Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sylwia Smolinska | -- | 1279 | 2023-08-31 19:20:04 | | | |
| 2 | Camila Xu | Meta information modification | 1279 | 2023-09-01 03:00:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Smolinska, S.; Antolín-Amérigo, D.; Popescu, F.; Jutel, M. Thymic Stromal Lymphopoietin, Its Receptors and Molecular Interactions. Encyclopedia. Available online: https://encyclopedia.pub/entry/48716 (accessed on 07 February 2026).
Smolinska S, Antolín-Amérigo D, Popescu F, Jutel M. Thymic Stromal Lymphopoietin, Its Receptors and Molecular Interactions. Encyclopedia. Available at: https://encyclopedia.pub/entry/48716. Accessed February 07, 2026.
Smolinska, Sylwia, Darío Antolín-Amérigo, Florin-Dan Popescu, Marek Jutel. "Thymic Stromal Lymphopoietin, Its Receptors and Molecular Interactions" Encyclopedia, https://encyclopedia.pub/entry/48716 (accessed February 07, 2026).
Smolinska, S., Antolín-Amérigo, D., Popescu, F., & Jutel, M. (2023, August 31). Thymic Stromal Lymphopoietin, Its Receptors and Molecular Interactions. In Encyclopedia. https://encyclopedia.pub/entry/48716
Smolinska, Sylwia, et al. "Thymic Stromal Lymphopoietin, Its Receptors and Molecular Interactions." Encyclopedia. Web. 31 August, 2023.
Copy Citation
Thymic stromal lymphopoietin (TSLP) is a pleiotropic cytokine that has emerged as a critical player in the development and progression of allergy and asthma. It is primarily produced by epithelial cells and functions as a potent immune system activator.
thymic stromal lymphopoietin
epithelium
allergy
1. Introduction
The cytokine TSLP was initially identified in conditioned medium from a murine thymic stromal cell line with a medullary phenotype as a growth factor for B and T cells and co-stimulator for thymocyte proliferation, consequently suggesting its role as lymphopoietin. The human TSLP homolog was found using in silico methods, detecting the homology to mouse TSLP by a computational screen of the human genomic databases [1][2]. TSLP was categorized later on as a epithelial-derived crucial mediator of the type 2 immune responses and is nowadays considered as a cytokine with multiple functions and critical roles in diverse physiological and pathological conditions, including asthma, allergic diseases, chronic inflammatory diseases and cancer [1]. Some of these include regulation of immune responses, acting as an early alarm signal with significant roles in epithelial barrier function, dendritic cell activation, type 2 innate lymphoid cells (ILC2s) activation and survival, immune cell recruitment, induction of T2 responses and regulation of B cell function, explaining its involvement in tissue homeostasis, host defence and microbiome regulation, and in the pathophysiology of allergic and inflammatory diseases. TSLP is a cytokine mainly derived from epithelial cells, which occupies an upstream position in the asthma inflammatory cascade [1][2].
2. TSLP, Its Receptors and Molecular Interactions
TSLP is a four α-helical type I cytokine which plays critical roles in diverse physiological and pathological conditions [1][2].
Various stimuli, including mechanical injury, ligands for Toll-like receptors (TLR2, TLR3) and NOD2, pro-inflammatory cytokines and allergen proteases can induce the expression of the alarmin cytokine TSLP in epithelial cells [1][3][4].
In the lungs, TSLP production by epithelial cells with a rapid cellular release is also triggered by viral infections, including those with respiratory syncytial viruses, rhinoviruses, influenza viruses and metapneumoviruses, which incite other danger signals and exacerbate inflammation [3][5][6].
Moreover, when challenged by allergen molecules, barrier cells such as bronchial and intestinal epithelial cells can overproduce reactive oxygen species (ROS) by up-regulating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and activating the nuclear transcription factor NF-κB signal pathway, leading to elevated cytokines including TSLP [7][8][9].
TSLP, mainly produced in response to pathogenic stimuli by lung and intestinal epithelial cells and skin keratinocytes, induces complex immune responses at barrier surface level by activation of dendritic cells (DCs), mast cells, eosinophils and lymphocytes into a type 2 polarizing phenotype [10][11]. Therefore, nowadays, TSLP is considered a master regulator of T2-driven inflammation [12].
TSLP activates intracellular signalling by forming a complex with its specific receptor, TSLPR (encoded by CRLF2) and IL-7Rα. The cognate receptor TSLPR allosterically triggers TSLP to potentiate the recruitment of the shared IL-7 receptor α-chain (IL-7Rα) by leveraging the cytokine molecule’s conformational heterogeneity, flexibility and electrostatics [13][14][15][16].
TSLP has a four-helix structure stabilized by three disulfide bridges, in which the four α-helices, designated αA to αD, are linked via a BC loop and two longer AB and CD loop regions. TSLP, positively charged, binds to TSLPR, negatively charged [15].
TSLP engages its C-terminal short tail, C-terminal half of the helix αD and continuous 10 residues in the long AB loop region (Figure 1, part ①) to interact with cytokine-binding homology region of the receptor TSLPR (site I) with cooperative recruitment of IL-7Rα (site II), allowing the membrane-proximal parts of the two receptors to engage in heterotypic receptor–receptor interactions (site III). [1][15].
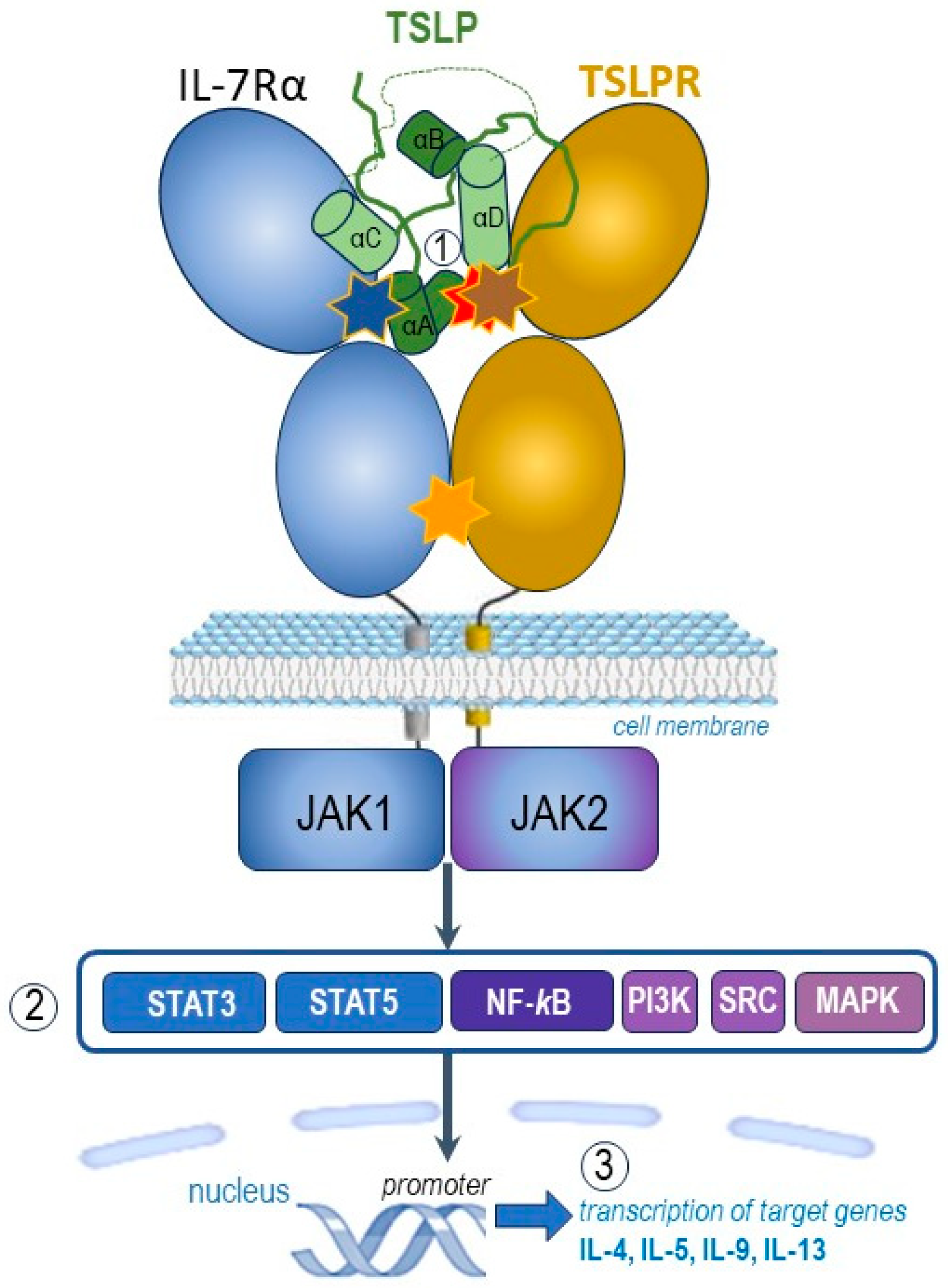
Figure 1. Graphic representation of the pro-inflammatory TSLP mediated complex and its intracellular signalling pathways (adapted after [1][13][14][15][16]). TSLP with an atypical open helical bundle core, wedges between TSLPR and IL-7Rα to mediate the T-shaped extracellular assembly. The brown star indicates site I (TSLP:TSLPR interface) with pronounced electrostatic complementarity, the blue star denotes site II (TSLP:IL-7Rα interface) and the orange one site III (IL-7Rα:TSLPR interface). TSLP, represented here in green, is the long-form TSLP (the common part with the short-form TSLP is presented in light green). The hidden red star highlights the binding region of tezepelumab overlapped by the binding region on TSLP on TSLPR. The parts noted 1, 2, and 3 of this figure represent complex molecular events at the receptor (1), transcription factors (2) and target genes (3) levels, as explained in the text.
Following the capture and rearrangement of TSLP by TSLPR at the cellular surface, IL-7Rα is recruited to initiate intracellular pro-inflammatory JAK-STAT pathways (Figure 1, part ②). The JAK-STAT signalling pathway is an important chain of interactions between proteins in a cell, which involves Janus kinase (JAK) and signal transducer and activator of transcription (STAT) [1][12].
TSLP intracellular signalling cascades are initiated by JAK1/JAK2, molecules involved in phosphorylation of STAT3/5, but also include NF-κB (nuclear factor κB), MAPK (mitogen-activated protein kinases), PI3K (phosphoinositide 3 kinase), SRC (sarcoma tyrosine kinase) pathways. TSLP activates JAK1 (via IL-7Rα) and JAK2 (via TSLPR). JAK1 and JAK2 then activate signal transducer and activator of transcription 5 (STAT5) and, to a lesser extent, STAT3 and other transcription factors [1][13][14][15][16].
Finally, the transcription of target genes is promoted (Figure 1, part ③), including type 2 pro-inflammatory cytokines, such as IL-5 (key cytokine in eosinophilic inflammation), IL-9 (important cytokine in allergic inflammation), IL-4 and IL-13 (key cytokines in type 2 inflammation) [1][12].
Altogether, the binding of TSLP to its heterodimeric receptor complex TSLPR and IL-7Rα initiates intracellular activation of several downstream protein tyrosine kinases with phosphorylation of transcription factors which initiate the transcription of some target genes including those encoding pro-inflammatory cytokines involved in allergic and eosinophilic inflammation [12][16].
TSLP engages the C-terminal part of the helix αD (residues 142–152), the C-terminal tail extending from αD (residues 153–158), with an important triplet of arginine residues, and a continuous sequence of 10 residues in the AB loop region (residues 60–69) to interact with a complementary epitope shaped at the cytokine-binding homology region of the receptor TSLPR (Figure 2). Key interactions between the C-terminal helix of TSLP and the binding groove of TSLPR were depicted. The strong binding is mediated by a network of hydrophobic interactions and hydrogen bonds between TSLP Leu156 and TSLPR Leu39 [12][15]. In addition, the AB loop may act as a link between the two receptor binding regions on TSLPR (sites I and II), balanced to dispatch the binding event to TSLPR at site I to prime TSLP for the cooperative recruitment of IL-7Rα at site II. The AB loop provides a physical link to αA, which is central to defining site II. Human TSLP-Leu45 interacts with both TSLPR and IL-7Rα [15].
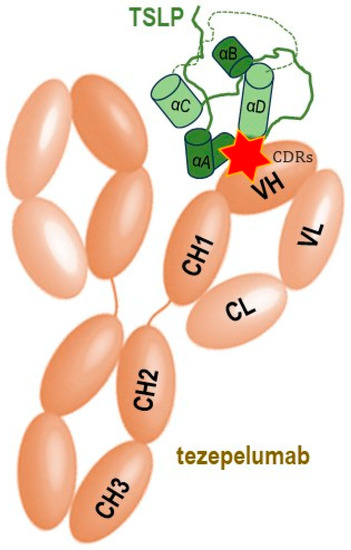
Figure 2. Schematic simplified representation of the molecular binding of the monoclonal antibody tezepelumab to the human TSLP with subsequent blocking of its interaction with the heterodimeric TSLP receptor, thus impeding the formation of the TSLPR: TSLP: IL-7Rα ternary complex on effector cells. The CDRs of the VH of tezepelumab target TSLP at the C-terminal region of helix αD and AB loop region (marked by red star), while the VL does not interact with TSLP (adapted after [15]). TSLP represented here in green is the long-form TSLP (the common part with the short-form TSLP is highlighted in light green).
Tezepelumab, a fully human monoclonal IgG2λ antibody currently indicated for the treatment of severe asthma, specifically binds human TSLP at the level of its ligation site for TSLPR, thereby impeding the TSLP-TSLPR interaction [16]. The complementarity determining regions of the VH (variable heavy chain domain) of tezepelumab target TSLP at the C-terminal region of helix D and AB loop region, while the VL (variable light chain fragment) does not interact with TSLP at all. Tezepelumab competes against a critical part of the TSLPR binding region on TSLP but remains completely clear of the IL-7Rα binding region on the other side of the TSLP helical bundle [15].
References
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2022, 23, 24–37.
- Quentmeier, H.; Drexler, H.G.; Fleckenstein, D.; Zaborski, M.; Armstrong, A.; Sims, J.E.; Lyman, S.D. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 2001, 15, 1286–1292.
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.-R.P.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258.
- Lv, J.; Yu, Q.; Lv, J.; Di, C.; Lin, X.; Su, W.; Wu, M.; Xia, Z. Airway epithelial TSLP production of TLR2 drives type 2 immunity in allergic airway inflammation. Eur. J. Immunol. 2018, 48, 1838–1850.
- Lee, H.-C.; Headley, M.B.; Loo, Y.-M.; Berlin, A.; Gale, M.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus–infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5.
- Stier, M.T.; Bloodworth, M.H.; Toki, S.; Newcomb, D.C.; Goleniewska, K.; Boyd, K.L.; Quitalig, M.; Hotard, A.L.; Moore, M.L.; Hartert, T.V.; et al. Respiratory syncytial virus infection activates IL-13–producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2016, 138, 814–824.e11.
- Rodriguez-Coira, J.; Villaseñor, A.; Izquierdo, E.; Huang, M.; Barker-Tejeda, T.C.; Radzikowska, U.; Sokolowska, M.; Barber, D. The Importance of Metabolism for Immune Homeostasis in Allergic Diseases. Front. Immunol. 2021, 12, 692004.
- Hristova, M.; Habibovic, A.; Veith, C.; Janssen-Heininger, Y.M.; Dixon, A.E.; Geiszt, M.; van der Vliet, A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 2016, 137, 1545–1556.e11.
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005, 115, 2169–2179.
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680.
- Bell, B.D.; Kitajima, M.; Larson, R.P.; Stoklasek, T.A.; Dang, K.; Sakamoto, K.; Wagner, K.-U.; Kaplan, D.H.; Reizis, B.; Hennighausen, L.; et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat. Immunol. 2013, 14, 364–371.
- Adhikary, P.P.; Tan, Z.; Page, B.D.; Hedtrich, S. TSLP as druggable target—A silver-lining for atopic diseases? Pharmacol. Ther. 2020, 217, 107648.
- Pandey, A.; Ozaki, K.; Baumann, H.; Levin, S.D.; Puel, A.; Farr, A.G.; Ziegler, S.F.; Leonard, W.J.; Lodish, H.F. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000, 1, 59–64.
- Park, L.S.; Martin, U.; Garka, K.E.; Gliniak, B.; Di Santo, J.P.; Muller, W.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A.; Farr, A.G.; et al. Cloning of the Murine Thymic Stromal Lymphopoietin (Tslp) Receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000, 192, 659–670.
- Verstraete, K.; Peelman, F.; Braun, H.; Lopez, J.; Van Rompaey, D.; Dansercoer, A.; Vandenberghe, I.; Pauwels, K.; Tavernier, J.; Lambrecht, B.N.; et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 2017, 8, 14937.
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Gallelli, L.; Terracciano, R.; Vatrella, A. Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma. Int. J. Mol. Sci. 2021, 22, 4369.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
901
Revisions:
2 times
(View History)
Update Date:
01 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




