Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Peter Mikuš and Version 2 by Rita Xu.
Structural data are classified and analyzed for almost seventy complexes of the general formula Pt(η
3
–P
1
X
1
P
2
)(Y) (X
1
= O, N, C, S, Si) and (Y = various monodentate ligands), in which the respective η
3
–P
1
X
1
P
2
ligand forms a pair of five-membered metallocyclic rings with a common X
1
atom of the P
1
C
2
X
1
C
2
P
2
type.
- heterotridentate
- Pt(η3–P1C2X1C2P2)(Y)
- trans-influence
1. Introduction
The chemistry of platinum coordination complexes has been intensively studied and developed for more than five decades, focusing on the relationship between structure and reactivity. The chemistry of platinum is important in the fields of biochemistry [1], catalysis [2], spectroscopy [3][4][3,4], and coordination theory. Very recently, Horiuchi and Umakoshi published a review that focused on the importance of and advances in the synthetic, structural, thermodynamic, electronic, and photophysical properties of Pt-based heteropolynuclear complexes [5].
Significant attention has been paid to organomonophosphines, representing soft donor ligands in the chemistry of platinum. There are a large number of published structural studies on such complexes that have been classified and analyzed [6]. Another group of related structural studies is devoted to Pt(II) complexes with organodiphosphines [7][8][7,8]. Recently, rwesearchers analyzed and classified structural data for the following compositions: Pt(η4–P4L), Pt(η4–P3 SiL), Pt(η4–P2N2L), Pt(η4–P2S2L), Pt(η4–P2C2L), Pt(η4–PN3L), and Pt(η4–PN2OL) [9]. As can be seen, P-donor ligands prevail by far. η4–ligands form 10-, 11-, 12-, 14-, and 16-membered metallocycles. A distorted square planar geometry around Pt(II) atoms with cis-configuration prevails by far.
From an application point of view, multifunctional ligands responsible for secondary catalyst–substrate interactions over the course of a catalytic transformation play increasingly important roles in contemporary catalysis, as has been demonstrated also within these groups of platinum complexes with P-donor ligands. Pincer-type complexes constitute a family of compounds that have recently attracted significant interest. They play important roles in organometallic reactions and mechanisms, catalysis, and the design of new materials (see, e.g., reviews [10][11][12][13][14][15][16][10,11,12,13,14,15,16]). The high thermal stability of such complexes, particularly those based on an aromatic backbone, permits their use as catalysts at elevated temperatures in various catalytic applications. Bulky bis-chelating pincer-type ligands are effective in the stabilization of highly unsaturated cationic complexes and the stabilization of reactive species [10][11][12][13][14][15][16][17][10,11,12,13,14,15,16,17].
2. Pt(η3–P1C2X1C2P2)(Y), (X1 = O1,N1, C1, S1, or Si1)
There are 69 complexes in which heterotridentate organodiphosphines create a pair of “equal” five-membered metallocyclic rings with a common X1 atom. These tridentate ligands with monodentate Y ligands construct a square planar geometry with various degrees of distortion around Pt(II) atoms. These complexes are centrosymmetric. Groups of X1 = O1, N1, or C1 structures, which were mentioned for several representatives in theour previous work devoted to any type of n-member metallocycle rings (n = 5,6,7) but different types of atoms between P1 and X1 [18][18], are analyzed in detail in this work, along with a new group of X1 = S1, Si1 structures, highlighting structural aspects related to distortion.2.1. Pt(η3–P1O1P2)(P3)
Monoclinic [Pt{η3-Ph2P(C15H12O)PPh2}{η1–P3(C5H4N)(Ph)2}](CF3SO3)2•0.5H2O [19] (at 150 K) is the only example of the P1C2O1C2P2 metallocycle type. The heterotridentate η3-P1O1P2 ligand with monodentate P3L creates a distorted square planar geometry around a Pt(II) atom. The total mean Pt–L bond distance elongates in the following sequences: Pt (η3-P1O1P2)(Y), Y = P3L (1 example): Pt–L: 2.189 (3) Å (O1, trans to P3) < 2.239 (2) Å (P3) < 2.302 (2,11) Å (P1,2, mutually trans)2.2. Pt(η3–P1N1P2)(Y), (Y = N2 L, (x1), CL(x9), Cl(x7), P3L(x2))
There are nineteen examples of the P1C2N1C2P2 metallocyclic type with a common N1 atom. Monoclinic [Pt{η3-Ph2P(C12H8N)PPh2}(N2C5H5)]CF3SO3.toluene [20] is the only example in which a N2 donor ligand completed a square planar geometry around a Pt(II) atom (PtP1N1P2N2). The structure of [Pt{η3-Ph2P(C12H8N)PPh2}(N2C5H5)]+ [20] is shown in Figure 1 as an example.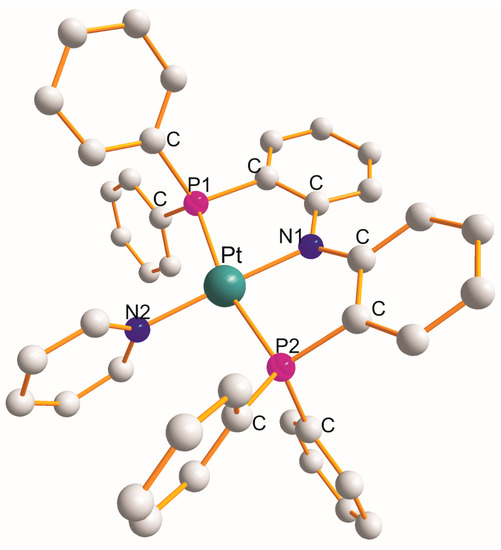
Figure 1. Structure of [Pt{η3-Ph2P(C12H8N)PPh2}(py)].
2.3. Pt(η3–P1C1P2)(Y), (Y = OL (x4), NL(x4), C2L (x9), Cl (x12), Br (x2))
There are over thirty examples of the P1C2C1C2P2 metallocycle type. In four complexes, monoclinic [Pt{η3-(CF3)2P(C8H7)P(CF3)2}(H2O)].SbF6 [30], triclinic [Pt{η3-Ph2P(C8H7)Ph2P}(H2O)]CF3SO3 [31], triclinic [Pt{η3-Ph2P(C8H7)PPh2}(OMe)]0.5C6H6 [31], and orthorhombic [Pt{η3-Pri2P(C20H11)PPri2}(OOCCF3)] [32] (Figure 2), a monodentate OL ligand completed a square planar geometry (PtP1C1P2O).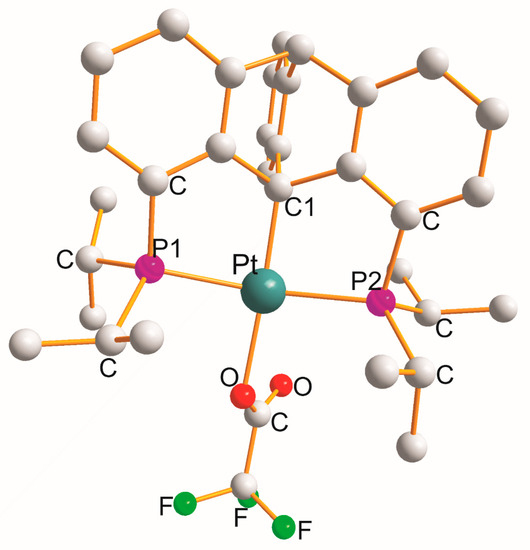
Figure 2. Structure of [Pt{η3-Pri2P(C20H11)PPri2}(OOCCF3)].
2.4. Pt(η3–P1S1P2)(Y), (Y = CH3 (x1), Cl (x2), P3Ph3 (x2), I (x1))
There are six complexes in which each heterotridentate ligand creates a P1C2S1C2P2 metallocycle. Monoclinic [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(CH3)]PF6.CH3CN [49][48] (at 100 K; Figure 3) is the only example with a (PtP1S1P2C) chromophore. In monoclinic [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(Cl)]PF6.CH3CN [49][48] and triclinic [Pt{η3-Ph2P(CH2)2S(=O)(CH2)2PPh2}(Cl)]ClO4 [50][49], the Cl− anion completed a square planar geometry (PtP1S1P2Cl).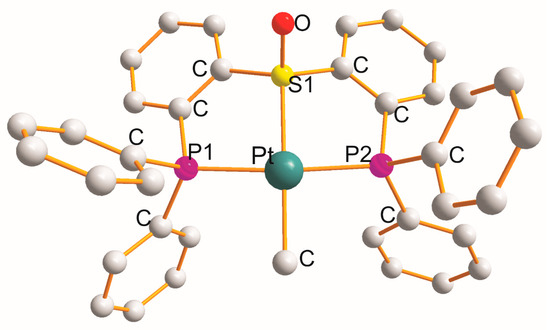
Figure 3. Structure of [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(CH3)].
2.5. Pt(η3–P1Si1P2)(Y), (Y = H (x2), OL (x1), NL (x1), CL (x1), Cl (x5), P3L (x1))
There are fourteen complexes in which each heterotridentate ligand creates a pair of “equal” five-membered metallocyclic rings with a common Si1 atom of the P1C2Si1C2P2 type. In two monoclinic [Pt{η3-cy2P(C6H4)Si(Me)(C6H4)Pcy2}(H)].0.5 pentane [53][52] (at 150 K) and [Pt{η3-cy2P(C6H4)Si(Me)(C6H4)Pcy2}(H)].1.25 pentane [53][52] (at 93 K), hydride completed a square planar geometry (PtP1Si1P2H). Triclinic [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(OEt2)]{B(C6F5)3 (CH2Ph)}.OEt2 [54][53] (Figure 4) is the only example with a monodentate OEt2 ligand (PtP1Si1P2O).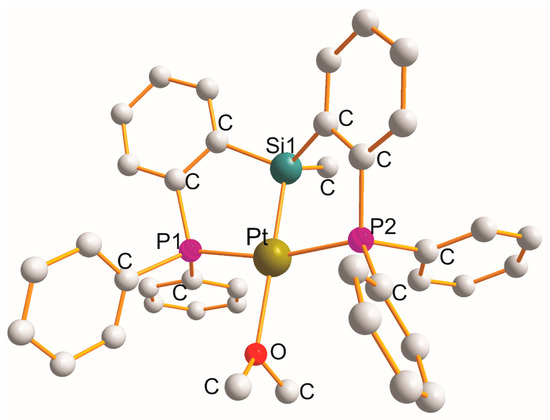
Figure 4. Structure of [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(OEt2)].
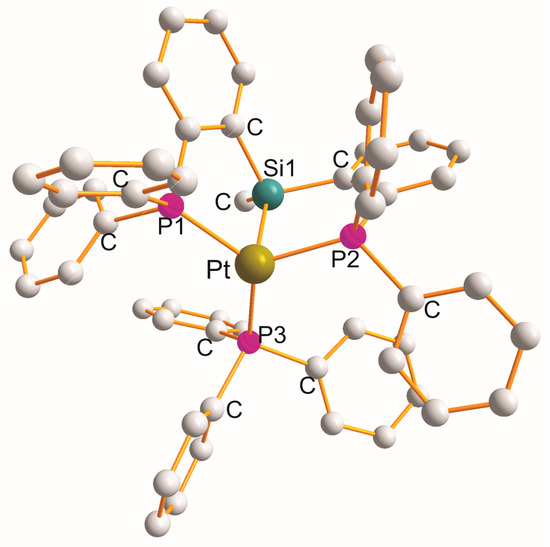
Figure 5. Structure of [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2}(PPh3)].
