Continuous industrial advancement has become the primary sources of lethal waste into the land and water sources. Mining of minerals, hefty input in farming exercises, armed force and military preparation, and the development of residue caused because of human exercises have been other noteworthy components of soil contamination in the course of the most recent not many decades [1]. The oil business and related items are additionally significant sources of soil pollution. Inappropriate management results in the release of these contaminants into the environment without proper treatments.
Surfactant-enhanced soil remediation technology offers numerous advantages over conventional soil remediation techniques. One key advantage is its effectiveness in removing a wide range of contaminants from the soil. Surfactants, or surface-active agents, have unique properties that enable them to break down and solubilize various pollutants, including hydrocarbons, heavy metals, and pesticides. This versatility makes surfactant-enhanced soil remediation applicable to a broad spectrum of soil contamination scenarios. Another significant advantage is the ability of surfactants to increase the availability and mobility of contaminants in the soil (
Table 1). By reducing the interfacial tension between the contaminant and soil particles, surfactants enhance the desorption and solubility of contaminants, allowing them to be more easily extracted or degraded. This process significantly accelerates the remediation timeframe, reducing the overall cost and effort required to clean up the contaminated soil. Surfactant-enhanced soil remediation technology is also highly compatible with other remediation methods. It can be integrated with techniques such as soil washing, soil vapor extraction, and bioremediation to enhance their efficiency. The surfactants facilitate the release of contaminants from the soil matrix, making them more amenable to extraction or degradation by other remediation methods. This synergy allows for a more comprehensive and effective approach to soil remediation. This method is relatively non-invasive and environmentally friendly compared to some alternative methods. It minimizes the need for soil excavation or transportation, reducing disruption to the site and the associated costs. The surfactants used in the process are typically biodegradable and pose minimal risk to human health and ecosystems when applied appropriately
[3][4][5].
Table 1. Comparative performances of surfactants in soil remediation
[5].
Soil Source/
Contaminated Sites |
Soil Texture |
Scale of Remediation |
Major Contaminants |
Surfactant and Use |
Effectiveness of
Remediation |
Agricultural soil from Crete
island, Greece |
56% sand, 35.5% silt, and
8.5% clay |
Laboratory |
Cd (II) |
10-2 M SDS, 38 V
electrokinetic leaching for
18 days |
94% Removal efficiency of
Cd after 18 days |
Heavy-metal- contaminated soil from a
metallurgy plant, Mexico |
39% clay, 36% loam, and
24% sand |
Laboratory |
Heavy metals like Cd, Zn,
Cu, Ni |
20 mL 0.5%
Texapon-40
mixed with 6 g soil, 24 h
stirring |
Cd, Ni, and Zn were
removed by 83.2%, 82.8%,
and 86.6% |
Organics-contaminated soil
in Pyeongtaek, Korea |
Sandy soil with 0.8% clay |
Laboratory |
1,2,4-trichlorobenzene
(TCB) |
4 wt% SDS + 10 wt% NaCl,
the volume of leachate
was 3750 mL |
97% Removal efficiency for
TCB |
Soil from the campus of
Nankai University,
Tianjin, China |
|
Laboratory |
Aldicarb (carbamate
pesticide) |
50 mL HTAB (200 mg/L) to
5 g contaminated soil |
56% Desorption ratio of
aldicarb |
Clay soil collected from
Manitoba Province,
Canada |
Crushed and screened clay
soil |
Laboratory |
Benzene series,
naphthalene and
phenanthrene |
1.5% (w/w) CTAB, the
hydraulic gradient was 2.8 |
Organic pollutants were
removed by 58.8–98.9% |
Fuel-oil-contaminated soil
near Algiers, Algeria |
94% silt, 2.4% sand, and
2.9% clay |
Field demonstration |
Diesel |
8 mM SDS, 48 h leaching at
3.2 mL/min flow velocity |
97% Removal efficiency for
diesel |
Underground storage tank
site in Oklahoma |
Sandy silt, silty clay, and
silt |
Full-scale remediation |
Diesel fuel and gasoline
fuel NAPL |
AOT/Calfax 16 L-35
(0.94 wt% total
concentration) 0.2–0.4 wt%
NaCl |
75–99% Benzene reduction,
65–99% TPH reduction |
An incinerator plant in
Czech Republic |
80% sand, 17% silt, and 3%
clay |
Field demonstration |
PCBs |
Spolapon AOS 146 solution
(40 g/L CMC value) |
56% Efficacy for PCBs
decontamination |
Alameda Point Naval Air
Station Site, Alameda, CA |
Homogeneous
sands and clay |
Field demonstration |
DNAPL, especially TCA and
TCE |
Dowfax (5 wt%), sodium
dihexyl sulfosuccinate
(2 wt%), NaCl and CaCl2 |
95% DNAPL removal and
93% surfactant recovery |
Millican Field, Pearl
Harbor, Hawaii |
Geological layers of highly
fractured volcanic tuff |
Field demonstration |
Petroleum, LNAPLS |
4 wt% Isalchem 123 (PO) 7.7
sodium ether sulfate with
8% SBA cosolvent |
87.5% of the LNAPL in soil
was recovered |
Chevron Cincinnati Facility
in Hooven, OH |
Fine sand and silt, clay |
Full-scale remediation |
BTEX, LNAPLs |
Mixture of Alfoterra
123-4-PO sulfate, 8%
2-butanol, Emcol-CC-9 and
calcium chloride |
LNAPL reduced from 8% to
less than 1% residual
saturation |
2. Petroleum Hydrocarbons (PHC)
Petroleum is a notable environmental pollutant that is commonly found in industrial waste, leaking fuel tanks, and crude oil spills. PHCs get into the upper and lower layers of soil due to unintended fuel or crude oil leakage from the vast underground pipeline networks. The more water-soluble PHCs from the leakage can easily penetrate into the subsoils and shallow aquifers, thus forming contaminated plumes. In addition, most of the spilled PHCs remain in a non-aqueous, liquid form, or as residuals, leading to displacement of the air and water spaces in the soil matrix
[3]. Petroleum hydrocarbons have a considerable effect on the chemical, physical, and microbial characteristics of soil by inducing nitrogen fixation and creation of organic matter. The increased amount of organic matter in the soil leads to deflocculation and consequently decreases soil texture, making it more prone to erosion
[4].
The specific composition of petroleum hydrocarbons (PHCs) may differ from place to place, but their negative characteristics remain the same. Substances that are highly hazardous and of great concern include benzene and polycyclic aromatic hydrocarbons (PAHs). The threat of exposure through skin contact or ingestion is proportional to the capability of a particular element to adhere to soil particles and enter vegetation through root absorption, which may then enter the food chain. Furthermore, a compound’s ability to evaporate directly from the soil or through contaminated water sources due to its release is linked to the risk of inhalation. In addition, a chemical’s solubility and density have an effect on surface or subterranean water sources. Human health is jeopardized by the intake of a chemical mixture that evaporates from polluted soil.
Benzene has been linked to an increased risk of leukemia at certain levels of exposure. In addition, exposure to certain chlorinated solvents can lead to depression of the central nervous system, damage to the liver and kidneys, as well as skin rashes, headaches, nausea, fatigue, and eye irritation. The United States Environmental Protection Agency (USEPA) has emphasized that contact, inhalation, or ingestion of soil toxins can be fatal in extreme cases. Prolonged inhalation of toluene concentrations of more than 100 ppm can cause headaches, fatigue, nausea, and drowsiness
[5].
3. Agrochemicals (Pesticides, Herbicides, and Fertilizers)
Nowadays, agrochemicals are commonly employed in agricultural production to maximize crop growth by destroying damaging insects, diseases, and unwelcome weeds. However, such use of agrochemicals has become a potential threat to food safety, human and environmental health, ecological equilibrium, and preserving soil biodiversity. In the long-term, if not used properly, agrochemicals can lead to a change in population of beneficial bacteria, which can result in the emergence of antibiotic resistance. The use of agrochemicals in farming systems can have a detrimental effect on soil microorganisms that are primarily involved in nutrient cycling processes, such as nitrogen fixation, releasing phosphorus, and other essential nutrient transformations. The long-term effects of agrochemicals can have a detrimental impact on both land and sea creatures. Several health issues, such as intense poisoning, skin complications, endocrine disruption, birth defects, miscarriages, fertility issues, and reduced sperm count, have been linked to the exposure to such chemicals. Additionally, common side effects, including itching, eye irritation, vision problems, nausea and dehydration, have been documented. Additionally, exposure to large doses of pesticides has been known to delay pregnancy in women
[6].
4. Polycyclic or Polynuclear Aromatic Hydrocarbons (PAHs)
Polycyclic aromatic hydrocarbons (PAHs) and polynuclear aromatic hydrocarbons (PAHs) are comprised of merely carbon (C) and hydrogen (H) atoms despite the fact that nitrogen, sulfur, and oxygen atoms can be swapped and generate heterocyclic aromatic compounds inside the aromatic benzene ring. These molecules are formed by pyrolyzing organic material and burning it inefficiently. The emission of PAHs into the atmosphere is caused by both natural and human activities, such as industrial burning of fossil fuels, petroleum catalytic cracking, residential wood burning, volcanic activities, vehicle emissions, and forest fires. The hydrophobic nature of PAHs, which are carcinogenic micropollutants, stops them from being broken down in the environment. Growing concerns about their detrimental health impacts has propelled the execution of numerous studies on the remediation of PAHs contaminated soil
[7].
5. Chlorinated Solvents
Different types of solvents such as halogenated non-polar aromatics, aliphatic, heterocyclics, and other polar organic compounds are utilized in various industrial and commercial purposes like dry cleaning, making adhesives, and cleaning and degreasing metal surfaces. The most commonly used chlorinated solvents include trichloroethylene, trichloroethane, methyl chloride, and tetrachloroethylene. Unfortunately, due to improper use, handling, and disposal, these solvents have contaminated both land and groundwater, leading to decreased soil fertility and nitrogen fixation
[8].
6. Asbestos
Asbestos is a mineral that is found in nature and was used in a variety of products, including construction materials, for a long time. Unfortunately, exposure to asbestos can lead to severe illnesses, like lung cancer and mesothelioma. Even though it is mostly linked to its application as insulation, asbestos can also be present in soil. Contamination of the soil with asbestos can happen when materials that contain the mineral are not disposed of correctly or when natural deposits of asbestos are disturbed. The fibers can become airborne, posing a risk for those living in the vicinity or working there.
For years, it has been extensively established that asbestos inhalation has detrimental effects on human health. As serpentinite and metabasite rocks are the chief source of asbestos, many studies have centered on studying their mineralogical and geochemical composition (NOA). Additionally, the soil derived from these rocks should also be studied as it reflects the mineralogical and geochemical makeup of the parent rocks and could contain hazardous fibers. Asbestos inhalation can lead to lung scarring, lung cancer, and mesothelioma
[9].
7. Heavy Metals
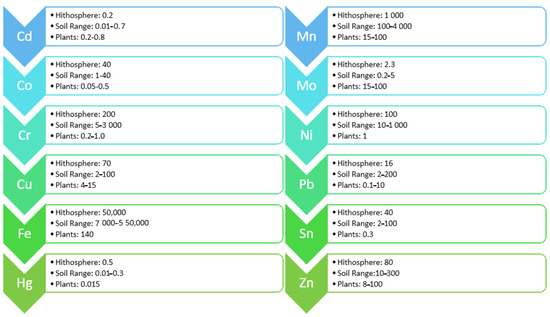
Across the planet, an issue of growing concern is environmental contamination due to heavy metal particles. These are discharged into the soil by a range of human activities, including industrial manufacturing, mining, smelting, and disposal of hazardous waste. Plants absorb the metals, which then become part of the food chain (Figure 1). As, Cd, Pb, and Hg are all carcinogenic but can also have other disastrous effects on humans, such as impacting the nervous system and causing renal malfunction. In addition, arsenic can be damaging to the skin, respiratory, and cardiovascular systems.
Figure 1. Heavy metal concentration (Ug/gm dry matter) in the lithosphere, soils, and plants.