Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Sułkowska-Ziaja | -- | 4455 | 2023-09-01 08:10:14 | | | |
| 2 | Sirius Huang | Meta information modification | 4455 | 2023-09-04 04:03:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sułkowska-Ziaja, K.; Trepa, M.; Olechowska-Jarząb, A.; Nowak, P.; Ziaja, M.; Kała, K.; Muszyńska, B. Antibacterial Activity of Substances of Fungal Origin. Encyclopedia. Available online: https://encyclopedia.pub/entry/48730 (accessed on 07 February 2026).
Sułkowska-Ziaja K, Trepa M, Olechowska-Jarząb A, Nowak P, Ziaja M, Kała K, et al. Antibacterial Activity of Substances of Fungal Origin. Encyclopedia. Available at: https://encyclopedia.pub/entry/48730. Accessed February 07, 2026.
Sułkowska-Ziaja, Katarzyna, Monika Trepa, Aldona Olechowska-Jarząb, Paweł Nowak, Marek Ziaja, Katarzyna Kała, Bożena Muszyńska. "Antibacterial Activity of Substances of Fungal Origin" Encyclopedia, https://encyclopedia.pub/entry/48730 (accessed February 07, 2026).
Sułkowska-Ziaja, K., Trepa, M., Olechowska-Jarząb, A., Nowak, P., Ziaja, M., Kała, K., & Muszyńska, B. (2023, September 01). Antibacterial Activity of Substances of Fungal Origin. In Encyclopedia. https://encyclopedia.pub/entry/48730
Sułkowska-Ziaja, Katarzyna, et al. "Antibacterial Activity of Substances of Fungal Origin." Encyclopedia. Web. 01 September, 2023.
Copy Citation
The phenomenon of drug resistance in micro-organisms necessitates the search for new compounds capable of combating them. Fungi emerge as a promising source of such compounds as they produce a wide range of secondary metabolites with bacteriostatic or fungistatic activity. These compounds can serve as alternatives for commonly used antibiotics.
antibacterial activity
compounds of fungal origin
1. Compounds of Fungal Origin with Antibacterial Activity
Numerous scientific studies indicate the antimicrobial activity of individual compounds and specific extracts obtained from fungal fruiting bodies.
It is believed that the presence of fungal fruiting bodies with such properties is due to defense mechanisms formed by fungi to survive in the environment. As the challenge of bacterial resistance to existing antibiotics grows, a variety of naturally occurring compounds exhibiting antimicrobial activity against pathogenic organisms is garnering increasing attention. Notably, one of the first compounds with antibacterial activity was the antibiotic substance sparassol, which was isolated from Sparassis crispa in 1920 [1] (Table 1). Over the following decades, the antibiotic activity of more than 2000 macromycetes species was subsequently validated.
Table 1. Chemical structures of examples of compounds with antibacterial activity of fungal origin.
| Group of Compounds |
Compound | Species | Chemical Formula | Reference |
|---|---|---|---|---|
| Benzoic acid derivative |
Sparassol | Sparassis crispa | 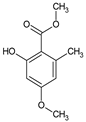 |
[1] |
| Sesquiterpenes (C15) |
Merulidial | Merulius tremellosus | 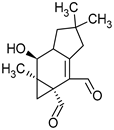 |
[2] |
| Pilatin | Flagelloscypha pilatii | 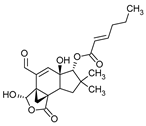 |
[3] | |
| Hypnophilin | Pleurotellus hypnophilus | 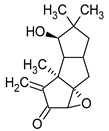 |
[4] | |
| Pleurotellol | Pleurotellus hypnophilus | 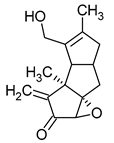 |
[4] | |
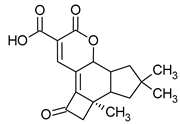
| Lentinellic acid | Lentinellus omphalodes Lentinellus ursinus |
 |
[5] | |
| Armillaric acid | Armillaria mellea | 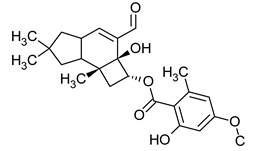 |
[6] | |
| Enokipodin A | Flammulina velutipes | 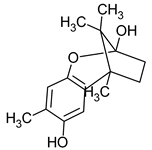 |
[7] | |
| Coriolin | Coriolus consors | 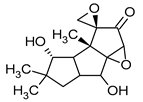 |
[8] | |
| Diterpenes (C20) |
Pleuromutilin | Clitopilus passeckerianus | 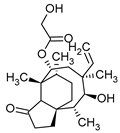 |
[9] |
| Striatin A | Cyathus striatus | 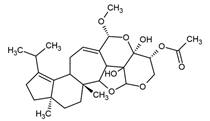 |
[10] | |
| Triterpenes (C30) |
Sulphurenic acid | Laetiporus sulphureus | 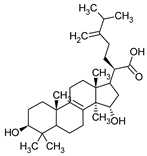 |
[11] |
| Ganodermanontriol | Ganoderma lucidum | 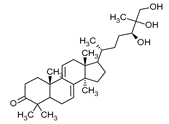 |
[12] | |
| Meroterpenoids (C40) |
Ganomycin A | Ganoderma pfeiferii | 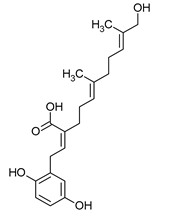 |
[13] |
| Acetylene derivatives |
Scorodonin | Marasmius scorodonius | 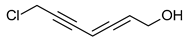 |
[14] |
| Sterols | Ganoderiol | Ganoderma lucidum | 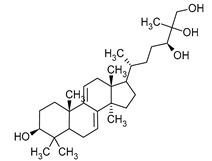 |
[12] |
Key Antibacterial Compounds of Fungal Origin
Fungi are known for producing a wide variety of compounds endowed with antibacterial activity [15]. These compounds exhibit a dual nature, yielding a broad spectrum of activity while also manifesting selective efficacy against specific bacterial strains. Antibiotics are substances that hinder the growth and division of bacteria. The term “antibiotic” was coined by microbiologist Selman Waksman, who discovered two antibiotics: streptomycin and neomycin [16]. Nowadays, antibiotics encompass a spectrum spanning natural substances, semisynthetic derivatives, and synthetic analogs. These agents selectively target various bacterial structures, leading to either a bactericidal or bacteriostatic effect. Antibiotics are categorized into distinct groups based on factors such as their mode of action, chemical structure, or spectrum of activity. For instance, certain antibiotics inhibit the synthesis of bacterial cell walls (e.g., β-lactams), while others impede protein production (e.g., chloramphenicol, tetracycline), or interfere with bacterial RNA and DNA nucleic acids (e.g., quinolones) [17]. In the early 20th century, small doses of penicillin proved highly effective in controlling a significant proportion of bacterial infections. However, as the use of penicillin increased, its effectiveness waned against infections. This phenomenon, identified as antibiotic resistance, stems from the defense mechanisms micro-organisms develop to counteract antibiotics. It is important to note that antibiotic resistance is not a recent occurrence but rather an outcome of bacteria evolving various mechanisms to protect themselves from harmful substances within their environment. This process allows them to quickly adapt to adverse changes [18]. Filamentous fungi, particularly those belonging to the genera Penicillium, Cephalosporium, Aspergillus, and Fusidium, are vital organisms in pharmaceutical biotechnology, particularly in the pharmaceutical industry. These fungi are well-known prolific producers of antibiotics, and alongside actinomycetes, they are recognized as the primary sources of antibiotics [19]. Among the most significant antibiotic classes produced by fungi are penicillins, cephalosporins, fusidans, fusafungin, and fumigacin (helvolic acid).
-
Penicillin
Penicillins belong to the β-lactam group of antibiotics (Figure 1). In terms of their chemical structure, they contain a thiazolidine ring conjugated with a β-lactam ring. The operational mechanism of penicillins, as well as other β-lactam antibiotics, entails binding to penicillin-binding proteins (PBPs), subsequently obstructing their function. Antibiotics within this grouping exhibit extremely low levels of general and organ toxicity against human cells because they only affect cells where peptidoglycan synthesis occurs. The commercial production of penicillins involves selected species such as Penicillium chrysogenum, Penicillium baculatum, Penicillium turbatum, Aspergillus persicinum, Aspergillus flavus, Aspergillus giganteus, Aspergillus nidulans, Aspergillus oryzae, and Aspergillus parasiticus [20][21]. Penicillins are primarily utilized in medicine to treat bacterial infections. They can be employed for addressing conditions such as impetigo, erysipelas, and acne, thereby contributing to the acceleration of wound healing [21].
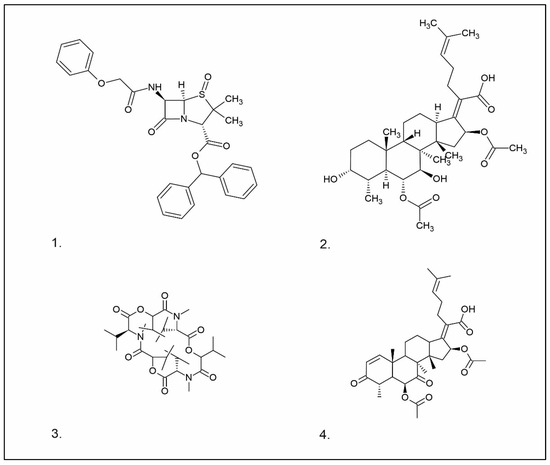
Figure 1. Chemical structures of 1. Penicillin; 2. Cephalosporin; 3. Fusafungine; and 4. helvolic acid.
-
Cephalosporin
The precursors of cephalosporins were initially isolated from cultures of Cephalosporium acremonium in 1948 by Giuseppe Brotzu [22][23] (Figure 1). The mechanism of action of cephalosporins is analogous to that of β-lactam antibiotics. These compounds find commercial production through strains of C. acremonium and Paecilomyces persicinius. Cephalosporins can be subdivided into several subgroups based on their chemical structure (P1–P5) [23]. Similar to all β-lactam antibiotics, cephalosporins inhibit the formation of bonds that connect peptidoglycan (murein) subunits, thus preventing the formation of a complete cell wall. They form covalent attachments to the active centers of bacterial enzymes, namely, carboxypeptidase and transpeptidase, leading to the inhibition of their actions. Consequently, they hinder the synthesis of bacterial cell walls. Cephalosporins treat bacterial infections of various origins, including both Gram-positive and Gram-negative bacteria. These antibacterial agents are employed in the treatment of infections caused by pathogens such as Staphylococcus aureus and Escherichia coli, among others [24]. Cephalosporins can be used to treat skin diseases caused by micro-organisms. In dermatology, they have been applied to address skin conditions such as folliculitis or postoperative infections [25].
-
Fusidans
One of the most well-recognized fusidans is fusidic acid. Fusidic acid has an inhibitory effect on the protein synthesis of Gram-positive bacteria. Initially isolated in 1962 from Fusidium coccineum, fusidic acid was subsequently extracted from Mucor ramannianus and Isaria kogana [26][27]. Currently, biotechnological methods are obtained to derive fusafungin from species such as Calcarisporium arbuscula, Fusidium coccophilum, and Mortierella ramanniana [26]. Sporting a steroidal configuration, fusidic acid functions as an antibiotic with bacteriostatic properties. Its operational mechanism inhibits the synthesis of bacterial proteins, thereby preventing the growth and multiplication of bacterial cells. Notably, fusidic acid exhibits a narrow spectrum of activity, with a primary focus on Gram-positive bacteria, particularly those that are resistant to penicillin, such as Staphylococcus strains. The administration of fusidic acid during treatment might lead to the emergence of resistant strains of Staphylococcus. This antibiotic can be used in the form of creams and ointments for the topical treatment of infections such as impetigo, boils, inflammation of sweat glands and hair follicles, atrophy, acne vulgaris, and infections caused by the genus Staphylococcus spp. [27][28]. Of significance, fusidic acid can permeate intact skin, with the extent of penetration influenced by factors such as antibiotic exposure duration and skin condition. The biological half-life of fusidic acid is approximately 4–5 h. After being absorbed into the bloodstream, fusidic acid undergoes significant metabolism in the liver. While it is primarily excreted through the bile, a minor portion is eliminated unchanged in the urine [29]. While these compounds are not typically directly utilized in cosmetics due to their medical nature, they do exhibit effectiveness against pathogens responsible for skin diseases, such as S. aureus and Staphylococcus epidermidis [27].
Fusafungine emerges as a peptide antibiotic that exerts a bacteriostatic effect on numerous pathogenic micro-organisms (Figure 1). In addition to its antibacterial attributes, it also independently demonstrates anti-inflammatory activity. The probable mechanism of action involves enhancing the activity of NK cells, stimulating lymphocytes to produce IL-2, and inhibiting proinflammatory cytokines [30]. Fusafungine has proven efficacy in treating pharyngitis, offering an alternative to systemic antibiotics, steroids, or anti-inflammatory drugs. Sourced from the entomopathogenic fungus Fusarium lateritium (Ascomycota), this compound boasts an expansive activity spectrum without inducing bacterial resistance. As an ionophore antibiotic, it amalgamates enniatins and exhibits a unique ability to selectively form complexes with potassium cations, thereby transporting them across the lipid membranes of liposomes [31]. The topical application of fusafungine has been utilized, while its aerosol form has shown promise in treating inflammation of the upper and lower respiratory tract. Clinical trials have confirmed the effectiveness of the aerosolized form of this medication [31].
Fumigacin and helvolic acid (Figure 1) encompass antibiotics and phytotoxic substances produced by fungi belonging to the Ascomycota category, including Aspergillus fumigatus, Cephalosporium caeruleus, and Sarocladium oryzae (known as plant pathogens), alongside the species Emericellopsis terricola. Possessing distinctive properties and a varied range of action, fumigacin draws parallels to cephalosporins, especially those within the P1 group [32].
Selected Compounds of Fungal Origin from the Group of Isoprenoids, Peptides, and Acetylene Derivatives
Other substances with antibiotic properties, isolated from macrofungi, are compounds classified as isoprenoids, peptides, nucleosides, and acetylene derivatives.
-
Isoprenoids
Isoprenoid compounds constitute a diverse group of secondary metabolites found in Basidiomycota. These compounds are intrinsically linked to the biogenetic pathway that originates from active acetate and proceeds through mevalonic acid, ultimately leading to the formation of “active isoprene.” Subsequent transformations of the latter undergo a series of transformations, resulting in the production of monoterpenes, sesquiterpenes, diterpenes, triterpenes, tetraterpenes, and steroids [33][34].
Merulidial, which contains an “unsaturated dialdehyde” functional group, emerges from liquid cultures of Merulius tremellosus (Table 1). This compound exhibits formidable activity against an array of Gram-positive bacteria, including Micrococcus roseus, Corynebacterium insidiosum, Bacillus brevis, Bacillus subtilis, Streptomyces viridochrontogenes, Sarcina lutea, and Arthrobacter citreus, as well as Gram-negative bacteria such as Proteus vulgaris [2][35]. Pilatin, a derivative of marasman (Table 1), is isolated from Flagelloscypha pilati. It proves effective in inhibiting the growth of Gram-negative bacteria, including Salmonella typhimurium, within a concentration range of 5–50 µg/mL [3]. From mycelial cultures of Pleurotellus hypnophilus, three metabolites with antibiotic activity have been identified. These include hypnophilin, pleurotellol, and pleurotellic acid (Table 1), all of which are sesquiterpenes derived from hirsutane. The common structural feature shared by all three metabolites is the α-methylenketone moiety [4]. Hypnophilin has been the subject of investigation due to its antimicrobial and antioxidant properties. Its potential utilization in skin care and cosmetics could be attributed to its antioxidant activity, which has the potential to safeguard the skin against oxidative stress and enhance overall skin health. Pleurotellol has been studied for its antibacterial and antifungal properties. In the context of skin care and cosmetics, pleurotellol’s antimicrobial activity could be relevant for formulations targeting skin conditions caused by microbial overgrowth [36]. Lentinellic acid, an iludane-type sesquiterpene (Table 1), exhibits robust antibacterial properties and has been isolated from two species of the genus Lentinellus: Lentinellus omphalodes and Lentinellus ursinus. It exhibits activity against Gram-positive bacteria, including B. brevis, Aerobacter aerogenes, and C. insidiosum, at concentrations ranging from 1 to 5 µL/mL [5]. Sesquiterpenoids with antimicrobial properties could potentially contribute to the development of novel skin care and cosmetic formulations aimed at addressing skin-related concerns caused by micro-organisms. Additionally, their potential antioxidant and anti-inflammatory activities may further enhance their suitability for cosmetic applications, promoting skin health and overall product quality. Lentinellic acid methyl ester also possesses antifungal activity [5]. In the context of skin care and cosmetics, this compound may have potential applications as a preservative in cosmetic formulations to help solve skin problems caused by fungal infections such as athlete’s foot or fungal acne [37].
Sulphurenic acid (Table 1) and eburicoic acid are triterpenes isolated from Laetiporus sulphureus [11]. Pleuromutilin, a diterpene compound, was isolated by Kavanagh in 1951 from a saprophytic fungus Clitopilus passeckerianus (formerly Pleurotus passeckerianus) (Table 1). Pleuromutilin and its derivatives inhibit bacterial protein synthesis by binding to the peptidyltransferase component of the 50S subunit of ribosomes [9]. Striatins A, B, and C are kyatan diterpenes isolated from Cyathus striatus (Table 1). These compounds exhibit antibiotic and cytotoxic effects at concentrations of 2 µg/mL. These compounds have been found in both the fruiting bodies and in vitro mycelium of the species. They demonstrate activity against various bacteria, including A. citreus, B. brevis, B. subtilis, E. coli, Leuconostoc mesenteroides, Mycobacterium phlei, Nocardia brasiliensis, P. vulgaris, Pseudomonas fluorescens, S. lutea, S. aureus, and Streptomyces viridochromogenes, along with the fungus Saccharomyces cerevisiae and the yeast Rhodotoula rubra. In the context of cosmetics and skin care, secondary metabolites such as striatins could have potential applications such as antimicrobial and antioxidant effects [10]. Armillaric acid, isolated from mycelial cultures of Armillaria mellea, is a sesquiterpene compound (Table 1) [6]. An aryl ester of this compound, known as melleolide, has exhibited antibacterial activity [38]. Flammulina velutipes mycelium has yielded four sesquiterpenes with antibacterial activity: enokipodins A, B, C, and D (Table 1). These compounds exhibit activity against B. subtilis, with enokipodins A and C also demonstrating activity against S. aureus Enokipodins, with a diverse range of biological properties that could have applications in cosmetics: antioxidant effects, skin brightening, and anti-inflammatory properties [7][39]. From the fruiting bodies of the saprophytic species Jahnoporus hirtus (Basidiomycota), a steroid named (24Z)-3,11-dioxolanosta-8,24-dien-26-oic acid has been isolated. This compound displays activity against Bacillus cereus and Enterococcus faecalis [40]. Ganomycin A and B (Table 1), isolated from Ganoderma pfeifferi, exhibit activity against B. subtilis, Micrococcus flavus, and S. aureus [13].
-
Peptides
One of the peptides produced by fungi is plectasin, isolated from the fruiting bodies of Pseudoplectania nigrella (Ascomycota). Plectasin belongs to the defensin group of peptides and carries a cationic character. Comprising 40 amino acids, it exhibits activity against Gram-positive bacteria such as S. aureus and Streptococcus pneumoniae, primarily affecting the stability of their cell membranes [41]. In vitro, plectasin’s impact on S. pneumoniae mirrors that of penicillin and vancomycin. In addition, this peptide targets Gram-positive bacteria of genera such as Streptococcus (S. pneumoniae, S. pyogenes), Staphylococcus (S. aureus, S. epidermidis), Enterococcus (E. faecalis, E. faecium), Corynebacterium (C. diphtheriae, C. jeikeium), and Bacillus (B. cereus, B. thuringiensis) [42]. Zervamicins, a group of peptides with antibacterial activity, is produced by Emericellopsis salmosynnnemata (Ascomycota) (Figure 2). These peptides, classified as peptaibols, are linear and characterized by a high content of α,α-dialkyl amino acids, such as α-aminoisobutyric acid [43][44]. Another set of peptaibols includes peptaibol boletusin, peptaibol chrysospermin-3, and peptaibol chrysospermin-5, all extracted from Boletus spp. These compounds demonstrate efficacy against B. subtilis, Corynebacterium lilium, and S. aureus. Peptaibol chrysospermin-3 also shows activity against various Streptococcus strains [45]. Derived from the fungal fermentation of Tolypocladium niveum or Aspergillus terreus strains (Figure 2), cyclosporin is a cyclic peptide. The cyclosporin family comprises cyclic peptides with specific amino acids and demonstrates a mild antibiotic effect. However, their primary utility lies in their role as immunosuppressive agents. Cyclosporin A, for instance, is used in medicine to prevent organ rejection in transplant patients and treat autoimmune diseases [46]. Enniatins, cyclic hexapolipeptides, are synthesized by various strains of the Fusarium genus within the family Nectriaceae (Ascomycota) (Figure 2). In addition to their antibiotic properties, they exhibit insecticidal and anticancer activities [47].
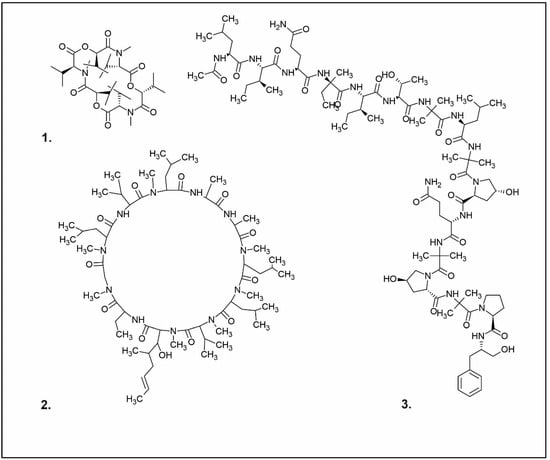
Figure 2. Examples of antibacterial compounds with peptides structure: 1. enniatin; 2. Cyclosporin; and 3. Zervamicin.
An example of a nucleoside exhibiting antibacterial activity is nebularine, which has been isolated from Clitocybe nebularis, a saprotrophic toxic species [48]. Notably, an enzyme with multifaceted attributes, ribonuclease, is sourced from the edible species Pleurotus sajor-caju. This enzyme demonstrates antimicrobial, antimitogenic, and antiproliferative effects and exhibits activity against Pseudomonas aeruginosa and S. aureus, functioning by targeting RNA [49].
-
Acetylene derivatives
Polyacetylenes stem from gradual desaturation processes involving saturated fatty acids, and they are a common occurrence within the fungal kingdom. Many acetylene derivatives identified in Basidiomycota exhibit antibacterial, cytotoxic, and antifungal activities, such as scorodonin, obtained from Marasmius scorodonius (Table 1), and 1-hydroxy-2-nonyn-3-one, extracted from Ischnoderma benzoinum [14]. Scorodonin derived from fungi holds potential for diverse cosmetic uses due to its antioxidant, anti-inflammatory, and skin-lightening properties [50]. Aqueveque has isolated two polyacetylenic compounds, namely, hepta-4,6-diyn-3-ol and 7-chloro-hepta-4,6-diyn-3-ol, from Gymnophilus spectabilis. These compounds are thought to arise from the desaturation pathway of saturated fatty acids and serve as precursors for the synthesis of polyacetylene compounds in fungi. Their antibacterial activity against Gram-positive and Gram-negative bacteria, as well as their antifungal activity, can be attributed to the presence of unsaturated triple bonds [51].
Other Compounds of Fungal Origin with Antibacterial Activity
Pleurotin, a derivative of quinone, along with leucopleurotin and dihydropleurotinic acid, has been isolated from Pleurotus griseus (now classified as Hohenbuehelia grisea). These compounds exhibit activity against Gram-positive bacteria and specific pathogenic fungi. Pleurotin has shown antimicrobial activity against certain bacteria and fungi. In the context of cosmetics, its antimicrobial properties could be explored for potential use as a natural preservative to prevent microbial growth in cosmetic products [52]. Oxalic acid, isolated from the mycelium of Lentinus edodes, shows activity against B. cereus, S. aureus, and E. faecalis [53]. Oxalic acid derived from fungi presents an array of cosmetic uses due to its exfoliating, brightening, antibacterial, and antioxidant properties [54]. Another compound, cloratin A, a benzoic acid derivative, has been isolated from the saprotrophic inedible fungus Xylaria intracolarata. This compound displays activity against E. coli, Klebsiella pneumoniae, P. aeruginosa, and Salmonella enterica, with particularly potent inhibitory activity observed against K. pneumoniae, surpassing the control group [55]. Antibacterial activity is also evident in anthraquinone derivatives such as 6–methylxanthopurpurin-3-O-methyl ether, (1 S, 3 S), austrocortilutein, (1 S, 3 R), austrocortilutein, (1 S, 3 S), austrocortirubin, and torosachryson, isolated from Cortinarius basirubencens. Compounds of erythroglaucine and emodin, isolated from other Cortinarius species, also demonstrated efficacy against S. aureus [56]. A fraction labeled B from Pycnoporus sanguineus, mainly composed of loccosee-3-one, exhibited activity against S. aureus and various strains of Streptococcus (A, B, C, and G). Compounds isolated from G. pfeifferi showed moderate activity against E. coli, Proteus mirabilis, and Serratia marcescens. Quinoline, isolated from the fungus Leucopaxillus albissimus, showed activity against Achromobacter xyloxidans, Acinetobacter baumannii, Burkholderia cenocepacia, Burkholderia loccose, Burkholderia multivorans, Cytophaga johnsonae, and P. aeruginosa, with the highest activity observed against C. johnsonae [57].
Polysaccharides, such as β-glucans, chitin, and its derivative chitosan, are vital components of the fungal cell wall. Chitosan, featuring amino sugars in its composition, exhibits antibacterial activity. Notably, chitosan is found not only in fungi but also present in the shells of arthropods such as crabs, shrimp, squid, and crayfish [58]. Exhibiting a wide antibacterial activity, chitosan proves effective against certain Gram-negative bacteria, Gram-positive bacteria, and fungi. Specifically, it has shown a higher effect on Gram-positive bacteria, including Listeria monocytogenes, Bacillus megaterium, B. cereus, S. aureus, Lactobacillus plantarum, L. brevis, and L. bulgaris. While it does display activity against Gram-negative bacteria, such as E. coli, P. fluorescens, S. typhimurium, and Vibrio parahaemolyticus, its potency is comparatively weaker [59][60].
Recent studies indicate that chitosan can be obtained biotechnologically from the cell wall of the filamentous fungus Rhizopus oryzae. Its antibacterial properties have been tested against E. coli, K. pneumoniae, and S. aureus [61][62]. A summary of the antibacterial activity of compounds derived from fungi is provided in Table 2.
Table 2. Summary of antibacterial activity of compounds of fungal origin.
| Species | Extract | Bacteria | References |
|---|---|---|---|
| Aspergillus giganteus Aspergillus nidulans Aspergillus oryzae Aspergillus parasiticus Aspergillus persicinum Aspergillus flavus Penicilium baculatum Penicilium chrysogenum Penicillium turbatum Penicillum chrysogenum |
Penicillins | Gram-positive bacteria Diplococcus spp. Enterococcus spp. Staphylococcus spp. Streptococcus spp. Gram-negative bacterial Clostridium spp. Enterobacteriaceae spp. |
[20][21] |
| Cephalosporium acremonium | Cephalosporins | Gram-positive bacteria: Diplococcus spp. Enterococcus spp. Staphylococcus spp. Streptococcus spp. Gram-negative bacteria: Clostridium spp. Enterobacteriaceae spp. |
[22][23][24][26][28][29] |
| Calcarisporium arbuscula Fusidium coccineum Isaria kogana Mucor ramannianus |
Fusidans | Gram-positive bacteria | [26][27][28][29] |
| Fusarium lateritium | Fusafungine | Streptococcus pyogenes Streptococcus pneumoniae Staphylococcus epidermidis Moraxella catarrhalis Legionella pneumophila Mycoplasma pneumoniae |
[30][31] |
| Aspergillus fumigatus Cephalosporium caeruleus Emericellopsis terricola Sarocladium oryzae |
Fumigacin (helvolic acid) |
Gram-negative bacteria | [32] |
| Merulius tremellosus | Merulidial | Gram-positive bacteria: Arthrobacter citreus Bacillus brevis Bacillus subtilis Corynebacterium insidiosum Sarcina lutea Streptomyces viridochrontogenes Gram-negative bacteria: Proteus vulgaris |
[2] |
| Flagelloscypha pilati | Pilatin | Salmonella typhimurum | [3] |
| Pleurotellus hypnophillus | Hypnophilin Pleurotellol Pleurotellic acid |
Bacillus brevis Salmonella typhimurium |
[4] |
| Lentinellus omphalodes Lentinellus ursinus |
Lentinellic acid | Bacillus brevis Aerobacter aerogenes Corynebacterium insidosum |
[5] |
| Laetiporus sulphureus | Sulphurenic acid Eburicoic acid |
Gram-positive bacteria | [11] |
| Clitopilus passeckerianus | Pleuromutilin | Mycoplasma spp. Brachyspira hyodysenteriae Brachyspira pilosicoli |
[9] |
| Cyathus striatus | Striatins A, B, C | Arthrobacter citreus Bacillus brevis Bacillus subtilis Escherichia coli Leuconostoc mesenteroides Mycobacterium phlei Nocardia brasiliensis Proteus vulgaris Pseudomonas fluorescens Sarcina lutea Staphylococcus aureus Streptomyces viridochromogenes |
[10] |
| Armillaria mellea | Armillaric acid | Gram-positive bacteria | [6] |
| Flammulina velutipes | Enokipodin | Bacillus subtilis Staphylococcus aureus |
[39] |
| Jahnoporus hirtus | (24Z)-3,11-Dioxolanosta-8,24-dien-26-oic acid | Bacillus cereus Enterococcus faecalis |
[40] |
| Ganoderma pfeifferi | Ganomycin A | Bacillus subtilis Micrococcus flavus Staphylococcus aureus |
[13] |
| Pseudoplectania nigrella | Plectasin | Bacillus cereus Bacillus thuringiensi Corynebacterium diphtheriae Corynebacterium jeikeium Enterococcus faecalis Enterococcus faecium Staphylococcus aureus Staphylococcus epidermidis |
[41][42] |
| Clitocybe nebularis | Nebularine | Mycobacterium tuberculosis | [48] |
| Pleurotus sajor–caju | Ribonuclease | Pseudomonas aeruginosa Staphylococcus aureus |
[49] |
| Gymnophilus spectabilis | Hepta-4,6-diyn-3-ol 7-Chloro-hepta-4,6-diyn-3-ol |
Gram-positive/Gram-negative | [51] |
| Hohenbuehelia grisea | Pleurotin | Gram-positive bacteria | [52] |
| Albatrellus flettii | Confluentin Grifolin Neogrifolin |
Bacillus cereus Enterococcus faecalis |
[40] |
| Lentinula edodes | Oxalic acid | Bacillus cereus Staphylococcus aureus Streptococcus faecalis |
[53] |
| Cortinarius basirubencens | Austrocortilutein Austrocortilutein Austrocortirubin Torosachryson |
Staphylococcus aureus | [56] |
| Boletus spp. | Boletusin Chrysospermin |
Bacillus subtilis Corynebacterium lilium Staphylococcus aureus |
[45] |
| Pycnoporus sanguineus | Phenoxazin-3-one | Staphylococcus aureus Streptococcus spp. |
[57] |
| Ganoderma pfeifferi | Terpenes | Escherichia coli Proteus mirabilis Serratia marcescens |
[13] |
| Xylaria intracolarata | Cloratin A | Escherichia coli Klebsiella pneumonia Pseudomonas aeruginosa Salmonella enteritidis |
[55] |
| Leucopaxillus albissimus | Chinoline | Achromobacter xyloxidans Acinetobacter baumannii Burkholderia cenocepacia Burkholderia loccose Burkholderia multivorans Cytophaga johnsonae Pseudomonas aeruginosa |
[57] |
2. Extracts of Fungal Origin with Antibacterial Activity
A considerable number of studies have focused on evaluating the antibacterial activity of natural raw materials, often by investigating the analysis of complete extracts. Notably, several types of extracts have been extensively examined, including aqueous, ethanol, methanol, chloroform, dichloromethane, ether, and acetone extracts.
Ganoderma lucidum stands as a prominent fungal raw material in East Asian traditional medicine, including TCM [63]. Notably, diverse extracts including aqueous, ethanol, methanol, and acetone have demonstrated comparable efficacy against gentamicin sulfate, an aminoglycoside antibiotic. This effectiveness extends to various bacterial species: E. coli, S. aureus, K. pneumoniae, B. subtilis, S. typhimurium, and P. aeruginosa [64]. Other studies have confirmed that acetone extract of G. lucidum exhibits antibacterial activity, mainly against Gram-negative K. pneumoniae bacteria. Additionally, a synergistic interaction was observed when combining G. lucidum extracts with antimicrobial agents such as ampicillin, cefazolin, oxytetracycline, and chloramphenicol. This synergy was particularly pronounced with cefazolin against B. subtilis and Klebsiella oxytoca [65]. Conversely, a chloroform extract from the edible mycorrhizal fungus Hygrophorus agathosmus exhibited inhibition against various pathogenic bacteria, including E. coli, Enterobacter aerogenes, S. typhimurium, P. aeruginosa, S. aureus, S. epidermidis, and B. subtilis. Furthermore, this extract demonstrated inhibitory effects on Candida albicans and S. cerevisiae [66]. In a similar vein, a dichloromethane extract from Suillus collitinus observed activity against Gram-positive bacteria, including S. epidermidis and B. subtilis. This extract exhibited substantial antibacterial activity, with MIC values of 7.81 µg/mL, surpassing the reference antibiotic streptomycin (MIC = 15.62 µg/mL). For S. aureus, MIC values remained equal to those of streptomycin, at 15.62 µg/mL [66]. Finally, the methanolic extract of Hypholoma fasciculare, a saprotrophic poisonous fungus, exhibited notable antibacterial activity against Gram-positive bacteria such as B. cereus, B. subtilis, and S. aureus [67]. Turkoglu conducted a study investigating the antibacterial activity of ethanol extracts from L. sulphureus. The extract displayed inhibitory activity against the growth of Gram-positive bacteria, including B. subtilis, B. cereus, Micrococcus luteus, and M. flavus [68]. Another study analyzed the antibacterial activity of different extracts (chloroform, ethyl acetate, and water) from Lentinula edodes fruiting bodies. These extracts showed antibacterial activity against Streptococcus spp., Actinomyces spp., Lactobacillus spp., Prevotella spp., and Porphyromonas spp., which are known to cause various oral infections. Specifically, chloroform extracts exhibited bactericidal activity against both growing and resting bacterial cells of Streptococcus mutans and Prevotella intermedia, while the other two extracts exhibited bacteriostatic activity against both growing and resting bacterial cells of S. mutans and resting bacterial cells of P. intermedia [69]. Furthermore, a low molecular weight fraction study was conducted on an extract of L. edodes formulated as a mouthwash and administered to a group of volunteers [70]. Methanolic extract from the mycelium of Leucopaxillus giganteus, an inedible saprophytic species, showed antibacterial properties against Gram-positive bacteria in the order of potency: S. aureus > B. cereus > B. subtilis. This study also revealed that diammonium hydrogen phosphate was the preferred nitrogen source for enhancing the production of bioactive compounds inhibiting the growth of Gram-positive bacteria [71]. Studies on methanolic extracts of Phellinus rimosus and Navesporus loccose demonstrated moderate antibacterial activity against Gram-positive bacteria B. subtilis and S. aureus [72]. Ethanolic extracts from Pleurotus ostreatus and Meripilus giganteus exhibited broad-spectrum antibacterial activity, particularly against S. lutea [73]. Evaluating extracts from fruiting bodies and mycelial cultures of Trametes versicolor, researchers found varying antibacterial activity based on the type of solvent used for the extraction (water, organic solvents, or mixtures). The study revealed significant antibacterial activity against Gram-positive bacteria, with lower activity against Gram-negative bacteria. This effect was attributed to coriolin, a sesquiterpene compound found in Trametes (formerly Coriolus) spp. (Table 1). Extracts from Clavariadelphus loccose and T. versicolor have exhibited activity against a range of bacteria, including E. coli, E. aerogenes, S. typhimurium, S. aureus, and B. subtilis [66]. Aqueous extracts of Cordyceps sinensis and Cordyceps militaris, which are species that parasitize invertebrates, have demonstrated antibacterial activity against S. aureus, probably as a result of an increase in phagocytic macrophage activity and cytokine expression [73]. Ethanol extracts containing polysaccharides from Grifola loccose fruiting bodies have been tested against Gram-positive bacteria such as S. aureus, E. faecalis, B. cereus, L. monocytogenes, and Gram-negative bacteria such as E. coli, Salmonella enteritidis, Shigella sonnei, and Yersinia enterocolitica. The most notable antibacterial activity was observed against B. cereus [74]. Acetyl acetate extracts from various species growing in Brazil, including Phellinus sp., Gloeoporus thelephoroides, Hexagonia hydnoides, and Nothopanus hygrophanus, demonstrated inhibition of growth against bacteria such as B. cereus, L. monocytogenes, and S. aureus [75]. Aqueous, ethanol, methanol, and xylene extracts of Agaricus bisporus and P. sajor-caju, both saprophytic edible fungi, have shown antibacterial activity against E. coli, E. aerogenes, P. aeruginosa, and K. pneumoniae. Consumption of these fungi may provide natural protection against common pathogenic organisms [76]. Methanolic extracts of Hydnum repandum, an edible saprophytic species, have demonstrated activity against the Gram-negative bacteria P. aeruginosa [77]. The methanolic extract of the fruiting bodies of Lepista nuda, another edible fungus, exhibited antibacterial activity against E. coli and P. aeruginosa [78]. Dichloromethane extract from S. collitinus displayed activity against a range of bacteria, including E. coli, E. aerogenes, S. typhimurium, S. aureus, and S. epidermidis, B. subtilis, as well as C. albicans and S. cerevisiae [66][79]. Regarding L. sulphureus, both ethanolic and aqueous extracts from its fruiting bodies have shown antibacterial effects against various strains, including B. subtilis, B. cereus, M. luteus, M. flavus, and K. pneumoniae. Among these strains, M. flavus exhibited the highest susceptibility, while K. pneumoniae showed resistance. Although the efficacy of the active extracts was lower compared to commercial drugs, they still demonstrated potential as antibacterial agents. Furthermore, the aqueous extract of L. sulphureus fruiting bodies has shown antibacterial effects against M. flavus and L. monocytogenes [68][80]. Notably, the extract displayed significant efficacy against L. monocytogenes, a strain resistant to streptomycin [80]. A summary of the antibacterial activity of fungal extracts can be found in Table 3.
Table 3. Summary of antibacterial activity of fungal origin extracts.
| Species | Extract | Bacteria | References |
|---|---|---|---|
| Ganoderma lucidum | Acetone extract Aqueous extract Ethanol extract Methanol extract |
Bacillus subtilis Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa Salmonella typhimurium Staphylococcus aureus |
[64] |
| Ganoderma lucidum | Acetone extract | Bacillus subtilis Klebsiella oxytoca |
[65] |
| Hygrophorus agathosmus |
Chloroform extract | Bacillus subtilis Enterobacter aerogenes Escherichia coli Pseudomonas aeruginosa Salmonella typhimurium Staphylococcus aureus Staphylococcus epidermidis |
[66] |
| Suillus collitinus | Dichloromethanol extract | Bacillus subtilis Staphylococcus epidermidis |
[66] |
| Hypholoma fasciculare |
Methanol extract | Bacillus cereus Bacillus subtilis Staphylococcus aureus |
[67] |
| Laetiporus sulphureus | Ethanol extract | Bacillus cereus Bacillus subtilis Micrococcus flavus Micrococcus luteus |
[68] |
| Lentinula edodes | Chloroform extract Acetate-ethyl extract |
Actinomyces spp. Lactobacillus spp. Porphyromonas spp. Prevotella spp. Streptococcus spp. |
[69] |
| Leucopaxillus giganteus | Methanol extract (mycelial cultures) |
Bacillus cereus Bacillus subtilis Staphylococcus aureus |
[71] |
| Navesporus loccose Phellinus rimosus |
Methanol extract | Bacillus subtilis Staphylococcus aureus |
[72] |
| Pleurotus ostreatus Meripilus giganteus |
Ethanol extract | Sarcina lutea | [73] |
| Trametes versicolor | Methanol extract | Gram-positive bacteria | [81] |
| Grifola frondosa | Ethanol extracts/polysaccharides |
Bacilluscereus | [74] |
| Gloeoporus thelephoroides Hexagonia hydnoides Phellinus spp. |
Acetate-ethyl extract | Bacillus cereus | [75] |
| Nothopanus hygrophanus |
Acetate-ethyl extract | Listeria monocytogenes Staphylococcus aureus |
[75] |
| Agaricus bisporus Pleurotus sajor–caju |
Aqueous extract Ethanol extract Methanol extract Xylene extract |
Enterobacter aerogenes Escherichia coli 390 Escherichia coli 739 Klebsiella pneumoniae Pseudomonas aeruginosa |
[76] |
| Hydnum repandum | Methanol extract | Pseudomonas aeruginosa | [77] |
| Lepista nuda | Methanol extract | Escherichia coli Pseudomonas aeruginosa |
[78] |
| Suillus collitinus | Dichloromethane extract | Bacillus subtilis Candida albicans Enterobacter aerogenes Escherichia coli Salmonella typhimurium Staphylococcus aureus Staphylococcus epidermidis |
[66] |
| Hygrophorus agathosmus |
Chloroform extract | Bacillus subtilis Enterobacter aerogenes Salmonella typhimurium Staphylococcus aureus Staphylococcus epidermidis |
[79] |
| Laetiporus sulphureus | Ethanol extract Aqueous extract |
Bacillus subtilis Bacillus cereus Micrococcus luteus Micrococcus flavus Klebsiella pneumoniea Listeria monocytogenes |
[68][80] |
References
- Sharma, N.; Tapwal, A.; Verma, R.; Kumar, D.; Nepovimova, E.; Kuca, K. Medicinal, nutritional, and nutraceutical potential of Sparassis Crispa s. lat.: A review. IMA Fungus 2022, 13, 8.
- Quack, W.; Anke, T.; Oberwinkler, F.; Giannetti, B.M.; Steglich, W. Antibiotics from Basidiomycetes. V. Merulidial, a new antibiotic from the basidiomycete Merulius tremellosus Fr. J. Antibiot. 1978, 31, 737–741.
- Heim, J.; Anke, T.; Mocek, U.; Steffan, B.; Steglich, W. Antibiotics from Basidiomycetes. XXIX: Pilatin, a new antibiotically active marasmane derivative from cultures of Flagelloscypha pilatii Agerer. J. Antibiot. 1988, 41, 1752–1757.
- Kupka, J.; Anke, T.; Giannetti, B.M.; Steglich, W. Antibiotics from Basidiomycetes. XIV. Isolation and biological characterization of hypnophilin, pleurotellol, and pleurotellic acid from Pleurotellus hypnophilus (Berk.) Sacc. Arch. Microbiol. 1981, 130, 223–227.
- Stärk, A.; Anke, T.; Mocek, U.; Steglich, W.; Kirfel, A.; Will, G. Lentinellic acid, a biologically active protoilludane derivative from Lentinellus species (Basidiomycetes). Z Naturforsch. C J. Biosci. 1988, 43, 177–183.
- Obuchi, T.; Kondoh, H.; Watanabe, N.; Tamai, M.; Omura, S.; Jun-Shan, Y.; Xiao-Tian, L. Armillaric acid, a new antibiotic produced by Armillaria mellea. Planta. Med. 1990, 56, 198–201.
- Tabuchi, A.; Fukushima-Sakuno, E.; Osaki-Oka, K.; Futamura, Y.; Motoyama, T.; Osada, H.; Ishikawa, N.K.; Nagasawa, E.; Tokimoto, K. Productivity and bioactivity of enokipodins A–D of Flammulina rossica and Flammulina velutipes. Biosci. Biotechnol. Biochem. 2020, 84, 876–886.
- Chen, H.P.; Liu, J.K. Secondary Metabolites from Higher Fungi. Prog. Chem. Org. Nat. 2017, 106, 1–201.
- Novak, R.; Shlaes, D.M. The pleuromutilin antibiotics: A new class for human use. Curr Opin Investig Drugs 2010, 11, 182–191.
- Hofle, G.; Oberwinkler, F. The striatins-new antibiotics from the Basidiomycete Cyathus striatus (Huds. Ex Pers.) Willd. J. Antibiot. 1977, 30, 221–225.
- Šiljegović, J.D.; Stojković, D.S.; Nikolić, M.M.; Glamočlija, J.M.; Soković, M.D.; Ćirić, A.M. Antimicrobial activity of aqueous extract of Laetiporus sulphureus (Bull.: Fr.) Murill. Zb Matice. Srp. Prir. Nauk. 2011, 299–305.
- Ma, B.; Ren, W.; Zhou, Y.; Ma, J.; Ruan, Y.; Wen, C.N. Triterpenoids from the spores of Ganoderma lucidum. N. Am. J. Med. Sci. 2011, 3, 495–498.
- Mothana, R.A.A.; Jansen, R.; Jülich, W.D.; Lindequist, U. Ganomycins A and B, new antimicrobial farnesyl hydroquinones from the Basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 2000, 63, 416–418.
- Anke, T.; Kupka, J.; Schramm, G.; Steglich, W. Antibiotics from Basidiomycetes. X. Scorodonin, a new antibacterial and antifungal metabolite from Marasmius scorodonius (Fr.) Fr. J. Antibiot. 1980, 33, 463–467.
- Agrawal, K.; Verma, P. Fungal Metabolites: A Recent Trend and Its Potential Biotechnological Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering: Recent Advances in Application of Fungi and Fungal Metabolites: Current Aspects; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–14.
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441.
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Dutta, B.; Lahiri, D.; Nag, M.; Ghosh, S.; Dey, A.; Ray, R.R. Fungi in pharmaceuticals and production of antibiotics. In Applied Mycology: Entrepreneurship with Fungi; Springer International Publishing: Cham, Germany, 2022; pp. 233–257.
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of bioactive fungal secondary metabolites: Cytotoxic and antimicrobial compounds. Antibiotics 2022, 11, 1604.
- Bush, K.; Bradford, P.A. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247.
- Miller, E.L. The penicillins: A review and update. J. Midwifery Womens Health 2002, 47, 426–434.
- Bo, G. Giuseppe Brotzu and the discovery of cephalosporins. Clin. Microbiol. Infect. 2000, 6, 6–8.
- Fernandez, J.; Jimenez-Rodriguez, T.W.; Blanca-Lopez, N. Classifying Cephalosporins: From generation to cross-reactivity. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 346–354.
- Khan, D.A.; Banerji, A.; Bernstein, J.A.; Bilgicer, B.; Blumenthal, K.; Castells, M.; Ein, D.; Lang, D.M.; Phillips, E. Cephalosporin allergy: Current understanding and future challenges. J. Allergy Clin. Immunol. Pract. 2019, 7, 2105–2114.
- Harrison, C.J.; Bratcher, D. Cephalosporins: A review. Pediatr. Rev. 2008, 29, 264–273.
- Cordes, M.G. Fusidic Acid. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–4.
- Schöfer, H.; Simonsen, L. Fusidic acid in dermatology: An updated review. Eur. J. Dermatol. 2010, 20, 006–015.
- Long, J.; Ji, W.; Zhang, D.; Zhu, Y.; Bi, Y. Bioactivities and structure-activity relationships of fusidic acid derivatives: A review. Front. Pharmacol. 2021, 12, 759220.
- Fernandes, P. Fusidic Acid: A bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb. Perspect. Med. 2016, 6, a025437.
- Levy, D.; Bluzat, A.; Seigneuret, M.; Rigaud, J.L. Alkali cation transport through liposomes by the antimicrobial fusafungine and its constitutive enniatins. Biochem. Pharmacol. 1995, 50, 2105–2107.
- Lund, V.J.; Grouin, J.M.; Eccles, R.; Bouter, C.; Chabolle, F. Efficacy of fusafungine in acute rhinopharyngitis: A pooled analysis. Rhinology 2004, 42, 207–212.
- Tschen, J.S.M.; Chen, L.L.; Hsieh, S.T.; Wu, T.S. Isolation and phytotoxic effects of helvolic acid from plant pathogenic fungus Sarocladium Oryzae. Bot. Bull. Acad. Sin. 1997, 38, 251–256.
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124.
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710.
- Anke, H.; Sterner, O.; Steglich, W. Structure-activity relationships for unsaturated dialdehydes. Mutagenic, antimicrobial, cytotoxic, and phytotoxic activities of merulidial derivatives. J. Antibiot. 1989, 42, 738–744.
- Mehta, G.; Murthy, A.S.K. The first total synthesis of the novel triquinane natural products pleurotellol and pleurotellic acid. Tetrahedron. Lett. 2003, 44, 5243–5246.
- Cadelis, M.M.; Copp, B.R.; Wiles, S. A Review of fungal protoilludane sesquiterpenoid natural products. Antibiotics 2020, 9, 928.
- Midland, S.L.; Izac, R.R.; Wing, R.M.; Zaki, A.I.; Munnecke, D.E.; Sims, J.J. Melleolide, a new antibiotic from Armillaria mellea. Tetrahedron. Lett. 1982, 23, 2515–2518.
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial cuparene-type sesquiterpenes, enokipodins c and d, from a mycelial culture of Flammulina velutipes. J. Nat. Prod. 2001, 64, 932–934.
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.A.; Monte, A. Antibacterial Compounds from Mushrooms I: A Lanostane-type triterpene and prenylphenol derivatives from Jahnoporus hirtus and Albatrellus flettii and their activities against Bacillus cereus and Enterococcus faecalis. Planta. Med. 2010, 76, 182–185.
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980.
- Brinch, K.S.; Sandberg, A.; Baudoux, P.; Van Bambeke, F.; Tulkens, P.M.; Frimodt-Møller, N.; Høiby, N.; Kristensen, H.H. Plectasin shows intracellular activity against Staphylococcus aureus in Human THP-1 monocytes and in a mouse peritonitis model. Antimicrob. Agents Chemother. 2009, 53, 4801–4808.
- Raap, J.; Erkelens, K.; Ogrel, A.; Skladnev, D.A.; Brückner, H. Fungal biosynthesis of non-ribosomal peptide antibiotics and α, α-dialkylated amino acid constituents. J. Pept. Sci. 2005, 11, 331–338.
- Rossi, C.; Eid, M.; Rippa, S.; Castano, S.; Desbat, B.; Chopineau, J.; Béven, L. Exploring the membrane mechanism of the bioactive peptaibol ampullosporin a using lipid monolayers and supported biomimetic membranes. J. Biophys. 2010, 2010, 179641.
- Lee, S.J.; Yeo, W.H.; Yun, B.S.; Yoo, I.D. Isolation and sequence analysis of new peptaibol, boletusin, from Boletus spp. J. Pept. Sci. 1999, 5, 374–378.
- Isaac, C.E.; Jones, A.; Pickard, M.A. Production of cyclosporins by Tylypocladium niveum strains. Antimicrob. Agents Chemother. 1990, 34, 121–127.
- Jonsson, M. The Toxicity of Fusarium Mycotoxins Enniatin and Moniliformin; University of Helsinki: Helsinki, Finland, 2017; ISBN 978-952-225-164-0.
- McMorris, T.C.; Turner, W.B. Fungal metabolites. Mycologia 1972, 64, 464.
- Ngai, P.H.K.; Ng, T.B. A ribonuclease with antimicrobial, antimitogenic and antiproliferative activities from the edible mushroom Pleurotus sajor-caju. Peptides 2004, 25, 11–17.
- Garcia, P.; De Oliveira, A.; Batista, R. Occurrence, biological activities and synthesis of kaurane diterpenes and their glycosides. Molecules 2007, 12, 455–483.
- Aqueveque, P.; Becerra, J.; Palfner, G.; Silva, M.; Alarcon, J.; Anke, T.; Sterner, O. Antimicrobial activity of metabolites from mycelial cultures of Chilean Basidiomycetes. J. Chil. Chem. Soc. 2006, 51, 1057–1060.
- Sandargo, B.; Thongbai, B.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral 4-hydroxypleurogrisein and antimicrobial pleurotin derivatives from cultures of the nematophagous Basidiomycete Hohenbuehelia grisea. Molecules 2018, 23, 2697.
- Bender, S.; Dumitrache-Anghel, C.N.; Backhaus, J.; Christie, G.; Cross, R.F.; Lonergan, G.T.; Baker, W.L. A case for caution in assessing the antibiotic activity of extracts of culinary-medicinal shiitake mushroom (Agaricomycetideae). Int. J. Med. Mushrooms. 2003, 5, 6.
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Oxalic Acid, a Molecule at the Crossroads of Bacterial-Fungal Interactions. Adv. Appl. Microbiol. 2019, 106, 49–77.
- Quang, D.N.; Bach, D.D.; Hashimoto, T.; Asakawa, Y. Chemical constituents of the vietnamese inedible mushroom Xylaria intracolorata. Nat. Prod. Res. 2006, 20, 317–321.
- Beattie, K.D.; Rouf, R.; Gander, L.; May, T.W.; Ratkowsky, D.; Donner, C.D.; Gill, M.; Grice, I.D.; Tiralongo, E. Antibacterial metabolites from australian macrofungi from the genus Cortinarius. Phytochemistry 2010, 71, 948–955.
- Schwan, W.R.; Dunek, C.; Gebhardt, M.; Engelbrecht, K.; Klett, T.; Monte, A.; Toce, J.; Rott, M.; Volk, T.J.; LiPuma, J.J. Screening a mushroom extract library for activity against Acinetobacter baumannii and Burkholderia cepacia and the identification of a compound with anti-burkholderia activity. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 4.
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72.
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive packaging materials from edible chitosan polymer—Antimicrobial activity assessment on dairy-related contaminants. J. Food Sci. 2003, 68, 2788–2792.
- Kumar, M. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27.
- Jeihanipour, A.; Keikhosro, K.; Taherzadeh, M. Antimicrobial Properties of Fungal Chitosan. Res. J. Biol. Sci. 2007, 2, 239–243.
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475.
- Paterson, R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001.
- Quereshi, S.; Pandey, A.K.; Sandhu, S.S. Evaluation of antibacterial activity of different Ganoderma lucidum extracts. PJSR 2010, 3, 9–13.
- Yoon, S.Y.; Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch. Pharm. Res. 1994, 17, 438–442.
- Yamaç, M.; Bilgili, F. Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 2006, 44, 660–667.
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Chemical composition and biological properties of Portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862.
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2006, 101, 267–273.
- Hirasawa, M.; Shouji, N.; Neta, T.; Fukushima, K.; Takada, K. Three kinds of antibacterial substances from Lentinus edodes (Berk.) Sing. (Shiitake, an Edible Mushroom). Int. J. Antimicrob. Agents 1999, 11, 151–157.
- Signoretto, C.; Burlacchini, G.; Marchi, A.; Grillenzoni, M.; Cavalleri, G.; Ciric, L.; Lingström, P.; Pezzati, E.; Daglia, M.; Zaura, E. Testing a low molecular mass fraction of a mushroom (Lentinus edodes) extract formulated as an oral rinse in a cohort of volunteers. J. Biomed. Biotechnol. 2011, 2011, 857987.
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 2007, 105, 179–186.
- Sheena, N.; Ajith, T.A.; Mathew, A.; Janardhanan, K.K. Antibacterial activity of three macrofungi, Ganoderma lucidum, Navesporus floccosa and Phellinus rimosus occurring in South India. Pharm. Biol. 2003, 41, 564–567.
- Kalyoncu, F.; Oskay, M.; Sağlam, H.; Erdoğan, T.F.; Tamer, A.U. Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J. Med. Food 2010, 13, 415–419.
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; Jakovljevic, D.; Zizak, Z.; Pavlovic, V.; Levic, S.; Niksic, M.; Van Griensven, L.J.L.D. Biological potential of extracts of the wild edible basidiomycete mushroom Grifola frondosa. Food Res. Int. 2015, 67, 272–283.
- Rosa, L.H.; Gomes Machado, K.M.; Jacob, C.; Capelari, M.; Rosa, C.A.; Zani, C.L. Screening of Brazilian Basidiomycetes for antimicrobial activity. Mem. Inst. Oswaldo. Cruz. 2003, 98, 967–974.
- Tambekar, D.H.; Sonar, T.P.; Khodke, M.V.; Khante, B.S. The novel antibacterials from two edible mushrooms: Agaricus bisporus and Pleurotus sajor -caju. Int. J. Pharmacol. 2006, 2, 584–587.
- Ozen, T.; Darcan, C.; Aktop, O.; Turkekul, I. Screening of antioxidant, antimicrobial activities and chemical contents of edible mushrooms wildly grown in the Black Sea region of Turkey. Comb. Chem. High Throughput. Screen. 2011, 14, 72–84.
- Dulger, B.; Ergul, C.C.; Gucin, F. Antimicrobial activity of the macrofungus Lepista nuda. Fitoterapia 2002, 73, 695–697.
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta. Med. 2012, 78, 1707–1718.
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I.C.F.R.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451.
- Hleba, L. Antimicrobial activity of crude methanolic extracts from Ganoderma lucidum and Trametes versicolor. Anim. Sci. Biotechnol. 2014, 47, 89–93.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
04 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




