
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tatjana Stevanovic | -- | 3128 | 2023-09-01 08:47:32 | | | |
| 2 | Lindsay Dong | Meta information modification | 3128 | 2023-09-01 08:49:42 | | | | |
| 3 | Lindsay Dong | Meta information modification | 3128 | 2023-09-01 08:50:03 | | |
Video Upload Options
Forest trees are the world’s most important renewable natural resources in terms of their dominance among other biomasses and the diversity of molecules that they produce. Forest tree extractives include terpenes and polyphenols, widely recognized for their biological activity. These molecules are found in forest by-products, such as bark, buds, leaves, and knots, commonly ignored in forestry decisions. The present entry focuses on in vitro experimental bioactivity from the phytochemicals of Myrianthus arboreus, Acer rubrum, and Picea mariana forest resources and by-products with potential for further nutraceutical, cosmeceutical, and pharmaceutical development.
1. Introduction
2. Bioactive Molecules from Promising Forest Resources of Myrianthus arboreus, Acer rubrum, and Picea mariana
2.1. Myrianthus arboreus
| Molecule Name | Classification | Extract Type | Plant Tissue | Bioactivity | Ref. |
|---|---|---|---|---|---|
| Epicatechin | Flavonoid | Ethyl acetate fraction (EtOAc) of 70% ethanolic extract. | Stem bark | All compounds, except euscaphic acid, inhibited the in vitro action of α-amylase. Euscaphic acid stimulated the glucose uptake in C2C12 cells. Epigallocatechin, dulcisflavan, tormentic acid, and arjunolic acid showed hypoglycaemic and anti-hyperlipidaemic activities in treptozotocin (STZ)-induced diabetic rats. Dulcisflavan was considered the most active compound and an appropriate substrate for further drug development. |
[18] |
| Epigallocatechin | Flavonoid | ||||
| Dulcisflavan | Flavonoid | ||||
| Euscaphic acid | ursane-type triterpenoids | ||||
| Tormentic acid | ursane-type triterpenoids | ||||
| Arjunolic acid | oleanane-type pentacyclic triterpenoid | ||||
| 3β-O-trans-feruloyl-2α,19α-dihydroxyurs-12-en-28-oic acid (H1) | ursene-type pentacyclic triterpene | Ethyl acetate fraction (EtOAc) of 95% ethanolic extract. | Root bark | All compounds decreased in vitro the activity of hepatocellular glucose-6-phosphatase (G6Pase) and activated glycogen synthase via the phosphorylation of glycogen synthase kinase-3. The compound (H3) and isoorientin were determined to be the most potent in modulating glucose homeostasis in liver cells. |
[19][20] |
| 2α-acetoxy-3β-O-trans-feruloyl-19α-hydroxyurs-12-en-28-oic acid (H3) | ursene-type pentacyclic triterpene | ||||
| ursolic acid | pentacyclic triterpene | ||||
| isoorientin | C-glycosylflavone | ||||
| orientin | C-glycosylflavone | ||||
| 3,4-dihydroxybenzaldehyde | phenolic aldehyde | ||||
| 3β,6β-dihydroxyolean-12-en-29-oic acid (myrianthinic acid) |
pentacyclic triterpene | Ethyl acetate fraction (EtOAc) of methanolic extract. | Stem bark | - | [21][22] |
| 2β/3β, 24-trihydroxy-olean-12-en-28-oic acid (arboreic acid) |
oleanane-type triterpenoid | ||||
| protocatechuic acid | phenolic acid | 70% methanolic extract (MAL) | Leaves | MAL administration significantly reduced body weight gain, basal glycemia, and insulin resistance in mice receiving a high-fat diet (HFD). MAL significantly downregulated the mRNA expression of IL-6, IL-1β, and TNF-α, known as obesity-associated inflammatory markers. MAL improved the altered expression of adipokines (leptin and adiponectin) in obese mice. |
[23] |
| methylumbelliferone fucopyranoside | hydroxycoumarin | ||||
| tectoridin | glycosyloxyisoflavone | ||||
| vanillic acid | phenolic acid | ||||
| medicagenic acid | triterpenoid | ||||
| brahmic acid | pentacyclic triterpenoid | ||||
| arjunolic acid | oleanane-type pentacyclic triterpenoid |
M. arboreous root bark and stem bark are used by local communities to treat diabetes and its complications [13]. Diabetes mellitus is a metabolic syndrome characterized by hyperglycemia accompanied by alterations of fat, protein, and carbohydrate metabolism that results from defects in insulin secretion, reduced insulin action, or both [10]. During diabetes, high amounts of free radicals are generated from glucose oxidation and nonenzymatic glycation of proteins as well as an impairment of antioxidant enzymes, contributing to oxidative stress and the development of diabetic co-morbidities, such as cataracts, nephropathy, encephalopathy, and cardiovascular diseases [10].
The ethyl acetate fraction (EAc) from the ethanolic extract (EtOH) of the stem bark of M. arboreus acts as an antioxidant in vitro, also stimulating glucose uptake in C2C12 myotubes and 3T3-L1 adipocytes by inhibiting alpha-glucosidase and alpha-amylase activity [24]. This extract also provoked a significant decrease in body weight, total protein, HDL-cholesterol, plasma glucose, LDL-cholesterol, triglycerides, serum urea, and serum creatinine in streptozotocin-induced diabetic rats [25].
2.2. Acer rubrum
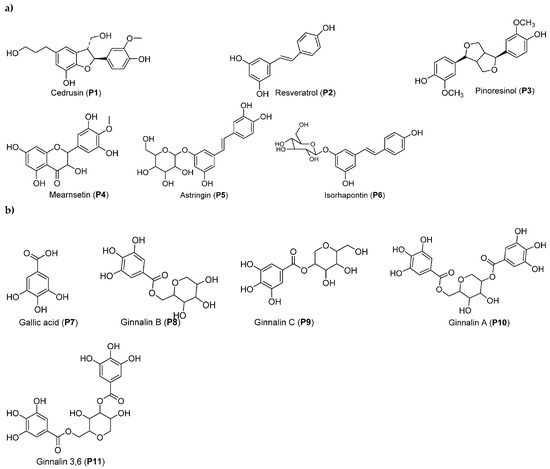
2.3. Picea mariana
3. Challenges and Opportunities for Forest Extract Valorization
References
- García-Pérez, M.-E.; Stevanovic, T. Les polyphénols bioactifs de ressources alimentaires et forestieres: Importance pour la santé. In Chimie Pour la Transformation Durable de la Resource Lignocellulosique; Presses Universitaires de Bordeaux: Pessac, France, 2019; Volume II, ISBN 979-10-300-0326-0.
- Ahmadu, T.; Ahmad, K. An Introduction to Bioactive Natural Products and General Applications. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2021; pp. 41–91. ISBN 978-3-030-54027-2.
- Mondal, S.; Soumya, N.P.P.; Mini, S.; Sivan, S.K. Bioactive Compounds in Functional Food and Their Role as Therapeutics. Bioact. Compd. Health Dis. 2021, 4, 24–39.
- Desborough, M.J.R.; Keeling, D.M. The Aspirin Story—From Willow to Wonder Drug. Br. J. Haematol. 2017, 177, 674–683.
- Zhang, L.; Han, Z.; Granato, D. Chapter One—Polyphenols in Foods: Classification, Methods of Identification, and Nutritional Aspects in Human Health. In Advances in Food and Nutrition Research; Granato, D., Ed.; Application of Polyphenols in Foods and Food Models; Academic Press: Cambridge, MA, USA, 2021; Volume 98, pp. 1–33.
- Tanase, C.; Coșarcă, S.; Muntean, D. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182.
- Arisandi, R.; Ashitani, T.; Takahashi, K.; Marsoem, S.N.; Lukmandaru, G. Lipophilic Extractives of the Wood and Bark from Eucalyptus pellita F. Muell Grown in Merauke, Indonesia. J. Wood Chem. Technol. 2020, 40, 146–154.
- Umezawa, T. Chemistry of Extractives. In Wood and Cellulosic Chemistry, 2nd ed.; Revised, and Expanded; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-8247-0024-9.
- Terpenes and Terpenoids: Recent Advances; BoD-Books on Demand; InTechOpen: London, UK, 2021; ISBN 978-1-83881-916-3.
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341.
- Czajka, M.; Fabisiak, B.; Fabisiak, E. Emission of Volatile Organic Compounds from Heartwood and Sapwood of Selected Coniferous Species. Forests 2020, 11, 92.
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers Phytochemicals: A Valuable Forest with Therapeutic Potential. Molecules 2021, 26, 3005.
- Kasangana, P.B.; Stevanovic, T. Chapter 1-Studies of Pentacyclic Triterpenoids Structures and Antidiabetic Properties of Myrianthus Genus. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; Volume 68, pp. 1–27.
- Mamadalieva, N.Z.; Mamedov, N.A. Taxus Brevifolia a High-Value Medicinal Plant, as a Source of Taxol. In Medicinal and Aromatic Plants of North America; Máthé, Á., Ed.; Medicinal and Aromatic Plants of the World; Springer International Publishing: Cham, Switzerland, 2020; pp. 201–218. ISBN 978-3-030-44930-8.
- Karuppusamy, S.; Pullaiah, T. 6-Botany of Paclitaxel Producing Plants. In Paclitaxel; Swamy, M.K., Pullaiah, T., Chen, Z.-S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 155–170. ISBN 978-0-323-90951-8.
- Kazachenko, A.S.; Akman, F.; Vasilieva, N.Y.; Issaoui, N.; Malyar, Y.N.; Kondrasenko, A.A.; Borovkova, V.S.; Miroshnikova, A.V.; Kazachenko, A.S.; Al-Dossary, O.; et al. Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation. Int. J. Mol. Sci. 2022, 23, 1602.
- Okafor, J.C. Edible Indigenous Woody Plants in the Rural Economy of the Nigerian Forest Zone. For. Ecol. Manag. 1980, 3, 45–55.
- Harley, B.K.; Dickson, R.A.; Amponsah, I.K.; Ben, I.O.; Adongo, D.W.; Fleischer, T.C.; Habtemariam, S. Flavanols and Triterpenoids from Myrianthus arboreus Ameliorate Hyperglycaemia in Streptozotocin-Induced Diabetic Rats Possibly via Glucose Uptake Enhancement and α-Amylase Inhibition. Biomed. Pharmacother. 2020, 132, 110847.
- Kasangana, P.B.; Haddad, P.S.; Eid, H.M.; Nachar, A.; Stevanovic, T. Bioactive Pentacyclic Triterpenes from the Root Bark Extract of Myrianthus arboreus, a Species Used Traditionally to Treat Type-2 Diabetes. J. Nat. Prod. 2018, 81, 2169–2176.
- Kasangana, P.B.; Eid, H.M.; Nachar, A.; Stevanovic, T.; Haddad, P.S. Further Isolation and Identification of Anti-Diabetic Principles from Root Bark of Myrianthus arboreus P. Beauv.: The Ethyl Acetate Fraction Contains Bioactive Phenolic Compounds That Improve Liver Cell Glucose Homeostasis. J. Ethnopharmacol. 2019, 245, 112167.
- Ngounou, F.N.; Lontsi, D.; Sondengam, B.L. Myrianthinic Acid: A New Triterpenoid from Myrianthus arboreus. Phytochemistry 1988, 27, 301–303.
- Ngounou, F.N.; Lontsi, D.; Ayafor, J.F.; Sondengam, B.L. Arboreic Acid: A Pentacyclic Triterpenoid from Myrianthus arboreus. Phytochemistry 1987, 26, 3080–3082.
- Tijani, R.O.; Molina-Tijeras, J.A.; Vezza, T.; Ruiz-Malagón, A.J.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A.; Abiodun, O.O.; Galvez, J. Myrianthus arboreus P. Beauv Improves Insulin Sensitivity in High Fat Diet-Induced Obese Mice by Reducing Inflammatory Pathways Activation. J. Ethnopharmacol. 2022, 282, 114651.
- Harley, B.; Dickson, R.; Fleisher, T. Antioxidant, Glucose Uptake Stimulatory, α-Glucosidase and α-Amylase Inhibitory Effects of Myrianthus arboreus Stem Bark. Nat. Prod. Chem. Res. 2017, 5, 271.
- Dickson, R.; Harley, B.; Berkoh, D.; Ngala, R.; Titiloye, N.; Fleischer, T. Antidiabetic and Haematological Effect of Myrianthus arboreus P. Beauv. Stem Bark Extract in Streptozotocin-Induced Diabetic Rats. Int. J. Pharm. Sci. Res. 2016, 7, 4812–4826.
- Ma, H.; Guo, H.; Liu, C.; Wan, Y.; Seeram, N.P. Protective Effects of a Red Maple (Acer rubrum) Leaves Extract on Human Keratinocytes against H2O2-Induced Oxidative Stress. FASEB J. 2018, 32, 656.33.
- Bhatta, S.; Ratti, C.; Poubelle, P.E.; Stevanovic, T. Nutrients, Antioxidant Capacity and Safety of Hot Water Extract from Sugar Maple (Acer saccharum M.) and Red Maple (Acer rubrum L.) Bark. Plant Foods Hum. Nutr. 2018, 73, 25–33.
- Geoffroy, T.R.; Meda, N.R.; Stevanovic, T. Suitability of DPPH Spiking for Antioxidant Screening in Natural Products: The Example of Galloyl Derivatives from Red Maple Bark Extract. Anal. Bioanal. Chem. 2017, 409, 5225–5237.
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as Protagonists and Targets in Chronic Inflammation. Nat. Rev. Immunol. 2017, 17, 248–261.
- Meda, N.R.; Stevanovic, T.; Poubelle, P.E. Anhydroglucitol-Core Gallotannins from Red Maple Buds Modulate Viability of Human Blood Neutrophils. Toxicol. Vitr. 2019, 60, 76–86.
- Meda, N.R.; Poubelle, P.E.; Stevanovic, T. Antioxidant Capacity, Phenolic Constituents and Toxicity of Hot Water Extract from Red Maple Buds. Chem. Biodivers. 2017, 14, e1700028.
- González-Sarrías, A.; Li, L.; Seeram, N.P. Effects of Maple (Acer) Plant Part Extracts on Proliferation, Apoptosis and Cell Cycle Arrest of Human Tumorigenic and Non-Tumorigenic Colon Cells. Phytother. Res. 2012, 26, 995–1002.
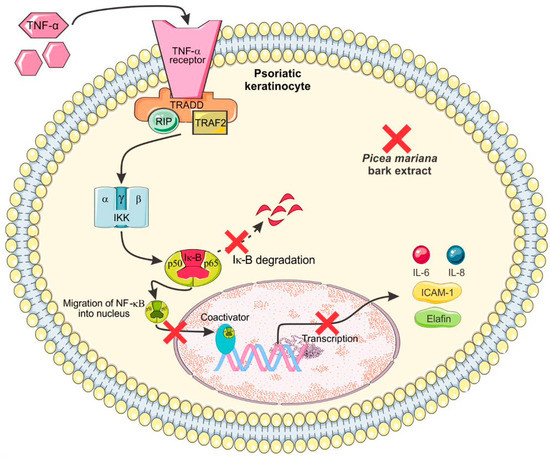
- García-Pérez, M.-E.; Allaeys, I.; Rusu, D.; Pouliot, R.; Janezic, T.S.; Poubelle, P.E. Picea Mariana Polyphenolic Extract Inhibits Phlogogenic Mediators Produced by TNF-α-Activated Psoriatic Keratinocytes: Impact on NF-ΚB Pathway. J. Ethnopharmacol. 2014, 151, 265–278.
- Downing, A.D.; Eid, H.M.; Tang, A.; Ahmed, F.; Harris, C.S.; Haddad, P.S.; Johns, T.; Arnason, J.T.; Bennett, S.A.L.; Cuerrier, A. Growth Environment and Organ Specific Variation in In-Vitro Cytoprotective Activities of Picea Mariana in PC12 Cells Exposed to Glucose Toxicity: A Plant Used for Treatment of Diabetes Symptoms by the Cree of Eeyou Istchee (Quebec, Canada). BMC Complement. Altern. Med. 2019, 19, 137.
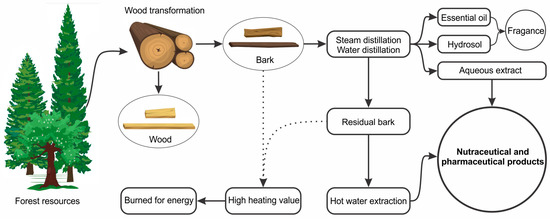
- Francezon, N.; Stevanovic, T. Integrated Process for the Production of Natural Extracts from Black Spruce Bark. Ind. Crops Prod. 2017, 108, 348–354.
- Valencia-Avilés, E.; García-Pérez, M.E.; Garnica-Romo, M.G.; de Dios Figueroa-Cárdenas, J.; Paciulli, M.; Martinez-Flores, H.E. Chemical Composition, Physicochemical Evaluation and Sensory Analysis of Yogurt Added with Extract of Polyphenolic Compounds from Quercus crassifolia Oak Bark. Funct. Foods Health Dis. 2022, 12, 502–517.
- Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Harte, F.; Medina-Torres, L.; Ochoa-Martínez, L.A.; Soto-García, M. Effect of High-Pressure Homogenization on the Physical and Antioxidant Properties of Quercus Resinosa Infusions Encapsulated by Spray-Drying. J. Food Sci. 2010, 75, N57–N61.
- Valencia Avilés, E. Efecto de la Adición de Aceite Vegetal y Nanocápsulas de un Extracto Polifenólico de Corteza de Quercus crassifolia a un Yogurt Medidos en Hámsteres Dislipidémicos. Ph.D Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, Mexico, 2019.
- Sheppard, J.P.; Chamberlain, J.; Agúndez, D.; Bhattacharya, P.; Chirwa, P.W.; Gontcharov, A.; Sagona, W.C.J.; Shen, H.; Tadesse, W.; Mutke, S. Sustainable Forest Management beyond the Timber-Oriented Status Quo: Transitioning to Co-Production of Timber and Non-Wood Forest Products—A Global Perspective. Curr. For. Rep. 2020, 6, 26–40.
- Mathela, M.; Bargali, H.; Sharma, M.; Sharma, R.; Kumar, A. Brainstorming on the Future of the Highly Threatened Medicinal Plants of the Western Himalaya, India. Curr. Sci. 2020, 118, 1485–1486.




