
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Claire Chyrsanthi Karpodini | -- | 4324 | 2023-08-31 12:42:09 | | | |
| 2 | Sirius Huang | + 1 word(s) | 4325 | 2023-09-01 03:44:41 | | |
Video Upload Options
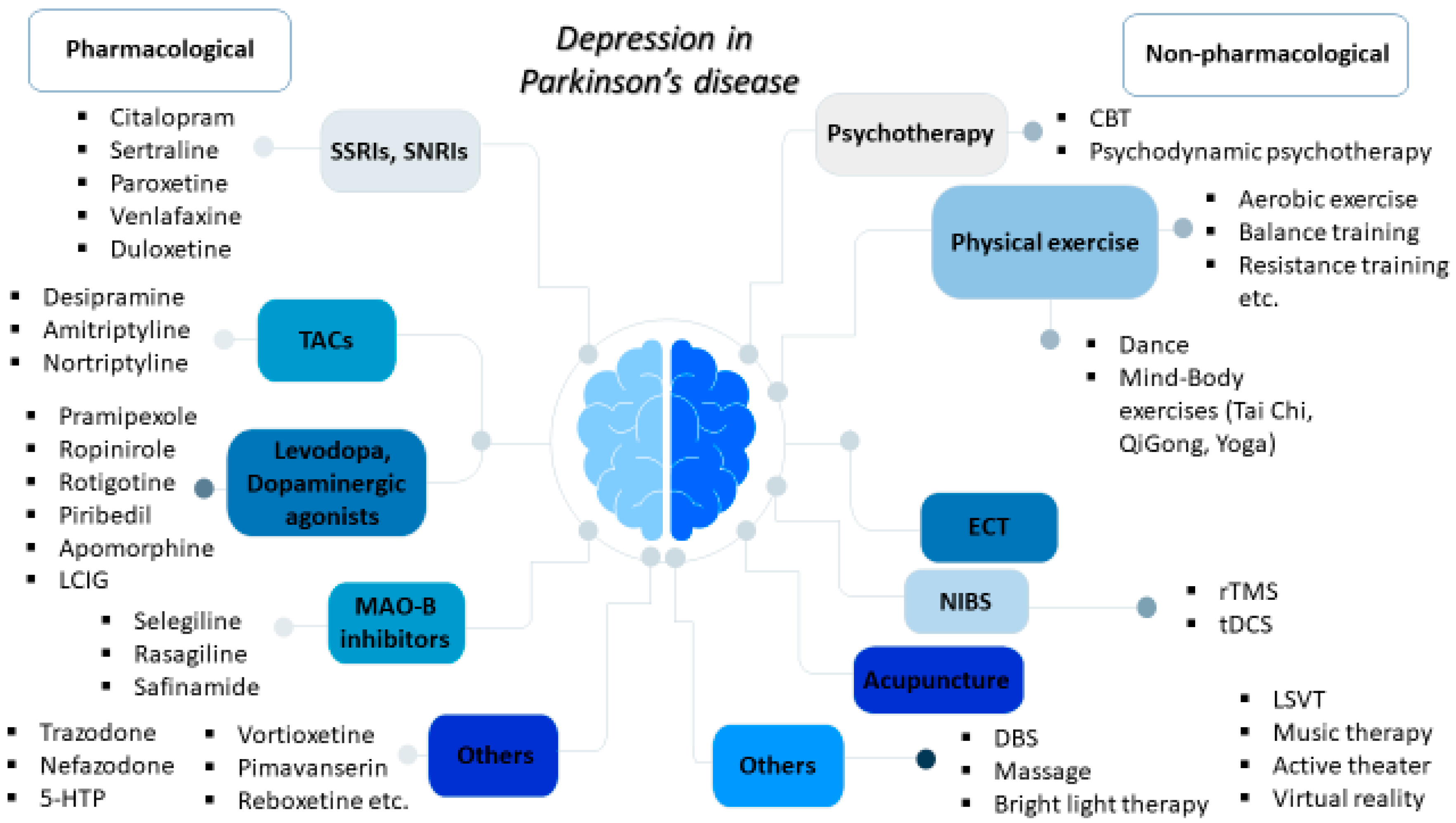
Depression represents one of the most common non-motor disorders in Parkinson’s disease (PD) and it has been related to worse life quality, higher levels of disability, and cognitive impairment, thereby majorly affecting not only the patients but also their caregivers. Available pharmacological therapeutic options for depression in PD mainly include selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants; meanwhile, agents acting on dopaminergic pathways used for motor symptoms, such as levodopa, dopaminergic agonists, and monoamine oxidase B (MAO-B) inhibitors, may also provide beneficial antidepressant effects. There is a growing interest in non-pharmacological interventions, including cognitive behavioral therapy; physical exercise, including dance and mind–body exercises, such as yoga, tai chi, and qigong; acupuncture; therapeutic massage; music therapy; active therapy; repetitive transcranial magnetic stimulation (rTMS); and electroconvulsive therapy (ECT) for refractory cases.
1. Introduction
2. Pharmacological Treatment Approaches for Depression in PD
| Pharmacological Treatments | |
|---|---|
| Type | Examples |
| SSRIs | citalopram, sertraline, paroxetine, etc. |
| SNRIs | venlafaxine, duloxetine, etc. |
| TCAs | desipramine, amitriptyline, nortriptyline, etc. |
| Levodopa | |
| Dopaminergic agonists | pramipexole, ropinirole, rotigotine, piribedil, apomorphine |
| MAO-B inhibitors | selegiline, rasagiline, safinamide |
| Others | 5-HTP, trazodone, nefazodone, vortioxetine, pimavanserin, reboxetine, rivastigmine, ketamine, exanetide |
3. Non-Pharmacological Treatments for Depression in PD
3.1. Psychotherapy (Cognitive Behavioral Therapy, Psychodynamic Psychotherapy)
3.2. Physical Activity and Exercise
3.3. Dance
3.4. Electroconvulsive Therapy (ECT)
3.5. Deep Brain Stimulation (DBS)
3.6. Non-Invasive Brain Stimulation (NIBS)
3.7. Bright Light Therapy (BLT)
3.8. Acupuncture
3.9. Lee Silverman Voice Treatment (LSVT)re
3.10. Other Non-Pharmacological Treatments (Music Therapy, Active Theater, Massage Therapy, Cognitive Training, Virtual Reality)
References
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories from 1990 to 2019. Front. Public Health 2021, 9, 776847.
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737.
- Angelopoulou, E.; Bozi, M.; Simitsi, A.M.; Koros, C.; Antonelou, R.; Papagiannakis, N.; Maniati, M.; Poula, D.; Stamelou, M.; Vassilatis, D.K.; et al. The relationship between environmental factors and different Parkinson’s disease subtypes in Greece: Data analysis of the Hellenic Biobank of Parkinson’s disease. Park. Relat. Disord. 2019, 67, 105–112.
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Role of Liver Growth Factor (LGF) in Parkinson’s Disease: Molecular Insights and Therapeutic Opportunities. Mol. Neurobiol. 2021, 58, 3031–3042.
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560.
- Prange, S.; Klinger, H.; Laurencin, C.; Danaila, T.; Thobois, S. Depression in Patients with Parkinson’s Disease: Current Understanding of its Neurobiology and Implications for Treatment. Drugs Aging 2022, 39, 417–439.
- Frisina, P.G.; Borod, J.C.; Foldi, N.S.; Tenenbaum, H.R. Depression in Parkinson’s disease: Health risks, etiology, and treatment options. Neuropsychiatr. Dis. Treat. 2008, 4, 81–91.
- Ryan, M.; Eatmon, C.V.; Slevin, J.T. Drug treatment strategies for depression in Parkinson disease. Expert Opin. Pharmacother. 2019, 20, 1351–1363.
- Shulman, L.M.; Taback, R.L.; Rabinstein, A.A.; Weiner, W.J. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2002, 8, 193–197.
- Borisovskaya, A.; Bryson, W.C.; Buchholz, J.; Samii, A.; Borson, S. Electroconvulsive therapy for depression in Parkinson’s disease: Systematic review of evidence and recommendations. Neurodegener. Dis. Manag. 2016, 6, 161–176.
- Wang, Y.; Sun, X.; Li, F.; Li, Q.; Jin, Y. Efficacy of non-pharmacological interventions for depression in individuals with Parkinson’s disease: A systematic review and network meta-analysis. Front. Aging Neurosci. 2022, 14, 1050715.
- Starkstein, S.E.; Brockman, S. Management of Depression in Parkinson’s Disease: A Systematic Review. Mov. Disord. Clin. Pract. 2017, 4, 470–477.
- Goodarzi, Z.; Hanson, H.M.; Jette, N.; Patten, S.; Pringsheim, T.; Holroyd-Leduc, J. Barriers and Facilitators for Guidelines with Depression and Anxiety in Parkinson’s Disease or Dementia. Can. J. Aging = La Rev. Can. Du Vieil. 2018, 37, 185–199.
- Orayj, K.; Almeleebia, T.; Vigneshwaran, E.; Alshahrani, S.; Alavudeen, S.S.; Alghamdi, W. Trend of recognizing depression symptoms and antidepressants use in newly diagnosed Parkinson’s disease: Population-based study. Brain Behav. 2021, 11, e2228.
- Skapinakis, P.; Bakola, E.; Salanti, G.; Lewis, G.; Kyritsis, A.P.; Mavreas, V. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. BMC Neurol. 2010, 10, 49.
- Chen, P.; Kales, H.C.; Weintraub, D.; Blow, F.C.; Jiang, L.; Mellow, A.M. Antidepressant treatment of veterans with Parkinson’s disease and depression: Analysis of a national sample. J. Geriatr. Psychiatry Neurol. 2007, 20, 161–165.
- Kampling, H.; Brendel, L.K.; Mittag, O. (Neuro)Psychological Interventions for Non-Motor Symptoms in the Treatment of Patients with Parkinson’s Disease: A Systematic Umbrella Review. Neuropsychol. Rev. 2019, 29, 166–180.
- Lopes, S.R.; Khan, S.; Chand, S. The Growing Role of Cognitive Behavior Therapy in the Treatment of Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2021, 34, 310–320.
- Mueller, C.; Rajkumar, A.P.; Wan, Y.M.; Velayudhan, L.; Ffytche, D.; Chaudhuri, K.R.; Aarsland, D. Assessment and Management of Neuropsychiatric Symptoms in Parkinson’s Disease. CNS Drugs 2018, 32, 621–635.
- Goldapple, K.; Segal, Z.; Garson, C.; Lau, M.; Bieling, P.; Kennedy, S.; Mayberg, H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry 2004, 61, 34–41.
- Hong, C.T.; Tan, S.; Huang, T.W. Psychotherapy for the Treatment of Anxiety and Depression in Patients with Parkinson Disease: A Meta-Analysis of Randomized Controlled Trials. J. Am. Med. Dir. Assoc. 2021, 22, 2289–2295.e2.
- Chen, J.; He, P.; Zhang, Y.; Gao, Y.; Qiu, Y.; Li, Y.; Zhang, Q.; Wang, L.; Huang, Z.; Zhao, J.; et al. Non-pharmacological treatment for Parkinson disease patients with depression: A meta-analysis of repetitive transcranial magnetic stimulation and cognitive-behavioral treatment. Int. J. Neurosci. 2021, 131, 411–424.
- Zhang, Q.; Yang, X.; Song, H.; Jin, Y. Cognitive behavioral therapy for depression and anxiety of Parkinson’s disease: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2020, 39, 101111.
- Troeung, L.; Egan, S.J.; Gasson, N. A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson’s disease. PLoS ONE 2013, 8, e79510.
- Luo, F.; Ye, M.; Lv, T.; Hu, B.; Chen, J.; Yan, J.; Wang, A.; Chen, F.; He, Z.; Ding, Z.; et al. Efficacy of Cognitive Behavioral Therapy on Mood Disorders, Sleep, Fatigue, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 793804.
- Koychev, I.; Okai, D. Cognitive-behavioural therapy for non-motor symptoms of Parkinson’s disease: A clinical review. Evid.-Based Ment. Health 2017, 20, 15–20.
- Xie, C.L.; Wang, X.D.; Chen, J.; Lin, H.Z.; Chen, Y.H.; Pan, J.L.; Wang, W.W. A systematic review and meta-analysis of cognitive behavioral and psychodynamic therapy for depression in Parkinson’s disease patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015, 36, 833–843.
- Angelopoulou, E.; Papachristou, N.; Bougea, A.; Stanitsa, E.; Kontaxopoulou, D.; Fragkiadaki, S.; Pavlou, D.; Koros, C.; Degirmenci, Y.; Papatriantafyllou, J.; et al. How Telemedicine Can Improve the Quality of Care for Patients with Alzheimer’s Disease and Related Dementias? A Narrative Review. Medicina 2022, 58, 1705.
- Latella, D.; Maresca, G.; Formica, C.; Sorbera, C.; Bringandi, A.; Di Lorenzo, G.; Quartarone, A.; Marino, S. The Role of Telemedicine in the Treatment of Cognitive and Psychological Disorders in Parkinson’s Disease: An Overview. Brain Sci. 2023, 13, 499.
- Wuthrich, V.M.; Rapee, R.M. Telephone-Delivered Cognitive Behavioural Therapy for Treating Symptoms of Anxiety and Depression in Parkinson’s Disease: A Pilot Trial. Clin. Gerontol. 2019, 42, 444–453.
- Dobkin, R.D.; Mann, S.L.; Weintraub, D.; Rodriguez, K.M.; Miller, R.B.; St Hill, L.; King, A.; Gara, M.A.; Interian, A. Innovating Parkinson’s Care: A Randomized Controlled Trial of Telemedicine Depression Treatment. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 2549–2558.
- Wu, P.L.; Lee, M.; Huang, T.T. Effectiveness of physical activity on patients with depression and Parkinson’s disease: A systematic review. PLoS ONE 2017, 12, e0181515.
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539.
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 1891–1900.
- Xu, X.; Fu, Z.; Le, W. Exercise and Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 45–74.
- Tian, J.; Kang, Y.; Liu, P.; Yu, H. Effect of Physical Activity on Depression in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6849.
- Feller, D.; Fox, I.; Gozzer, P.; Trentin, F.; Papola, D. Exercise for Depressive Symptoms in Parkinson Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2023, 104, 331–339.
- Kim, R.; Lee, T.L.; Lee, H.; Ko, D.K.; Jeon, B.; Kang, N. Effects of Exercise on Depressive Symptoms in Patients With Parkinson Disease: A Meta-analysis. Neurology 2023, 100, e377–e387.
- Gollan, R.; Ernst, M.; Lieker, E.; Caro-Valenzuela, J.; Monsef, I.; Dresen, A.; Roheger, M.; Skoetz, N.; Kalbe, E.; Folkerts, A.K. Effects of Resistance Training on Motor- and Non-Motor Symptoms in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Park. Dis. 2022, 12, 1783–1806.
- Braz de Oliveira, M.P.; Rigo Lima, C.; da Silva, S.L.A.; Firmino Vaz Figueira, E.C.; David Truax, B.; Smaili, S.M. Effect of aquatic exercise programs according to the International Classification of Functionality, Disability and Health domains in individuals with Parkinson’s disease: A systematic review and meta-analysis with GRADE quality assessment. Disabil. Rehabil. 2023, 1–14.
- Kattenstroth, J.C.; Kolankowska, I.; Kalisch, T.; Dinse, H.R. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front. Aging Neurosci. 2010, 2, 1724.
- Karpodini, C.C.; Dinas, P.C.; Angelopoulou, E.; Wyon, M.A.; Haas, A.N.; Bougiesi, M.; Papageorgiou, S.G.; Koutedakis, Y. Rhythmic cueing, dance, resistance training, and Parkinson’s disease: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 875178.
- Wang, L.L.; Sun, C.J.; Wang, Y.; Zhan, T.T.; Yuan, J.; Niu, C.Y.; Yang, J.; Huang, S.; Cheng, L. Effects of dance therapy on non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2022, 34, 1201–1208.
- Zhang, Q.; Hu, J.; Wei, L.; Jia, Y.; Jin, Y. Effects of dance therapy on cognitive and mood symptoms in people with Parkinson’s disease: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2019, 36, 12–17.
- Yang, Y.; Wang, G.; Zhang, S.; Wang, H.; Zhou, W.; Ren, F.; Liang, H.; Wu, D.; Ji, X.; Hashimoto, M.; et al. Efficacy and evaluation of therapeutic exercises on adults with Parkinson’s disease: A systematic review and network meta-analysis. BMC Geriatr. 2022, 22, 813.
- Takamiya, A.; Seki, M.; Kudo, S.; Yoshizaki, T.; Nakahara, J.; Mimura, M.; Kishimoto, T. Electroconvulsive Therapy for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 50–58.
- Strome, E.M.; Zis, A.P.; Doudet, D.J. Electroconvulsive shock enhances striatal dopamine D1 and D3 receptor binding and improves motor performance in 6-OHDA-lesioned rats. J. Psychiatry Neurosci. JPN 2007, 32, 193–202.
- Fall, P.A.; Ekman, R.; Granerus, A.K.; Thorell, L.H.; Walinder, J. ECT in Parkinson’s disease. Changes in motor symptoms, monoamine metabolites and neuropeptides. J. Neural Transm. Park. Dis. Dement. Sect. 1995, 10, 129–140.
- Troster, A.I.; Jankovic, J.; Tagliati, M.; Peichel, D.; Okun, M.S. Neuropsychological outcomes from constant current deep brain stimulation for Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 433–440.
- Dafsari, H.S.; Petry-Schmelzer, J.N.; Ray-Chaudhuri, K.; Ashkan, K.; Weis, L.; Dembek, T.A.; Samuel, M.; Rizos, A.; Silverdale, M.; Barbe, M.T.; et al. Non-motor outcomes of subthalamic stimulation in Parkinson’s disease depend on location of active contacts. Brain Stimul. 2018, 11, 904–912.
- Giannini, G.; Francois, M.; Lhommee, E.; Polosan, M.; Schmitt, E.; Fraix, V.; Castrioto, A.; Ardouin, C.; Bichon, A.; Pollak, P.; et al. Suicide and suicide attempts after subthalamic nucleus stimulation in Parkinson disease. Neurology 2019, 93, e97–e105.
- Weiss, E.F.; Malik, R.; Santos, T.; Ceide, M.; Cohen, J.; Verghese, J.; Zwerling, J.L. Telehealth for the cognitively impaired older adult and their caregivers: Lessons from a coordinated approach. Neurodegener. Dis. Manag. 2021, 11, 83–89.
- Cartmill, T.; Skvarc, D.; Bittar, R.; McGillivray, J.; Berk, M.; Byrne, L.K. Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease: A Meta-Analysis of Mood Effects. Neuropsychol. Rev. 2021, 31, 385–401.
- Elgebaly, A.; Elfil, M.; Attia, A.; Magdy, M.; Negida, A. Neuropsychological performance changes following subthalamic versus pallidal deep brain stimulation in Parkinson’s disease: A systematic review and metaanalysis. CNS Spectr. 2018, 23, 10–23.
- Wang, J.W.; Zhang, Y.Q.; Zhang, X.H.; Wang, Y.P.; Li, J.P.; Li, Y.J. Cognitive and Psychiatric Effects of STN versus GPi Deep Brain Stimulation in Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0156721.
- Combs, H.L.; Folley, B.S.; Berry, D.T.; Segerstrom, S.C.; Han, D.Y.; Anderson-Mooney, A.J.; Walls, B.D.; van Horne, C. Cognition and Depression Following Deep Brain Stimulation of the Subthalamic Nucleus and Globus Pallidus Pars Internus in Parkinson’s Disease: A Meta-Analysis. Neuropsychol. Rev. 2015, 25, 439–454.
- Zheng, H.B.; Liu, B.; Shen, J.; Xie, F.; Ji, Q.M.; Zhu, X.Y. Non-invasive brain stimulation for treating psychiatric symptoms in Parkinson’s disease: A systematic review and meta-analysis. J. Clin. Neurosci. 2022, 106, 83–90.
- Brunoni, A.R.; Teng, C.T.; Correa, C.; Imamura, M.; Brasil-Neto, J.P.; Boechat, R.; Rosa, M.; Caramelli, P.; Cohen, R.; Del Porto, J.A.; et al. Neuromodulation approaches for the treatment of major depression: Challenges and recommendations from a working group meeting. Arq. De Neuro-Psiquiatr. 2010, 68, 433–451.
- Makkos, A.; Pal, E.; Aschermann, Z.; Janszky, J.; Balazs, E.; Takacs, K.; Karadi, K.; Komoly, S.; Kovacs, N. High-Frequency Repetitive Transcranial Magnetic Stimulation Can Improve Depression in Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Neuropsychobiology 2016, 73, 169–177.
- Shin, H.W.; Youn, Y.C.; Chung, S.J.; Sohn, Y.H. Effect of high-frequency repetitive transcranial magnetic stimulation on major depressive disorder in patients with Parkinson’s disease. J. Neurol. 2016, 263, 1442–1448.
- Brys, M.; Fox, M.D.; Agarwal, S.; Biagioni, M.; Dacpano, G.; Kumar, P.; Pirraglia, E.; Chen, R.; Wu, A.; Fernandez, H.; et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology 2016, 87, 1907–1915.
- Xie, C.L.; Chen, J.; Wang, X.D.; Pan, J.L.; Zhou, Y.; Lin, S.Y.; Xue, X.D.; Wang, W.W. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: A meta-analysis of randomized controlled clinical trials. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015, 36, 1751–1761.
- Zhang, W.; Deng, B.; Xie, F.; Zhou, H.; Guo, J.F.; Jiang, H.; Sim, A.; Tang, B.; Wang, Q. Efficacy of repetitive transcranial magnetic stimulation in Parkinson’s disease: A systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine 2022, 52, 101589.
- Lesenskyj, A.M.; Samples, M.P.; Farmer, J.M.; Maxwell, C.R. Treating refractory depression in Parkinson’s disease: A meta-analysis of transcranial magnetic stimulation. Transl. Neurodegener. 2018, 7, 8.
- Hai-Jiao, W.; Ge, T.; Li-Na, Z.; Deng, C.; Da, X.; Shan-Shan, C.; Liu, L. The efficacy of repetitive transcranial magnetic stimulation for Parkinson disease patients with depression. Int. J. Neurosci. 2020, 130, 19–27.
- Li, S.; Jiao, R.; Zhou, X.; Chen, S. Motor recovery and antidepressant effects of repetitive transcranial magnetic stimulation on Parkinson disease: A PRISMA-compliant meta-analysis. Medicine 2020, 99, e19642.
- Qin, B.; Chen, H.; Gao, W.; Zhao, L.B.; Zhao, M.J.; Qin, H.X.; Yang, M.X. Effectiveness of high-frequency repetitive transcranial magnetic stimulation in patients with depression and Parkinson’s disease: A meta-analysis of randomized, controlled clinical trials. Neuropsychiatr. Dis. Treat. 2018, 14, 273–284.
- Zhou, L.; Guo, Z.; Xing, G.; Peng, H.; Cai, M.; Chen, H.; McClure, M.A.; He, L.; Xiong, L.; He, B.; et al. Antidepressant Effects of Repetitive Transcranial Magnetic Stimulation Over Prefrontal Cortex of Parkinson’s Disease Patients With Depression: A Meta-Analysis. Front. Psychiatry 2018, 9, 769.
- Fregni, F.; Ono, C.R.; Santos, C.M.; Bermpohl, F.; Buchpiguel, C.; Barbosa, E.R.; Marcolin, M.A.; Pascual-Leone, A.; Valente, K.D. Effects of antidepressant treatment with rTMS and fluoxetine on brain perfusion in PD. Neurology 2006, 66, 1629–1637.
- Cardoso, E.F.; Fregni, F.; Martins Maia, F.; Boggio, P.S.; Luis Myczkowski, M.; Coracini, K.; Lopes Vieira, A.; Melo, L.M.; Sato, J.R.; Antonio Marcolin, M.; et al. rTMS treatment for depression in Parkinson’s disease increases BOLD responses in the left prefrontal cortex. Int. J. Neuropsychopharmacol. 2008, 11, 173–183.
- Avissar, M.; Powell, F.; Ilieva, I.; Respino, M.; Gunning, F.M.; Liston, C.; Dubin, M.J. Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul. 2017, 10, 919–925.
- Huang, H.T.; Huang, T.W.; Hong, C.T. Bright Light Therapy for Parkinson Disease: A Literature Review and Meta-Analysis of Randomized Controlled Trials. Biology 2021, 10, 1205.
- Lin, F.; Su, Y.; Weng, Y.; Lin, X.; Weng, H.; Cai, G.; Cai, G. The effects of bright light therapy on depression and sleep disturbances in patients with Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. Sleep Med. 2021, 83, 280–289.
- Tyrer, A.E.; Levitan, R.D.; Houle, S.; Wilson, A.A.; Nobrega, J.N.; Meyer, J.H. Increased Seasonal Variation in Serotonin Transporter Binding in Seasonal Affective Disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 2447–2454.
- Rutten, S.; Vriend, C.; Smit, J.H.; Berendse, H.W.; van Someren, E.J.W.; Hoogendoorn, A.W.; Twisk, J.W.R.; van der Werf, Y.D.; van den Heuvel, O.A. Bright light therapy for depression in Parkinson disease: A randomized controlled trial. Neurology 2019, 92, e1145–e1156.
- Paus, S.; Schmitz-Hubsch, T.; Wullner, U.; Vogel, A.; Klockgether, T.; Abele, M. Bright light therapy in Parkinson’s disease: A pilot study. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 1495–1498.
- Sun, W.; Yan, J.; Wu, J.; Ma, H. Efficacy and Safety of Light Therapy as a Home Treatment for Motor and Non-Motor Symptoms of Parkinson Disease: A Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935074.
- Wen, X.; Li, K.; Wen, H.; Wang, Q.; Wu, Z.; Yao, X.; Jiao, B.; Sun, P.; Ge, S.; Wen, C.; et al. Acupuncture-Related Therapies for Parkinson’s Disease: A Meta-Analysis and Qualitative Review. Front. Aging Neurosci. 2021, 13, 676827.
- Li, Q.; Wu, C.; Wang, X.; Li, Z.; Hao, X.; Zhao, L.; Li, M.; Zhu, M. Effect of acupuncture for non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 995850.
- Ning, B.; Wang, Z.; Wu, Q.; Deng, Q.; Yang, Q.; Gao, J.; Fu, W.; Deng, Y.; Wu, B.; Huang, X.; et al. Acupuncture inhibits autophagy and repairs synapses by activating the mTOR pathway in Parkinson’s disease depression model rats. Brain Res. 2023, 1808, 148320.
- Fox, C.; Ebersbach, G.; Ramig, L.; Sapir, S. LSVT LOUD and LSVT BIG: Behavioral Treatment Programs for Speech and Body Movement in Parkinson Disease. Park. Dis. 2012, 2012, 391946.
- Dashtipour, K.; Johnson, E.; Kani, C.; Kani, K.; Hadi, E.; Ghamsary, M.; Pezeshkian, S.; Chen, J.J. Effect of exercise on motor and nonmotor symptoms of Parkinson’s disease. Park. Dis. 2015, 2015, 586378.
- Choi, Y.; Kim, D. Effects of Task-Based LSVT-BIG Intervention on Hand Function, Activity of Daily Living, Psychological Function, and Quality of Life in Parkinson’s Disease: A Randomized Control Trial. Occup. Ther. Int. 2022, 2022, 1700306.
- Burt, J.; Ravid, E.N.; Bradford, S.; Fisher, N.J.; Zeng, Y.; Chomiak, T.; Brown, L.; McKeown, M.J.; Hu, B.; Camicioli, R. The Effects of Music-Contingent Gait Training on Cognition and Mood in Parkinson Disease: A Feasibility Study. Neurorehabilit. Neural Repair 2020, 34, 82–92.
- Elefant, C.; Baker, F.A.; Lotan, M.; Lagesen, S.K.; Skeie, G.O. The effect of group music therapy on mood, speech, and singing in individuals with Parkinson’s disease—A feasibility study. J. Music Ther. 2012, 49, 278–302.
- Modugno, N.; Iaconelli, S.; Fiorlli, M.; Lena, F.; Kusch, I.; Mirabella, G. Active theater as a complementary therapy for Parkinson’s disease rehabilitation: A pilot study. TheScientificWorldJournal 2010, 10, 2301–2313.
- Sproesser, E.; Viana, M.A.; Quagliato, E.M.; de Souza, E.A. The effect of psychotherapy in patients with PD: A controlled study. Park. Relat. Disord. 2010, 16, 298–300.
- Angelopoulou, E.; Anagnostouli, M.; Chrousos, G.P.; Bougea, A. Massage therapy as a complementary treatment for Parkinson’s disease: A Systematic Literature Review. Complement. Ther. Med. 2020, 49, 102340.
- Leung, I.H.; Walton, C.C.; Hallock, H.; Lewis, S.J.; Valenzuela, M.; Lampit, A. Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 2015, 85, 1843–1851.
- Li, R.; Zhang, Y.; Jiang, Y.; Wang, M.; Ang, W.H.D.; Lau, Y. Rehabilitation training based on virtual reality for patients with Parkinson’s disease in improving balance, quality of life, activities of daily living, and depressive symptoms: A systematic review and meta-regression analysis. Clin. Rehabil. 2021, 35, 1089–1102.
- Lee, N.Y.; Lee, D.K.; Song, H.S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147.





