Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peter Mikuš | -- | 2300 | 2023-09-05 04:21:48 | | | |
| 2 | Rita Xu | -7 word(s) | 2293 | 2023-09-05 04:48:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Melník, M.; Mikušová, V.; Mikuš, P. Pt(η3–P1C2X1C2P2)(Y) Derivative Types. Encyclopedia. Available online: https://encyclopedia.pub/entry/48798 (accessed on 07 March 2026).
Melník M, Mikušová V, Mikuš P. Pt(η3–P1C2X1C2P2)(Y) Derivative Types. Encyclopedia. Available at: https://encyclopedia.pub/entry/48798. Accessed March 07, 2026.
Melník, Milan, Veronika Mikušová, Peter Mikuš. "Pt(η3–P1C2X1C2P2)(Y) Derivative Types" Encyclopedia, https://encyclopedia.pub/entry/48798 (accessed March 07, 2026).
Melník, M., Mikušová, V., & Mikuš, P. (2023, September 05). Pt(η3–P1C2X1C2P2)(Y) Derivative Types. In Encyclopedia. https://encyclopedia.pub/entry/48798
Melník, Milan, et al. "Pt(η3–P1C2X1C2P2)(Y) Derivative Types." Encyclopedia. Web. 05 September, 2023.
Copy Citation
Structural data are classified and analyzed for almost seventy complexes of the general formula Pt(η3–P1X1P2)(Y) (X1 = O, N, C, S, Si) and (Y = various monodentate ligands), in which the respective η3–P1X1P2 ligand forms a pair of five-membered metallocyclic rings with a common X1 atom of the P1C2X1C2P2 type.
heterotridentate
Pt(η3–P1C2X1C2P2)(Y)
trans-influence
1. Introduction
The chemistry of platinum coordination complexes has been intensively studied and developed for more than five decades, focusing on the relationship between structure and reactivity. The chemistry of platinum is important in the fields of biochemistry [1], catalysis [2], spectroscopy [3][4], and coordination theory. Very recently, Horiuchi and Umakoshi published a review that focused on the importance of and advances in the synthetic, structural, thermodynamic, electronic, and photophysical properties of Pt-based heteropolynuclear complexes [5].
Significant attention has been paid to organomonophosphines, representing soft donor ligands in the chemistry of platinum. There are a large number of published structural studies on such complexes that have been classified and analyzed [6]. Another group of related structural studies is devoted to Pt(II) complexes with organodiphosphines [7][8]. Recently, researchers analyzed and classified structural data for the following compositions: Pt(η4–P4L), Pt(η4–P3 SiL), Pt(η4–P2N2L), Pt(η4–P2S2L), Pt(η4–P2C2L), Pt(η4–PN3L), and Pt(η4–PN2OL) [9]. As can be seen, P-donor ligands prevail by far. η4–ligands form 10-, 11-, 12-, 14-, and 16-membered metallocycles. A distorted square planar geometry around Pt(II) atoms with cis-configuration prevails by far.
From an application point of view, multifunctional ligands responsible for secondary catalyst–substrate interactions over the course of a catalytic transformation play increasingly important roles in contemporary catalysis, as has been demonstrated also within these groups of platinum complexes with P-donor ligands. Pincer-type complexes constitute a family of compounds that have recently attracted significant interest. They play important roles in organometallic reactions and mechanisms, catalysis, and the design of new materials (see, e.g., reviews [10][11][12][13][14][15][16]). The high thermal stability of such complexes, particularly those based on an aromatic backbone, permits their use as catalysts at elevated temperatures in various catalytic applications. Bulky bis-chelating pincer-type ligands are effective in the stabilization of highly unsaturated cationic complexes and the stabilization of reactive species [10][11][12][13][14][15][16][17].
2. Pt(η3–P1C2X1C2P2)(Y), (X1 = O1,N1, C1, S1, or Si1)
There are 69 complexes in which heterotridentate organodiphosphines create a pair of “equal” five-membered metallocyclic rings with a common X1 atom. These tridentate ligands with monodentate Y ligands construct a square planar geometry with various degrees of distortion around Pt(II) atoms. These complexes are centrosymmetric. Groups of X1 = O1, N1, or C1 structures, which were mentioned for several representatives in the previous work devoted to any type of n-member metallocycle rings (n = 5,6,7) but different types of atoms between P1 and X1 [18], along with a new group of X1 = S1, Si1 structures, highlighting structural aspects related to distortion.
2.1. Pt(η3–P1O1P2)(P3)
Monoclinic [Pt{η3-Ph2P(C15H12O)PPh2}{η1–P3(C5H4N)(Ph)2}](CF3SO3)2•0.5H2O [19] (at 150 K) is the only example of the P1C2O1C2P2 metallocycle type. The heterotridentate η3-P1O1P2 ligand with monodentate P3L creates a distorted square planar geometry around a Pt(II) atom.
The total mean Pt–L bond distance elongates in the following sequences:
Pt (η3-P1O1P2)(Y), Y = P3L (1 example): Pt–L: 2.189 (3) Å (O1, trans to P3) < 2.239 (2) Å (P3) < 2.302 (2,11) Å (P1,2, mutually trans)
2.2. Pt(η3–P1N1P2)(Y), (Y = N2 L, (x1), CL(x9), Cl(x7), P3L(x2))
There are nineteen examples of the P1C2N1C2P2 metallocyclic type with a common N1 atom. Monoclinic [Pt{η3-Ph2P(C12H8N)PPh2}(N2C5H5)]CF3SO3.toluene [20] is the only example in which a N2 donor ligand completed a square planar geometry around a Pt(II) atom (PtP1N1P2N2). The structure of [Pt{η3-Ph2P(C12H8N)PPh2}(N2C5H5)]+ [20] is shown in Figure 1 as an example.
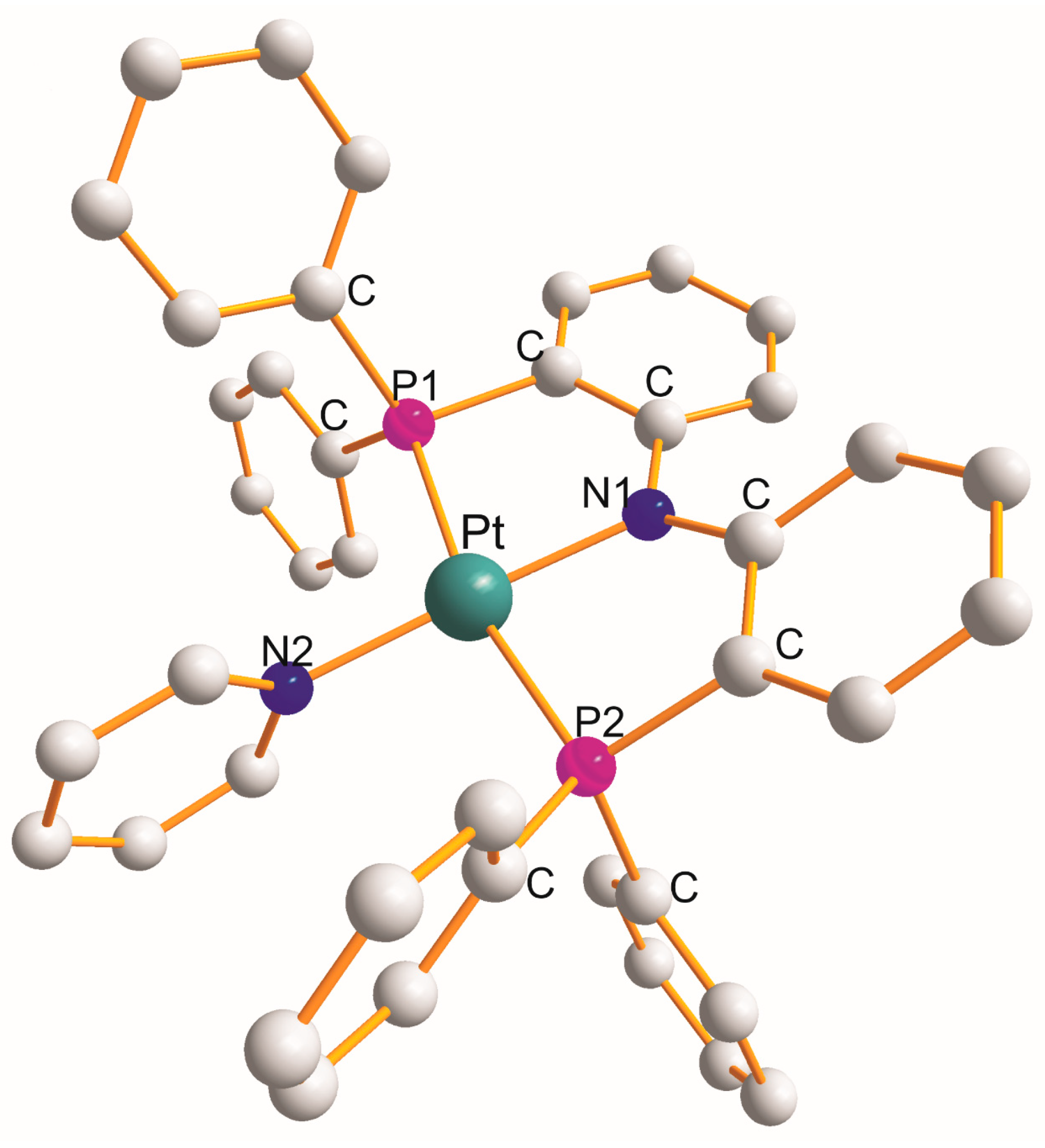
Figure 1. Structure of [Pt{η3-Ph2P(C12H8N)PPh2}(py)].
In the following eight complexes, triclinic [Pt{η3-But2P(C7H7N)PBut2}(CH3)]Cl (at 100 K), monoclinic [Pt{η3-But2P(C7H6N)PBut2}(CH3)] [21] (at 100 K), monoclinic [Pt{η3-Ph2P(C7H7N)PPh2}{C(=O)Et}]BF4.(CH2Cl2)5 [22] (at 100 K), triclinic [Pt{η3-Ph2P(C7H7N)PPh2}(CH2CHO)]BF4 [22], triclinic [Pt{η3-Ph2P(C7H7N)PPh2}(CH=CHPh)]BF4 [23], monoclinic [Pt{η3-Pri2P(C12H7F2N)PPri2}(C6H4F)]B(C6H5)4 (at 110 K) and monoclinic [Pt{η3-Pri2P(C12H7F2N)PPri2}. (p-toluene)]B(C6H5)4 [24] (at 110 K), and monoclinic [Pt{η3-Ph2P(C7H7N)PPri2}(η1-C11H15NO3)]BF4 [25] and a η3-P1N1P2 ligand with a monodentate CL create a distorted square planar geometry around a Pt(II) atom (PtP1N1P2C).
There are seven complexes, monoclinic [Pt{η3-Ph2P(C12H8N)PPh2}(Cl)](C6H6)5 [20], trigonal [Pt{η3-But2P(C7H7N)PBut2}(Cl)]Cl [21], orthorhombic [Pt{η3-But2P(C7H6N)PBut2}(Cl)] [21] (at 120 K), monoclinic [Pt{η3-Pri2P(C12H6F2N)PPri2}(Cl)]CHB11Cl11 [24] (at 110 K), monoclinic [Pt{η3-Ph2P(C14H12N)PPri2}(Cl)]C6H6 [26] (at 183 K) and triclinic [Pt{η3-Ph2P(C14H12N)Pcy2}(Cl)]C6H6 [26] (at 183 K), and monoclinic [Pt{η3-Ph2P(C7H8N)PPh2}(Cl)] [27] in which Cl− anions complete inner coordinate spheres (PtP1N1P2Cl).
In the remaining two complexes, triclinic [Pt{η3-(η2-(C24H44)P(C7H6N)P(C24H44)} (PPh3)].2CH2Cl2 [28] (at 103 K) and monoclinic [Pt{η3-(η2-C18H28)P(C7H6N)P(η2-C18H29)}(P3cy3)] [29] (at 103 K), a monodentate P3L is involved (PtP1N1P2P3).
The total mean Pt-L bond distance elongates in the following sequences:
Pt (η3-P1N1P2)(Y), Y = N2L, CL, Cl, P3L (19 examples): Pt–N1: (trans to Y): 2.024 (3) Å (N2) < 2.077 (2,5) Å (P3) < 2.128 (2,70) Å (C) < 2.201 (3,26) Å (Cl); Pt–Y: (trans to N1): 2.056 (3) Å (N2) < 2.072 (2,85) Å (C) < 2.277 (2,5) Å (P3) < 2.316 (2,17) Å (Cl); Pt–P1,2: (mutually trans) is 2.287 (2,17) Å
2.3. Pt(η3–P1C1P2)(Y), (Y = OL (x4), NL(x4), C2L (x9), Cl (x12), Br (x2))
There are over thirty examples of the P1C2C1C2P2 metallocycle type. In four complexes, monoclinic [Pt{η3-(CF3)2P(C8H7)P(CF3)2}(H2O)].SbF6 [30], triclinic [Pt{η3-Ph2P(C8H7)Ph2P}(H2O)]CF3SO3 [31], triclinic [Pt{η3-Ph2P(C8H7)PPh2}(OMe)]0.5C6H6 [31], and orthorhombic [Pt{η3-Pri2P(C20H11)PPri2}(OOCCF3)] [32] (Figure 2), a monodentate OL ligand completed a square planar geometry (PtP1C1P2O).

Figure 2. Structure of [Pt{η3-Pri2P(C20H11)PPri2}(OOCCF3)].
In four complexes, monoclinic [Pt{η3-(CF3)2P(C8H7)P(CF3)2} (NC5H5)]B(C6H5)4 [30], orthorhombic [Pt{η3-Ph2P(C20H13O4)PPh2}(N≡CCH3)]BF4 [32], monoclinic [Pt{η3-Ph2P(C20H11O2)PPh2}(NC5H5)]Cl}](NC5H5) [33], and monoclinic [Pt{η3-Ph2P(C20H11O4)PPh2} (N≡CCH3)]BF4. CH2Cl2 [33], monodentate NL ligands completed the inner coordination sphere PtP1N1P2N2.
There are nine complexes, tetragonal [Pt{η3-Ph2P(C24H19O2)PPh2}(CN)] [34], triclinic [Pt{η3-(CF3)2P(C8H7)P(CF3)2}(CO)]SbF6 [35], monoclinic [Pt{η3-(CF3)2P(C8H7)P(CF3)2}(CH3)] [35], monoclinic [Pt{η3-Pri2P(C8H7)PPri2}(CO)]CF3SO3 0.5C6H6 [36], monoclinic [Pt{η3-But2P(C8H7)PBut2}(η1-CHOMe)]CF3SO3.thf [36], orthorhombic [Pt{η3-But2P(C12H9)PBut2}(CO)]BF4 [37], monoclinic [Pt{η3-Ph2P(C8H7)PPh2}(η1-C12H19N2)] [38], monoclinic [Pt{η3-Ph2P(C6H7N2)PPh2}(η1-C3F2)] [39], and monoclinic [Pt{η3-Ph2P(C8H7)PPh2}(η1-C12H21N2)]2(BF4) [40], in which a monodentate C2L ligands are involved (PtP1C1P2C2).
In twelve complexes, triclinic [Pt{η3-Ph2P(C20H11O2)PPh2}(Cl)](CH3CN)4 [33], monoclinic [Pt{η3-Ph2P(C24H19O2)PPh2}(Cl)] [34], monoclinic [Pt{η3-(CF3)2P(C8H7)P(CF3)2}(Cl)]1.5C6H14 [35], orthorhombic [Pt{η3-But2P(C8H7)PBut2}(Cl)] [36], monoclinic [Pt{η3-But2P(C12H9)PBut2}(Cl)] [37], monoclinic [Pt{η3-But2P(C8H7)PBut2}(Cl)] [41], triclinic [Pt{η3-Pri2P(C8H7)PPri2}(Cl)] [42], monoclinic [Pt{η3-Ph2P(C14H7)PPh2}(Cl)] [43], monoclinic [Pt{η3-Ph2P(C8H7)PPh2}(Cl)]CH3CN [44], orthorhombic [Pt{η3-Ph2P(C18H11O8)PPh2}(Cl)]CH3CN [44], orthorhombic [Pt{η3-Ph2P(C18H11O8)PPh2}(Cl)]CH2Cl2 [45], and monoclinic [Pt{η3-Pri2P(C20H11)PPri2}(Cl)](CH3CN)2 [46], a Cl− anion completed inner coordination spheres around each Pt(II) atom (PtP1C1P2Cl).
A Br− anion is involved in two monoclinic complexes, [Pt{η3-Ph2P(C8H7)PPh2}(Br)] [47] and [Pt{η3-But2P(C8H7)PBut2}(Br)] [48].
The total mean PL-L bond distance elongates in the following sequences:
Pt (η3-P1C1P2)(Y), Y = OL, NL, C2L Cl, Br (31 examples): Pt–C1: (trans to Y): 2.001 (3,8) Å (N) < 2.027 (2,8) Å (O) ~ 2.027 (2,6) Å (Br) < 2.031 (2,12) Å (Cl) < 2.049 (2) Å (C2); Pt–Y: (trans to C1): 2.065 (7,12) Å (C2) < 2.085 (2,12) Å (N) < 2.132 (2,9) Å (O) < 2.400 (2,16) Å (Cl) < 2.467 (1,10) Å (Br); Pt–P1,2: (mutually trans) is 2.75 (2,12) Å.
2.4. Pt(η3–P1S1P2)(Y), (Y = CH3 (x1), Cl (x2), P3Ph3 (x2), I (x1))
There are six complexes in which each heterotridentate ligand creates a P1C2S1C2P2 metallocycle. Monoclinic [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(CH3)]PF6.CH3CN [49] (at 100 K; Figure 3) is the only example with a (PtP1S1P2C) chromophore. In monoclinic [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(Cl)]PF6.CH3CN [49] and triclinic [Pt{η3-Ph2P(CH2)2S(=O)(CH2)2PPh2}(Cl)]ClO4 [50], the Cl− anion completed a square planar geometry (PtP1S1P2Cl).
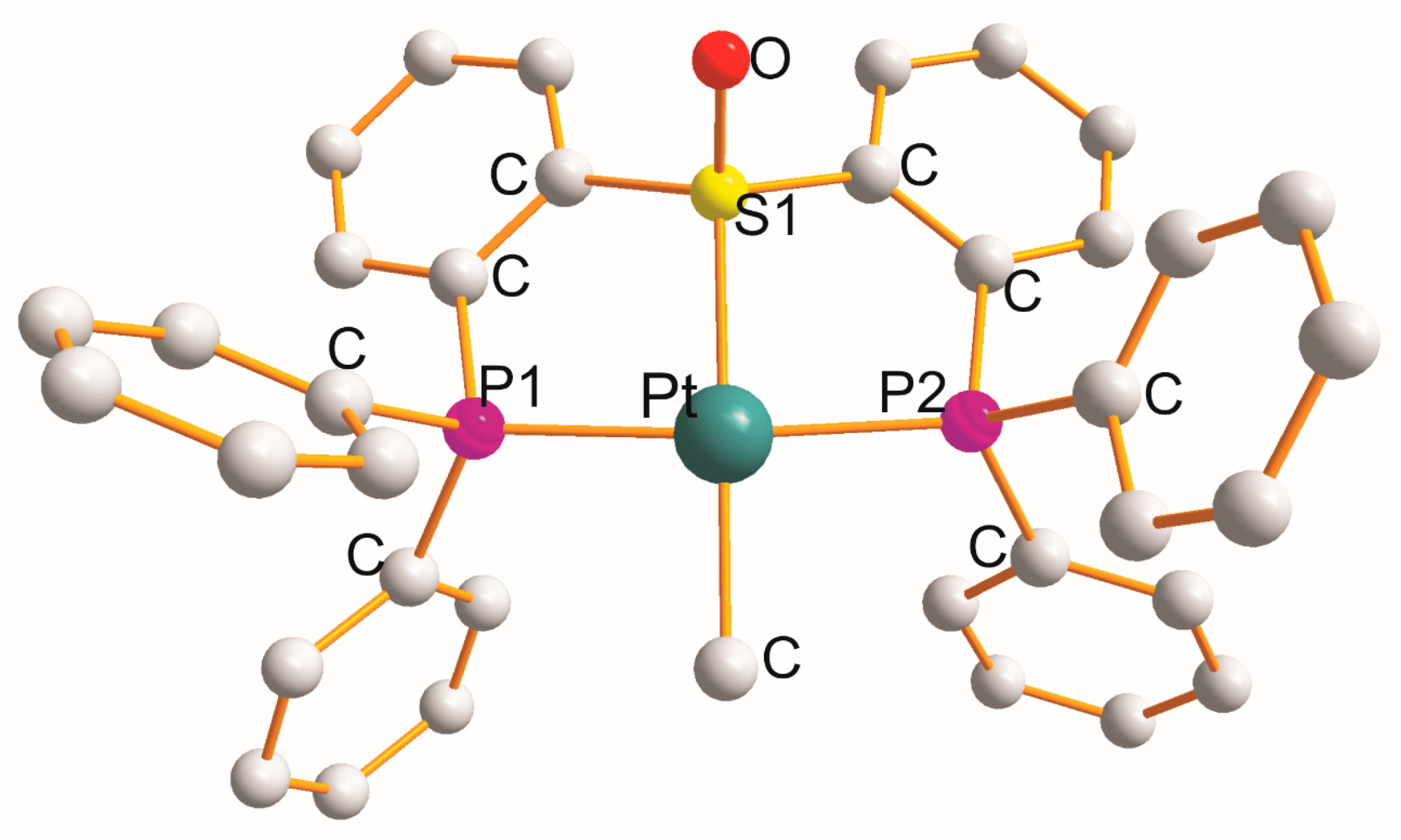
Figure 3. Structure of [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(CH3)].
In triclinic [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(P3Ph3)]0.5.CH2Cl [49] (at 100 K) and orthorhombic [Pt{η3-Ph2P(CH2)2S(CH2)2PPh2}(P3Ph3)]ClO4 [51] (at 100 K), the P3Ph3 are involved (PtP1S1P2P3).
In another triclinic [Pt{η3-Ph2P(C23H28S)PPh2}(I)].1.74 CH2Cl2 [52] (at 150 K), the I− anion is involved (PtP1S1P2I).
The total mean PL-L bond distance elongates in the sequences:
Pt (η3-P1S1P2)(Y), Y = CL, Cl, P3L, I (6 examples): Pt–S1: (trans to Y): 2.187 (2,5) Å (Cl) < 2.256 (2) Å (I) < 2.268 (2) Å (C) < 2.328 (2,15) Å (P3); Pt–Y: (trans to S1): 2.093 (2) Å (C) < 2.285 (2,3) Å (P3) < 2.317 (2,5) Å (Cl) < 2.510 (1) Å (I); Pt–P1,2: (mutually trans) is 2.300 (4,30) Å.
2.5. Pt(η3–P1Si1P2)(Y), (Y = H (x2), OL (x1), NL (x1), CL (x1), Cl (x5), P3L (x1))
There are fourteen complexes in which each heterotridentate ligand creates a pair of “equal” five-membered metallocyclic rings with a common Si1 atom of the P1C2Si1C2P2 type. In two monoclinic [Pt{η3-cy2P(C6H4)Si(Me)(C6H4)Pcy2}(H)].0.5 pentane [53] (at 150 K) and [Pt{η3-cy2P(C6H4)Si(Me)(C6H4)Pcy2}(H)].1.25 pentane [53] (at 93 K), hydride completed a square planar geometry (PtP1Si1P2H).
Triclinic [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(OEt2)]{B(C6F5)3 (CH2Ph)}.OEt2 [54] (Figure 4) is the only example with a monodentate OEt2 ligand (PtP1Si1P2O).
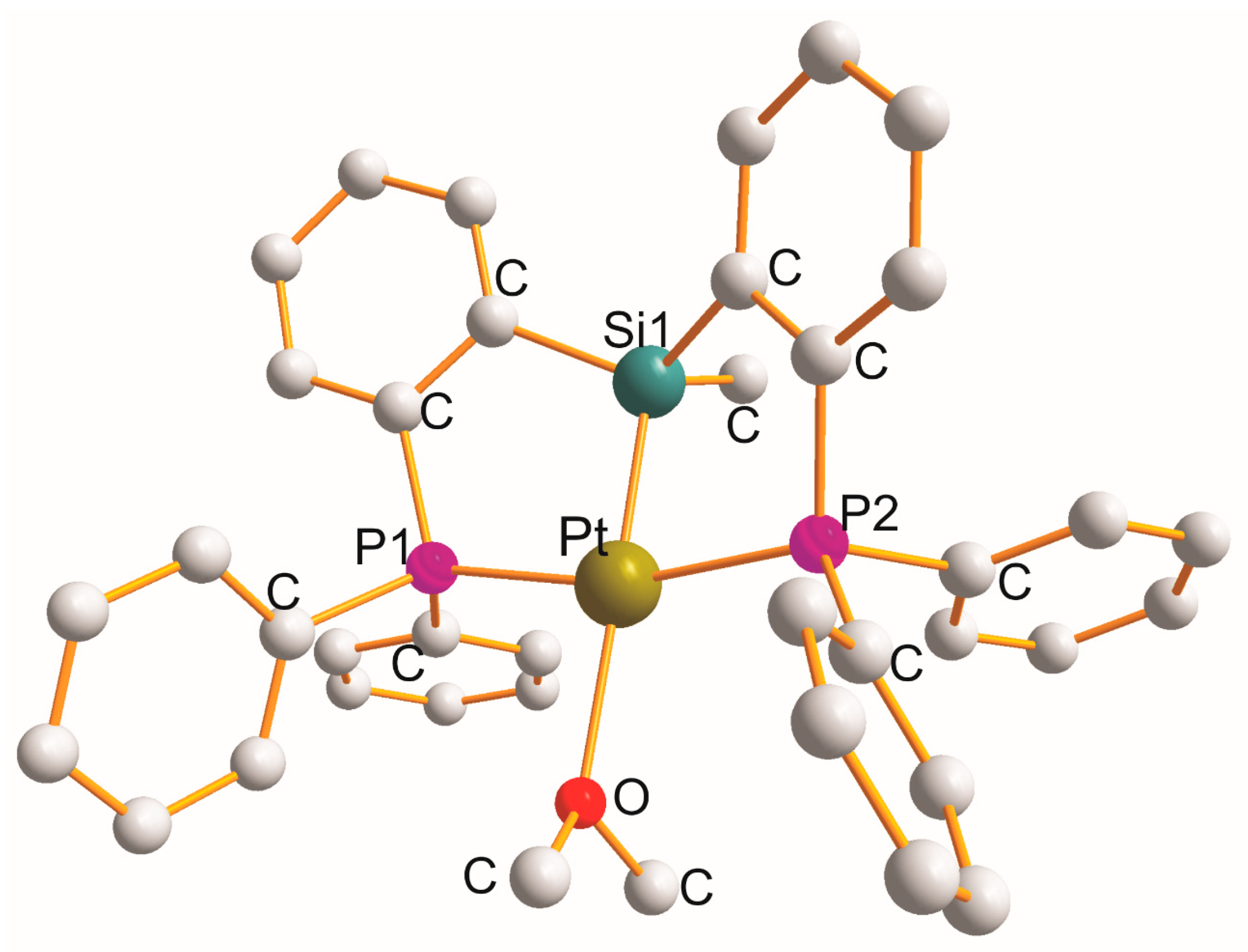
Figure 4. Structure of [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(OEt2)].
In another triclinic [Pt{η3-Pri2P1(C6H4)Si1(C6H4PPri2)(C6H4)P2Pri2}(NC5H5)]B(C8H3F6)4 [55] (at 100 K), a monodentate NC5H5 is involved (PtP1Si1P2N).
In the following four complexes: triclinic [Pt{η3-cy2P(C6H4)Si(Me)(C6H4)Pcy2}(Ph)]OEt2 [54] (at 173 K), triclinic [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(CH2Ph)]CH2Cl2 [54] (at 193 K), triclinic [Pt{η3-Pri2P)(C6H4)Si(OH)(C6H4)PPri2)}(CO)]B(C6F5)4 [56] (at 120 K), and orthorhombic [Pt{η3-Pri2P(C6H4)Si(H)(C6H4)PPri2}(mes)] [57] (at 110 K), monodentate CL ligands are involved (PtP1Si1P2C).
In the following five complexes: monoclinic [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(Cl)] [54] (at173 K), orthorhombic [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(ClAlCl3)](C6H5F)2 [54] (at 193 K), monoclinic [Pt{η3-Pri2P(C6H4)Si(OH)(C6H4)PPri2}(Cl)] [56] (at 120 K), monoclinic [Pt{η3-Pri2P(C6H4)Si(H)(C6H4)Pcy2}(Cl)] [57] (at 110 K), and monoclinic [Pt{η3-cy2P(C6H4)Si(Me)(C6H4)Pcy2}(Cl)] [58] (at 110 K), tridentate P1Si1P2 with Cl− anions construct inner coordination spheres around each Pt(II) atom (PtP1Si1P2Cl).
The total mean PL-L bond distance elongates in the following sequences:
Pt (η3-P1Si1P2)(Y), Y = H, OL, NL, CL, Cl, P3L (19 examples): Pt–Si1: (trans to Y): 2.276 (2) Å (O) < 2.279 (2,6) Å (Cl) < 2.315 (2) Å (N) < 2.331 (2,5) Å (H) < 2.339 (2,17) Å (C) < 2.369 (2) Å (P3); Pt–Y: 1.51 (1,2) Å (H) < 2.122 (2,6) Å (C) < 2.222 (2) Å (N) < 2.282 (2) Å (O) < 2.316 (2) Å (P3) < 2.451 (2,13) Å (Cl); Pt–P1,2: (mutually trans) is 2.289 (2,32) Å.
The structure of monoclinic [Pt{η3-Ph2P1(C6H4)Si1(Me)(C6H4)P2Ph2}(P3Ph3)] [59] (at 123 K) is shown in Figure 5. In a distorted trigonal–pyramidal geometry, three P atoms construct a trigonal plane, and the Si1 atom occupies a pyramid. The heterotridentate P1Si1P2 ligand forms a pair of “equal” five-membered metallocyclic rings with a common Si1 atom of the P1C2Si1C2P2 type, with the mean P1–Pt–Si1/Si1–Pt–P2 bite angles of 83.3 (1,8)°. The values for the remaining angles are 120.7 (2)° (P1–Pt–P2), 119.6 (2,2.4)° (P1–Pt–P3/P3–Pt–P2), and 108.9 (2)° (Si1–Pt–P3). The Pt-L bond distance elongates in the following order: 2.290 (2.11) Å (Pt–P1, Pt–P2) < 2.318 (2) Å (Pt–P3) < 2.369 (2) Å (Pt–Si1). This is the only example of such geometries.
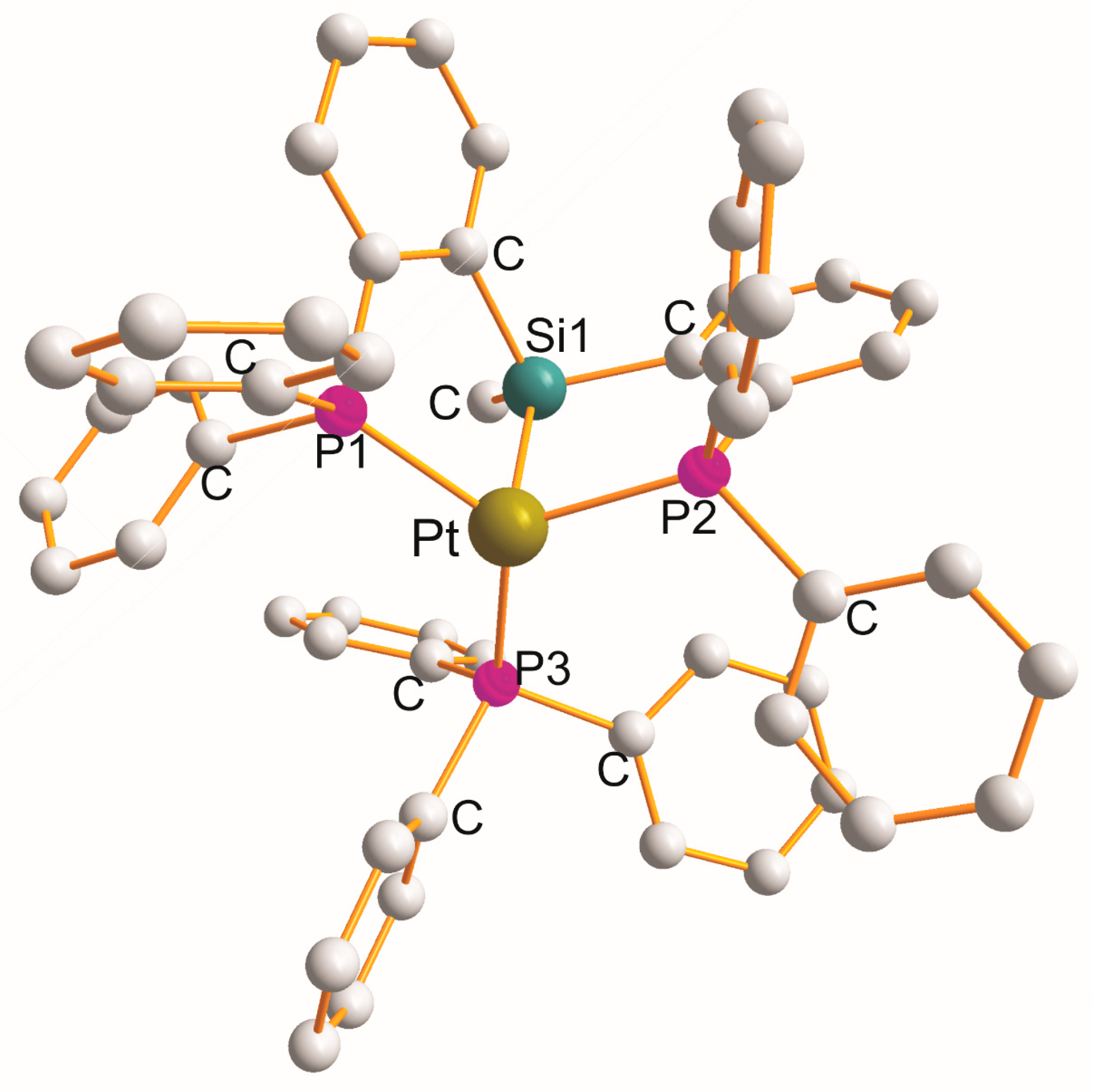
Figure 5. Structure of [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(PPh3)].
References
- Rosenberg, B.; Van Camp, L.; Trasko, Y.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386.
- Ashiq, M.; Danish, M.; Mohsin, M.; Bari, S.; Mukhtar, F. Chemistry of Platinum and Palladium Metal Complexes in Homogeneous and Heterogeneous Catalysis: A Mini Review. Int. J. Sci. Basic. Appl. Res. 2013, 7, 50–61. Available online: http://gssrr.org/index.php?journal=JournalOfBasicAndApplied&page=article&op=view&path%5B%5D=1134 (accessed on 25 May 2023).
- Chaaban, M.; Zhou, C.; Lin, H.; Chyic, B.; Ma, B. Platinum(ii) binuclear complexes: Molecular structures, photophysical properties, and applications. J. Mater. Chem. C 2019, 7, 5910–5924.
- Santos, T.M.R.; Andolpho, G.A.; Tavares, C.A.; Gonçalves, M.A.; Ramalho, T.C. Improving the Path to Obtain Spectroscopic Parameters for the PI3K—(Platinum Complex) System: Theoretical Evidences for Using 195Pt NMR as a Probe. Magnetochemistry 2023, 9, 89.
- Horiuchi, S.; Umakoshi, K. Recent advances in pyrazolato-bridged homo- and heterometallic polynuclear platinum and palladium complexes. Coord. Chem. Rev. 2023, 476, 214924.
- Melník, M.; Mikuš, P. Organomonophosphines in PtP2Cl2 Derivatives: Structural Aspects. Rev. Inorg. Chem. 2015, 35, 179–189.
- Melník, M.; Mikuš, P. Organodiphosphines in PtP2X2 (X = As, Ge or Te) Derivatives—Structural Aspects. Main. Group. Chem. 2020, 43, 132–137.
- Melník, M.; Mikuš, P. Distortion Isomers of Cis-PtP2X2 and Cis-PtP2XY Derivatives—Structural Aspects. Rev. Inorg. Chem. 2020, 40, 153–165.
- Albrecht, M.; van Koten, G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3750.
- van der Boom, M.E.; Milstein, D. Cyclometalated Phosphine-Based Pincer Complexes: Mechanistic Insight in Catalysis, Coordination, and Bond Activation. Chem. Rev. 2003, 103, 1759.
- Rybtchinski, B.; Milstein, D. Metal Insertion into C−C Bonds in Solution. Angew. Chem. Int. Ed. 1999, 38, 870.
- Singleton, J.T. The Uses of Pincer Complexes in Organic Synthesis. Tetrahedron 2003, 59, 1837–1857.
- Vigalok, A.; Milstein, D. Advances in metal chemistry of quinonoid compounds: New types of interactions between metals and aromatics. Acc. Chem. Res. 2001, 34, 798–807.
- Milstein, D. Challenging metal-based transformations. From single-bond activation to catalysis and metallaquinonoids. Pure Appl. Chem. 2003, 75, 445–460.
- Jensen, C.M. Iridium PCP pincer complexes: Highly active and robust catalysts for novel homogeneous aliphatic dehydrogenations. Chem. Commun. 1999, 2443–2449.
- Gandelman, M.; Konstantinovski, L.; Rozenberg, H.; Milstein, D. Interplay between solvent and counteranion stabilization of highly unsaturated rhodium(III) complexes: Facile unsaturation-induced dearomatization. Chem. Eur. J. 2003, 9, 2595–2602.
- Melník, M.; Mikuš, P. Tetradentate organophosphines in Pt(η4–A4L) (A = P4, P3Si), P2X2 (X2 = N2, S2, C2), (PX3) (X3 = N3, N2O): Structural aspects. Main Group Met. Chem. 2021, 44, 270–280.
- Melník, M.; Mikuš, P. Heterotridentate organophosphines in Pt(κ3–P1C1P2)(X), (X=H, OL, NL, CL, Cl, Br or I) and Pt(κ3–P1P2C1)(Cl) derivatives structural aspects. J. Mol. Struct. 2023, 1276, 134768.
- Lenero, K.Q.A.; Guari, Y.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Donnadiere, B.; Sabo-Etienne, S.; Chaudret, B.; Lutz, M.; Spek, A.L. Heterolytic activation of dihydrogen by platinum and palladium complexes. Dalton Trans. 2013, 42, 6495–6512.
- Liang, L.C.; Lin, J.M.; Lee, W.Y. Benzene C–H Activation by Platinum(Ii) Complexes of Bis(2-Diphenylphosphinophenyl)Amide. Chem. Commun. 2005, 19, 2462–2464.
- Feller, M.; Ben-Ari, E.; Iron, M.A.; Diskin-Posner, Y.; Leitus, G.; Shimon, L.J.W.; Konstantinovski, L.; Milstein, D. Cationic, Neutral, and Anionic PNP Pd II and Pt II Complexes: Dearomatization by Deprotonation and Double-Deprotonation of Pincer Systems. Inorg. Chem. 2010, 49, 1615–1625.
- Carlisle, S.; Matta, A.; Valles, H.; Bracken, J.B.; Miranda, M.; Yoo, J.; Hahn, C. Addition to Alkynes Promoted by a Dicationic Platinum(II) Complex. Organometallics 2011, 30, 6446–6457.
- Hahn, C. Structural Investigations of Platinum(II) Styrene and Styryl Complexes and Mechanistic Study of Vinylic Deprotonation. Organometallics 2010, 29, 1331–1338.
- DeMott, J.C.; Bhuvanesh, N.; Ozerov, O.V. Frustrated Lewis Pair-like Splitting of Aromatic C–H Bonds and Abstraction of Halogen Atoms by a Cationic +. Species. Chem. Sci. 2013, 4, 642–649.
- Cucciolito, M.E.; D’Amora, A.; Tuzi, A.; Vitagliano, A. Catalytic Hydroarylation of Olefins Promoted by Dicationic Platinum(II) and Palladium(II) Complexes. The Interplay of C−C Bond Formation and M−C Bond Cleavage. Organometallics 2007, 26, 5216–5223.
- Lansing, R.B.; Goldberg, K.I.; Kemp, R.A. Unsymmetrical RPNPR′ Pincer Ligands and Their Group 10 Complexes. Dalton Trans. 2011, 40, 8950–8958.
- Sacco, A.; Vasapollo, G.; Nobile, C.F.; Piergiovanni, A.; Pellinghelli, M.A.; Lanfranchi, M. Syntheses and Structures of 2-Diphenylphosphinomethylenide-6-Diphenylphosphinomethylenepyridine Complexes of Palladium(II) and Platinum(II); Crystal Structures of and . J. Organomet. Chem. 1988, 356, 397–409.
- Takeuchi, K.; Taguchi, H.; Tanigawa, I.; Tsujimoto, S.; Matsuo, T.; Tanaka, H.; Yoshizawa, I.K.; Ozawa, F. A Square-Planar Complex of Platinum(0). Angew. Chem. Int. Ed. Engl. 2016, 55, 15347–15350.
- Taguchi, H.; Chang, Y.H.; Takeuchi, K.; Ozawa, F. Catalytic Synthesis of an Unsymmetrical PNP-Pincer-Type Phosphaalkene Ligand. Organometallics 2015, 34, 1589–1596.
- Adams, J.J.; Arulsamy, N.; Roddick, D.M. Acceptor Pincer Coordination Chemistry of Platinum: Reactivity Properties of (CF3 PCP)Pt(L) + (L = NC5F5, C2H4). Organometallics 2009, 28, 1148–1157.
- Arunachalampillai, A.; Loganathan, N.; Wendt, O.F. Carboxylation of Pincer PCP Platinum Methoxide Complexes under Formation of Metalla Carbonates. Polyhedron 2012, 32, 24–29.
- Musa, S.; Shpruhman, A.; Gelman, D. New PC(sp3)P pincer complexes of platinum and palladium. J. Organomet. Chem. 2012, 699, 92–95.
- De-Botton, S.; Romm, R.; Bensoussan, G.; Hitrik, M.; Musa, S.; Gelman, D. Coordination Versatility of P-Hydroquinone-Functionalized Dibenzobarrelene-Based PC(Sp3)P Pincer Ligands. Dalton Trans. 2016, 45, 16040–16046.
- Jia, Y.X.; Yang, X.Y.; Tay, W.S.; Li, Y.; Pullarkat, S.A.; Xu, K.; Hirao, H.; Leung, P.H. Computational and Carbon-13 NMR Studies of Pt–C Bonds in P–C–P Pincer Complexes. Dalton Trans. 2016, 45, 2095–2101.
- Adams, J.J.; Lau, A.; Arulsamy, N.; Roddick, D.M. Synthesis and Platinum Coordination Chemistry of the Perfluoroalkyl Acceptor Pincer Ligand, 1,3-(CH2P(CF3)2)2C6H4. Inorg. Chem. 2007, 46, 11328–11334.
- Vuzman, D.; Poverenov, E.; Diskin-Posner, Y.; Leitus, G.; Shimon, L.J.W.; Milstein, D. Reactivity and Stability of Platinum(Ii) Formyl Complexes Based on PCP-Type Ligands. The Significance of Sterics. Dalton Trans. 2007, 48, 5692–5700.
- Schwartsburd, L.; Poverenov, E.; Shimon, L.J.W.; Milstein, D. Naphthyl-Based PCP Platinum Complexes. Nucleophilic Activation of Coordinated CO and Synthesis of a Pt(II) Formyl Complex. Organometallics 2007, 26, 2931–2936.
- Spek, A.L.; Dani, P.; van Koten, G. Crystal Structure of . Private Communication CCDB, IXICEX (2004). Available online: https://www.ccdc.cam.ac.uk/ (accessed on 28 April 2023).
- Hughes, R.P.; Williamson, A.; Incarvito, C.D.; Rheingold, A.L. Synthesis and Molecular Structure of a Perfluoroalkyl Complex of Platinum Containing a PCP Pincer Ligand. Organometallics 2001, 20, 4741–4744.
- Albrecht, M.; Dani, P.; Lutz, M.; Spek, A.L.; van Koten, G. Transcyclometalation Processes with Late Transition Metals: C-aRyl−H Bond Activation via Noncovalent C−H···Interactions. J. Am. Chem. Soc. 2000, 122, 11822–11833.
- Olsson, D.; Arunachalampillai, A.; Wendt, O.F. Synthesis and Characterisation of PCsp3P Phosphine and Phosphinite Platinum(Ii) Complexes. Cyclometallation and Simple Coordination. Dalton Trans. 2007, 46, 5427–5433.
- Poverenov, E.; Leitus, G.; Shimon, L.J.W.; Milstein, D. C-Metalated Diazoalkane Complexes of Platinum Based on PCP- and PCN-Type Ligands. Organometallics 2005, 24, 5937–5944.
- Fischer, S.; Wendt, O.F. chloroplatinum(II). Acta Cryst. Sect. E Struct. Rep. Online 2004, 60, m69–m70.
- Hu, J.; Xu, H.; Nguyen, M.H.; Yip, J.H.K. Photooxidation of a Platinum-Anthracene Pincer Complex: Formation and Structures of Pt II -Anthrone and -Ketal Complexes. Inorg. Chem. 2009, 48, 9684–9692.
- Yang, X.Y.; Tay, W.S.; Li, Y.; Pullarkat, S.A.; Leung, P.H. Versatile Syntheses of Optically Pure PCE Pincer Ligands: Facile Modifications of the Pendant Arms and Ligand Backbones. Organometallics 2015, 34, 1582–1588.
- Azerraf, C.; Gelman, D. New Shapes of PC(Sp3)P Pincer Complexes. Organometallics 2009, 28, 6578–6584.
- Bachechi, F. X-Ray Structural Analysis of NiII, PdII, and PtII Complexes with the Potentially Tridentate Ligand 1,3-Bis(Diphenylphosphinomethyl)Benzene, 1,3-C6H4(CH2PPh2)2. Struct. Chem. 2003, 14, 263–269.
- Williams, B.S.; Dani, P.; Lutz, M.; Spek, A.L.; van Koten, G. Development of the First P-Stereogenic PCP Pincer Ligands, Their Metallation by Palladium and Platinum, and Preliminary Catalysis. Helv. Chim. Acta 2001, 84, 3519–3530.
- Suess, D.L.M.; Peters, J.C. Late-Metal Diphosphinosulfinyl S(O)P 2 Pincer-Type Complexes. Organometallics 2012, 31, 5213–5222.
- Siah, S.Y.; Leung, P.H.; Mok, K.F. Palladium(II) and Platinum(II) Complexes with a Novel P—S(O)—P Tridentate Ligand. Polyhedron 1994, 13, 3253–3255.
- Andreasen, L.V.; Hazell, A.; Wernberg, O. (Triphenylphosphine-κP)Platinum(II) Diperchlorate Acetone Solvate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2002, 58, m385–m387.
- Emslie, D.J.H.; Harrington, L.E.; Jenkins, H.A.; Robertson, C.M.; Britten, J.F. Group 10 Transition-Metal Complexes of an Ambiphilic PSB-Ligand: Investigations into η3(BCC)-Triarylborane Coordination. Organometallics 2008, 27, 5317–5325.
- Suh, H.W.; Balcells, D.; Edwards, A.J.; Guard, L.M.; Hazari, N.; Mader, E.A.; Mercado, B.Q.; Repisky, M. Understanding the Solution and Solid-State Structures of Pd and Pt PSiP Pincer-Supported Hydrides. Inorg. Chem. 2015, 54, 11411–11422.
- Mitton, S.J.; McDonald, R.; Turculet, L. Synthesis and Characterization of Neutral and Cationic Platinum(II) Complexes Featuring Pincer-like Bis(Phosphino)Silyl Ligands: Si−H and Si−Cl Bond Activation Chemistry. Organometallics 2009, 28, 5122–5136.
- Tsay, C.; Mankad, N.P.; Peters, J.C. Four-Coordinate, Trigonal Pyramidal Pt(II) and Pd(II) Complexes. J. Am. Chem. Soc. 2010, 132, 13975–13977.
- Korshin, E.E.; Leitus, G.; Shimon, L.J.W.; Konstantinovski, L.; Milstein, D. Silanol-Based Pincer Pt(II) Complexes: Synthesis, Structure, and Unusual Reactivity. Inorg. Chem. 2008, 47, 7177–7189.
- DeMott, J.C.; Gu, W.; McCulloch, B.J.; Herbert, D.E.; Goshert, M.D.; Walensky, J.R.; Zhou, J.; Ozerov, O.V. Silyl–Silylene Interplay in Cationic PSiP Pincer Complexes of Platinum. Organometallics 2015, 34, 3930–3933.
- Brost, R.D.; Bruce, G.C.; Joslin, F.L.; Stobart, S.R. Phosphinoalkylsilyl Complexes. 12. Stereochemistry of the Tridentate Bis(Diphenylphosphinopropyl)Silyl (BiPSi) Framework: Complexation That Introduces “Face Discrimination” at Coordinatively Unsaturated Metal Centers. X-Ray Crystal and Molecular Structure. Organometallics 1997, 16, 5669–5680.
- Takaya, J.; Iwasawa, N. Reaction of Bis(o-Phosphinophenyl)Silane with M(PPh3)4(M = Ni, Pd, Pt): Synthesis and Structural Analysis of H2-(Si–H) Metal(0) and Pentacoordinate Silyl Metal(Ii) Hydride Complexes of the Ni Triad Bearing a PSiP-Pincer Ligand. Dalton Trans. 2011, 40, 8814–8821.
More
Information
Subjects:
Crystallography
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
597
Revisions:
2 times
(View History)
Update Date:
05 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





