Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rupal Mehta | -- | 1857 | 2023-08-31 14:52:09 | | | |
| 2 | Dean Liu | -9 word(s) | 1848 | 2023-09-01 02:55:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mehta, R.I.; Mehta, R.I. Giant Arachnoid Granulations. Encyclopedia. Available online: https://encyclopedia.pub/entry/48703 (accessed on 08 February 2026).
Mehta RI, Mehta RI. Giant Arachnoid Granulations. Encyclopedia. Available at: https://encyclopedia.pub/entry/48703. Accessed February 08, 2026.
Mehta, Rupal I., Rashi I. Mehta. "Giant Arachnoid Granulations" Encyclopedia, https://encyclopedia.pub/entry/48703 (accessed February 08, 2026).
Mehta, R.I., & Mehta, R.I. (2023, August 31). Giant Arachnoid Granulations. In Encyclopedia. https://encyclopedia.pub/entry/48703
Mehta, Rupal I. and Rashi I. Mehta. "Giant Arachnoid Granulations." Encyclopedia. Web. 31 August, 2023.
Copy Citation
Arachnoid granulations (AGs) are macroscopically visible arachnoid tissue outpouchings that protrude into bone, dura, and/or dural venous sinuses (DVSs).
GAG
giant arachnoid granulation
1. Introduction
Arachnoid granulations (AGs) are macroscopically visible arachnoid tissue outpouchings that protrude into bone, dura, and/or dural venous sinuses (DVSs) [1]. Historically, they have been defined by their juxtaposition and drainage into the superior sagittal sinus (SSS) and other DVSs. AGs primarily consist of collagen, immune cells, and cerebrospinal fluid (CSF)-filled spaces situated at brain borders [1]. These structures were described in 1543 by Vesalius [2] and were further characterized by Willis in 1664, Littre in 1684, Collins in 1685, Mery in 1701, and Pacchioni in 1705 [3][4][5]. However, they were only recently systematically characterized through detailed radiologic–pathologic investigation incorporating comprehensive analyses with cellular and molecular markers, thus enhancing knowledge of their anatomy and potential functions [1].
Typically, AGs are asymptomatic structures that abut dural tissues and measure only a few millimeters in diameter [1][6], but they occasionally enlarge to form so-called giant arachnoid granulations (GAGs) that may also associate with bone marrow spaces and, rarely, scalp dermal tissue. These structures may also cause clinical symptoms and/or nodular DVS filling defects on venography that manifest secondary to flow aberrations, DVS expansion, venous stenosis, and/or other suspected pathologies. GAGs have increasingly been reported in recent years with several cases involving the DVS and others found in extrasinus calvarial or diploic locations.
2. Reports and GAG Cases
The published literature from 1973 to 2023 yielded 41 publications describing GAGs in 169 persons [3][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46]. This incorporated reports of 146 persons each with a single GAG; 21 persons with at least two GAGs [7][8][9][10][11]; and 2 persons who each exhibited “multiple” GAGs, with three [12] or four [8] GAGs being discernible on presented imaging. Considering the above, the review yielded a total of at least 195 reported GAGs. Of these, 164 (84%) were intrasinus-type, 30 (15%) were calvarial-type, and 1 (1%) was mixed-type; the specific location was recorded in 182 (93%); clinical history was available for 92 (47%); imaging data were available in 140 (72%); and histologic data were available in 52 (27%) [3][9][10][13][14][15][16]. Sex was available for 147 of 169 persons (87%) [3][10][13][14][15][16]. Among the GAG cases with histology, 14 presented at surgery [3][12][13][14][15][16][45], and 27 presented at autopsy [9][10].
3. Demographic Features
Of the 147 persons with documented gender, GAGs involved 80 (54%) females and 67 (46%) males and were similarly distributed across sex (1.2:1 female-to-male ratio). The afflicted persons included infants [15], children [10][16][17][18][19][20], adolescents [17][21][22][23][24], and adults across a wide age spectrum [10][15][45] (range, 0.33 to 91 years; mean, 43 ± 20 years). Interestingly, one pediatric case was reported by parents since birth [16]. The mean number of GAGs per person was 1.0 and the overall number of GAGs recorded per decade of life is depicted in Figure 1A. Most persons with GAGs exhibited no comorbidities or past medical history but 4 of 169 persons (2%) were noted to be moderately obese or had cerebral small vessel disease [7][25][26]; 1 of 169 persons (1%) had a history of retrobulbar neuritis [3]; and 1 of 169 persons (1%) had staring episodes as well as a family history of seizures [10].
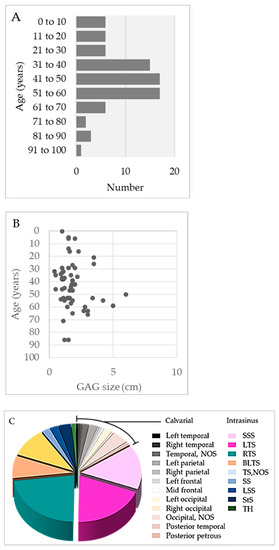
Figure 1. (A) Summary of the age distribution of persons with GAGs, according to decade of life. (B) GAG size distribution according to age. (C) Summary of GAG distribution by location.
4. GAG Size and Morphology
The size distribution of GAGs is summarized in Figure 1B. The mean diameter of GAGs was 1.9 cm ± 1.1 cm (standard deviation), though diameters ranged from 0.4 to 6.0 cm. Notably, five reported GAGs measured less than 1 cm in diameter (range: 4 to 9 mm) [26][32][33]. There was no direct correlation of GAG size with age [11]. The person’s age and GAG size were recorded for only 24 persons with recorded gender. Analyses among these cases revealed no statistically significant difference in mean age across male versus female individuals (females, 38 years; males, 45 years). However, a statistically significant difference was noted for GAG diameter across sex (females, 1.78 cm; males, 3.39 cm; p < 0.05) [3][13][15][16][20][23][26][27][28][29][30][31][32][33]. GAGs also varied in shape. Typically, GAGs were well-defined, nodular, round-to-ovoid structures, but others were irregular and a discrete, oblong vermiform shape was also characterized in one person (1%) [31].
5. Anatomical Distribution and Frequency
The majority of GAGs presented along SSS or transverse sinuses, or in parasagittal brain regions, whereas a subset involved the temporal bone (Figure 2C) and caused the compression of inner or middle ear structures [14][37]. Postmortem DVS studies reveal GAGs in 3.68–20% of adolescent and adult autopsies [9][10]. However, these analyses likely underestimate the true number of GAGs since they did not examine calvarial-type GAGs. Although imaging series are on record [11][18], no imaging study has analyzed the true prevalence of GAGs in live persons.
7. Reported GAG Histology
Fourteen calvarial-type or mixed-type GAGs were evaluated histologically [3][13][14][15][16][45]. Surgically resected bone and soft tissue elements from these structures were studied with routine hematoxylin and eosin (H&E) staining following the decalcification of bone tissues and revealed collagen and meningothelium. The workup of two cases incorporated immunohistochemistry analysis [13][45]. Both of these cases were analyzed with the use of the anti-EMA label, which confirmed the meningothelial component [13][45]. One case that underwent comprehensive immunohistochemistry workup additionally revealed the presence of S100-positive nerve twigs; CD68-positive, CD138-positive, or CD45-positive immune cells (consistent with the presence of foam cells or monocytes/macrophages, plasma cells, or lymphocytes, respectively) (Figure 2); and CD31-positive, CD34-positive, and D2-40-positive capillary vessels within the GAG (consistent with blood capillary vessels and/or lymphatic capillary vessels). A thrombosed vein, hemorrhage, lymphatic vascular obliteration, and meningothelial hyperplasia were also present within this reactive GAG (Figure 2) [45]. Histology on 8 of 14 (57%) GAGs confirmed diploic space infiltration by GAGs [3][13][14][15][16]. In 5 of 14 (36%) cases, a large CSF-filled central cavity was reported rather than a dense collagen core, and these were therefore reported to mimic unilocular cysts [3][13][15]. In 14 of 14 (100%) cases, the outer GAG surfaces were covered by arachnoid or dural cells, rather than by endothelium [3][13][14][15][16]. On a retrospective review of published histological images, 3 of 14 (21%) cases demonstrate apparent mononuclear immune cell infiltrate within the GAG core, though this was not characterized as immune cells in the original reports [14][15]. Moreover, 2 of 14 (14%) described the presence of fat cells, though a retrospective review of published histology images suggests that these were instead foam cells (i.e., lipid-laden monocytes/macrophages) that had been misinterpreted on histologic assessment [16].
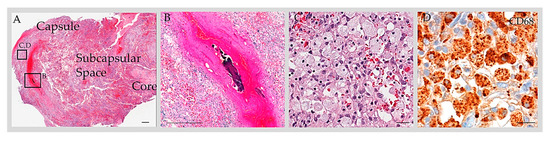
Figure 2. Anatomy of GAG. H&E-stained section of a GAG dome that was resected from an adult patient with post-traumatic headache revealed a multilaminar structure composed of a capsule, subcapsular space, and core (A). The subcapsular space contained blood and cells. On a high-power exam, a thrombosed vein (B) and foam cells (C) were present within the structure. Immunohistochemistry for CD68 highlighted a prominent number of cells, consistent with macrophages (D). Scale bars = (A,B), 100 µm; (C,D), 10 µm. Copped areas (black boxes in (A)) are shown in (B–D). Images reproduced from Int. J. Mol. Sci. 2023, 24, 11410 [45].
At least 27 DVS-type GAGs were identified on postmortem DVS examination [9][10][15]. However, their tissue composites were not analyzed in detail. The largest population-based anatomical study of DVS-type GAGs consisted of a postmortem investigation published by Haybaeck et al. [9] and incorporated data from H&E and Elastica van Gieson stains as well as immunohistochemistry preparations incorporating labels for vimentin, desmin, EMA, and S100. In this series, intrasinus GAGs were reported to consist of dense collagen and meningothelial cell clusters covered by an endothelial cell layer. Mamourian et al. [10] describe large, centrally-placed blood vessels within three DVS-type GAGs from two patients although characterization of the tissue component was limited.
8. Signs and Symptoms
While some reports define GAGs as normal AG variants of no known clinical significance [10][34], heterogeneous acute, subacute, and/or chronic signs and symptoms have been reported in association with many GAG cases. The most common presenting signs in persons with GAGs included headache (32 of 169 persons, 19%), vision change (10 of 169 persons, 6%), hearing change (9 of 169 persons, 5%), vertigo (6 of 169 persons, 4%), papilledema, and intracranial hypertension (each in 4 of 169 persons, 2%). Interestingly, 1 of 169 patients (1%) presented with a so-called laughing headache [7]. More ominous symptoms such as a change in consciousness, loss of consciousness, or seizure (each involving 2%) and meningism, neck pain, fever, and facial droop (each involving 1%) were also noted. Interestingly, 1 of 169 patients (1%) presented with repetitive hemorrhagic episodes, and 38 of 169 patients (22%) exhibited the herniation of brain parenchyma into a calvarial-type or DVS-type GAG, with involvement of cerebral cortical and/or cerebellar foliar tissue. A significant proportion of patients with herniated brain tissue, including one 5-year-old child, exhibited evidence of brain injury [8][17][18][20][38]. In a series of 27 patients, Gozgec et al. [17] reported a statistically significant positive correlation between the frequency of herniated brain damage and GAG size (p < 0.05).
Some afflicted persons indicated that symptoms had been ongoing for several years, or for decades prior to diagnosis [26][29][45]. Acute clinical events that exacerbated GAG symptoms were present in 9 of 169 persons (5%) and a relieving factor, i.e., internal jugular venous compression that mitigated pulsatile tinnitus, was noted in 6 of 169 persons (4%). A total of 3 of 169 (2%) patients with auditory changes complained of pulsatile tinnitus with “whooshing”, “swooshing”, or “sloshing” sounds [26][28][29]. All of these patients had transverse sinus or posterior temporal bone involvement by GAG [26][29], and one patient indicated that the frequency of the perceived auditory change was constant with her heartbeat [29]. Several patients indicated that GAG-associated symptoms had a significant impact on their quality of life or interfered with activities of daily living [7][17][26][29][45].
9. Imaging Features
Diagnosis of GAG on imaging workup was accomplished by visualization of round-to-ovoid, irregular or oblong, unilocular or multilocular cystic-appearing structures with or without internal septations, and with internal CSF-like density or signal intensity and communication with the subarachnoid space on MRI with and/or without contrast [8][13][15][17][19][20][22][23][24][25][26][28][30][31][36][37][38][39] (Figure 3A), MR angiogram/venogram [10][23][25][34][35][36][39][40][41][42], CT with and/or without contrast, or CT angiogram [8][21][25][27][28][31][34][35] (Figure 3B). GAGs were also identified as well-delineated focal calvarial defects on plain X-ray [13][15] or as focal filling defects within the DVS on conventional angiography and/or on cross-sectional studies [11]. GAGs with bone involvement caused smooth, evenly marginated impressions on the inner table of the skull and sometimes expanded into the diploic space, rarely eroding the outer skull table. Eight extrasinus-type GAGs that exhibited large “erosive” or “destructive” osteolytic calvarial defects were suspected to be malignant tumors [3][12][13][15][16]. MRI was the test of choice for differentiating GAGs from DVS thrombosis.
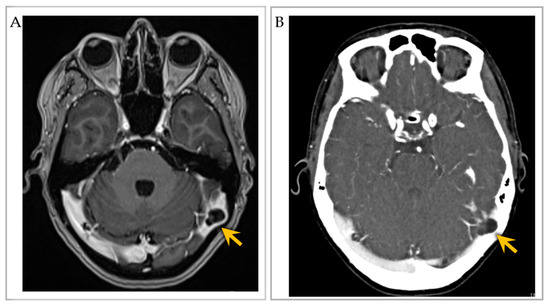
Figure 3. Imaging of an individual with multiple intrasinus-type GAGs. (A) Post-contrast T1-weighted brain MRI axial image shows two GAGs along the right and left lateral transverse sinuses, with severe sinus narrowing on the left (arrow). (B) CT venogram axial image further depicts the two GAGs with severe left lateral transverse sinus luminal narrowing (arrow). Images reproduced from Int. J. Mol. Sci. 2023, 24, 11410 [45].
While the internal GAG characteristics generally paralleled those of CSF on CT and MRI, GAGs more commonly demonstrated internal vascular (i.e., presumed veins) and/or soft tissue elements that were not easily observable in smaller AGs. In an imaging review, brain parenchymal herniation into GAGs was found in 22% of DVS-type GAGs [18]. The internal MRI signal was CSF-incongruent in a majority of GAG cases [11][18] and this differential signal was most commonly identifiable on high-resolution T2-weighted or T2-FLAIR sequences [18]. In a retrospective MRI analysis of DVS-type GAGs published by Ogul et al. [18], vessels were identified in 33 of 45 GAGs (73.3%) and were best observable by contrast-enhanced dynamic MR venography or post-contrast high-resolution T1-weighted MPRAGE sequences. An internal GAG vein was demonstrated in 22 out of 26 (84.6%) female patients by dynamic MR venography and was significantly more common than in males (p = 0.04), although the reason for this sex difference is unclear [18].
References
- Shah, T.; Leurgans, S.E.; Mehta, R.I.; Yang, J.; Galloway, C.A.; Bentley, K.L.d.M.; Schneider, J.A.; Mehta, R.I. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J. Exp. Med. 2023, 220, e20220618.
- Vesalius, A. Fabrica; Singer, C., Translator; Oxford University Press: Oxford, UK, 1952.
- Scully, R.B.; Mark, E.J.; McNeely, B.U. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 42-1984. A 29-year-old woman with a lytic lesion of a parietal bone. N. Engl. J. Med. 1984, 311, 1036–1043.
- Turner, L. The structure of arachnoid granulations with observations on their physiological and pathological significance. Ann. R. Coll. Surg. Engl. 1961, 29, 237–264.
- Willis, T. Cerebri Anatome; cui Accessit Nervorum Descriptio et Usus; Flesher: London, UK, 1664.
- Leach, J.L.; Jones, B.V.; Tomsick, T.A.; Stewart, C.A.; Balko, M.G. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: Differentiation from dural sinus disease. AJNR Am. J. Neuroradiol. 1996, 17, 1523–1532.
- Giraud, P.; Segal, O.; Chauvet, S. Laughing headache with giant pacchionian granulations. Headache 2013, 53, 681–683.
- Khan, N.M.; Shaw, B.H.; Wallace, C.J.; Metz, L. A Pacchionian Puzzle. Can. J. Neurol. Sci. 2020, 47, 231–232.
- Haybaeck, J.; Silye, R.; Soffer, D. Dural arachnoid granulations and “giant” arachnoid granulations. Surg. Radiol. Anat. 2008, 30, 417–421.
- Mamourian, A.C.; Towfighi, J. MR of giant arachnoid granulation, a normal variant presenting as a mass within the dural venous sinus. AJNR Am. J. Neuroradiol. 1995, 16, 901–904.
- Trimble, C.R.; Harnsberger, H.R.; Castillo, M.; Brant-Zawadzki, M.; Osborn, A.G. “Giant” arachnoid granulations just like CSF?: NOT!! AJNR Am. J. Neuroradiol. 2010, 31, 1724–1728.
- Lu, C.X.; Du, Y.; Xu, X.X.; Li, Y.; Yang, H.F.; Deng, S.Q.; Xiao, D.M.; Li, B.; Tian, Y.H. Multiple occipital defects caused by arachnoid granulations: Emphasis on T2 mapping. World J. Radiol. 2012, 4, 341–344.
- Chan, R.; de Tilly, L.N.; Bilbao, J. Magnetic resonance appearance of a giant cystic arachnoidal granulation presenting as an osteolytic parietal bone lesion: Case report. Can. Assoc. Radiol. J. 1999, 50, 126–129.
- Gacek, R.R. Arachnoid granulation cerebrospinal fluid otorrhea. Ann. Otol. Rhinol. Laryngol. 1990, 99, 854–862.
- Rosenberg, A.E.; O’Connell, J.X.; Ojemann, R.G.; Plata, M.J.; Palmer, W.E. Giant cystic arachnoid granulations: A rare cause of lytic skull lesions. Hum. Pathol. 1993, 24, 438–441.
- Beatty, R.M.; Hornig, G.W.; Hanson, E.J., Jr. Protruding arachnoid granulations mimicking dermoid cysts. J. Pediatr. Surg. 1989, 24, 411–413.
- Gozgec, E.; Ogul, H.; Izgi, E.; Kantarci, M. Tissue damage in herniated brain parenchyma into giant arachnoid granulations: Demonstration with high resolution MRI. Acta Radiol. 2021, 62, 799–806.
- Ogul, H.; Guven, F.; Izgi, E.; Kantarci, M. Evaluation of giant arachnoid granulations with high-resolution 3D-volumetric MR sequences at 3T. Eur. J. Radiol. 2019, 121, 108722.
- Park, H.; Lim, G.Y.; Eom, T.H. Giant arachnoid granulation in a child with benign intracranial hypertension: An unusual case. Childs Nerv. Syst. 2018, 34, 2525–2527.
- Sade, R.; Ogul, H. Herniation of the Cerebral Gyrus Into the Giant Arachnoid Granulation in a Child With Intermittent Headache. Headache 2016, 56, 750–752.
- Chin, S.C.; Chen, C.Y.; Lee, C.C.; Chen, F.H.; Lee, K.W.; Hsiao, H.S.; Zimmerman, R.A. Giant arachnoid granulation mimicking dural sinus thrombosis in a boy with headache: MRI. Neuroradiology 1998, 40, 181–183.
- De Keyzer, B.; Bamps, S.; Van Calenbergh, F.; Demaerel, P.; Wilms, G. Giant arachnoid granulations mimicking pathology. A report of three cases. Neuroradiol. J. 2014, 27, 316–321.
- Umeh, R.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. Giant Arachnoid Granulation Associated with Anomalous Draining Vein: A Case Report. Cureus 2017, 9, e1065.
- Taieb, G.; Dargazanli, C.; Prin, P.; Charif, M.; Ducros, A. Reversible giant arachnoid granulations. Neurology 2018, 91, 1107–1108.
- Kiroglu, Y.; Yaqci, B.; Cirak, B.; Karabulut, N. Giant arachnoid granulation in a patient with benign intracranial hypertension. Eur. Radiol. 2008, 18, 2329–2332.
- Gadot, R.; Hoang, A.N.; Raper, D.M.S.; Sweeney, A.D.; Juliano, M.; Lustrin, E.; Tanweer, O. Arachnoid Granulation Causing Unilateral Pulsatile Tinnitus Treated With Dural Venous Sinus Stenting. Otol. Neurotol. 2023, 44, 86–89.
- Peters, S.A.; Frombach, E.; Heyer, C.M. Giant arachnoid granulation: Differential diagnosis of acute headache. Australas. Radiol. 2007, 51, B18–B20.
- Deep, N.L.; Hoxworth, J.M.; Stevens, C.J.; Link, M.J.; Driscoll, C.L.; Wood, C.P. Giant Posterior Temporal Bone Arachnoid Granulations: CT and MRI Findings. Otol. Neurotol. 2016, 37, 963–966.
- Pereira, V.M.; Cancelliere, N.M.; Najafi, M.; MacDonald, D.; Natarajan, T.; Radovanovic, I.; Krings, T.; Rutka, J.; Nicholson, P.; Steinman, D.A. Torrents of torment: Turbulence as a mechanism of pulsatile tinnitus secondary to venous stenosis revealed by high-fidelity computational fluid dynamics. J. Neurointerv. Surg. 2021, 13, 732–737.
- Ayaz, E.; Atalay, B.; Baysal, B.; Senturk, S.; Aslan, A. Giant arachnoid granulation mimicking dural sinus thrombosis. North. Clin. Istanb. 2017, 4, 185–187.
- Mamaliga, T.; Hadi, M. An unusual vermiform giant arachnoid granulation. Radiol. Case Rep. 2019, 14, 1525–1528.
- Blaauw, G. Cranial extradural cyst. Acta Neurochir. 1979, 49, 81–86.
- Deprez, F.C.; Hernalsteen, D.; Bosschaert, P. Arachnoid Pacchioni’s granulation bulging in a transverse sinus of the brain. JBR-BTR 2010, 93, 104.
- Kan, P.; Stevens, E.A.; Couldwell, W.T. Incidental giant arachnoid granulation. AJNR Am. J. Neuroradiol. 2006, 27, 1491–1492.
- Choi, H.J.; Cho, C.W.; Kim, Y.S.; Cha, J.H. Giant arachnoid granulation misdiagnosed as transverse sinus thrombosis. J. Korean Neurosurg. Soc. 2008, 43, 48–50.
- Sunbulli, M.; Zak, I.; Chaturvedi, S. Giant arachnoid granulations. Neurology 2005, 64, 2150.
- Monté, A.S.; De Bleecker, J.L.; Buccauw, K.; Boulanger, T.; De Cocker, L.J. Giant arachnoid granulation of the posterior temporal bone wall mimicking a jugular foramen mass. Acta Neurol. Belg. 2015, 115, 421–422.
- Rodrigues, J.R.; Santos, G.R. Brain Herniation into Giant Arachnoid Granulation: An Unusual Case. Case Rep. Radiol. 2017, 2017, 8532074.
- Karegowda, L.H.; Rajagopal, K.; Krishnamurthy, S.K.; Lakshmana, S. Giant arachnoid granulation with a thrombosed dural arteriovenous fistula. BMJ Case Rep. 2018, 2018, bcr2018224851.
- Arjona, A.; Delgado, F.; Fernandez-Romero, E. Intracranial hypertension secondary to giant arachnoid granulations. J. Neurol. Neurosurg. Psychiatry 2003, 74, 418.
- Zheng, H.; Zhou, M.; Zhao, B.; Zhou, D.; He, L. Pseudotumor cerebri syndrome and giant arachnoid granulation: Treatment with venous sinus stenting. J. Vasc. Interv. Radiol. 2010, 21, 927–929.
- Yang, I.H.; Pereira, V.M.; Lenck, S.; Nicholson, P.; Orru, E.; Klostranec, J.M.; Krings, T.; Tsang, A.C.O. Endovascular treatment of debilitating tinnitus secondary to cerebral venous sinus abnormalities: A literature review and technical illustration. J. Neuro-Interv. Surg. 2019, 11, 841–846.
- Rosenberg, K.I.; Banik, R. Pseudotumor cerebri syndrome associated with giant arachnoid granulation. J. Neuro-Ophthalmol. 2013, 33, 417–419.
- Esposito, G.; Della Pepa, G.M.; Sturiale, C.L.; Gaudino, S.; Anile, C.; Pompucci, A. Hypertrophic arachnoid granulation of the occipital bone: Neuroradiological differential diagnosis. Clin. Neuroradiol. 2011, 21, 239–243.
- Mehta, R.I.; Mehta, R.I. Giant arachnoid granulations: Diagnostic workup and characterization in three symptomatic adults. Int. J. Mol. Sci. 2023, 24, 11410.
- Browder, J.; Kaplan, H.A.; Howard, E.M. Hyperplasia of pacchionian granulations. Arch. Pathol. 1973, 95, 315–316.
More
Information
Subjects:
Anatomy & Morphology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
569
Revisions:
2 times
(View History)
Update Date:
01 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




