloproteinase 9 contributes to gut microbe homeostasis in a model of infectious colitis. BMC Microbiol. 2012, 12, 105.
- Wei, Z.; Cao, S.; Liu, S.; Yao, Z.; Sun, T.; Li, Y.; Li, J.; Zhang, D.; Zhou, Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget 2016, 7, 46158–46172.
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630.
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409.
- Ciecierska, A.; Drywien, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324.
- Wang, H.; Chen, K.; Ning, M.; Wang, X.; Wang, Z.; Yue, Y.; Yuan, Y.; Yue, T. Intake of Pro- and/or Prebiotics as a Promising Approach for Prevention and Treatment of Colorectal Cancer. Mol. Nutr. Food Res. 2023, 67, e2200474.
- Zhang, J.; Wu, K.; Shi, C.; Li, G. Cancer Immunotherapy: Fecal Microbiota Transplantation Brings Light. Curr. Treat. Options Oncol. 2022, 23, 1777–1792.
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; Liu, Z. Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment. Front. Immunol. 2022, 13, 949490.
- Ting, N.L.; Lau, H.C.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425.
- Zhao, Y.; Zhan, J.; Wang, Y.; Wang, D. The Relationship Between Plant-Based Diet and Risk of Digestive System Cancers: A Meta-Analysis Based on 3,059,009 Subjects. Front. Public Health 2022, 10, 892153.
- Kato, I.; Sun, J. Microbiome and Diet in Colon Cancer Development and Treatment. Cancer J. 2023, 29, 89–97.
- Gomes, S.; Teixeira-Guedes, C.; Silva, E.; Baltazar, F.; Preto, A. Colon microbiota modulation by dairy-derived diet: New strategy for prevention and treatment of colorectal cancer. Food Funct. 2022, 13, 9183–9194.
- Jia, F.; Yu, Q.; Wang, R.; Zhao, L.; Yuan, F.; Guo, H.; Shen, Y.; He, F. Optimized Antimicrobial Peptide Jelleine-I Derivative Br-J-I Inhibits Fusobacterium Nucleatum to Suppress Colorectal Cancer Progression. Int. J. Mol. Sci. 2023, 24, 1469.
- Jang, H.I.; Rhee, K.J.; Eom, Y.B. Antibacterial and antibiofilm effects of alpha-humulene against Bacteroides fragilis. Can. J. Microbiol. 2020, 66, 389–399.
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic bacteria in colorectal cancer: Mechanisms and treatments. Cancer Biol. Med. 2021, 19, 147–162.
- Alam, Z.; Shang, X.; Effat, K.; Kanwal, F.; He, X.; Li, Y.; Xu, C.; Niu, W.; War, A.R.; Zhang, Y. The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer. J. Food Biochem. 2022, 46, e14302.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
958
Revisions:
2 times
(View History)
Update Date:
05 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




