
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | DINKORMA T. OUOLOGUEM | -- | 4452 | 2023-08-30 12:32:40 | | | |
| 2 | Fanny Huang | -1 word(s) | 4451 | 2023-09-01 10:15:33 | | |
Video Upload Options
Malaria elimination never succeed without the implementation of transmission-blocking strategies. The transmission of Plasmodium spp. parasites from the human host to the mosquito vector depends on circulating gametocytes in the peripheral blood of the vertebrate host. Once ingested by the mosquito during blood meals, these sexual forms undergo a series of radical morphological and metabolic changes to survive and progress from the gut to the salivary glands, where they will be waiting to be injected into the vertebrate host. The design of effective transmission-blocking strategies requires a thorough understanding of all the mechanisms that drive the development of gametocytes, gametes, sexual reproduction, and subsequent differentiation within the mosquito. The drastic changes in Plasmodium falciparum shape and function throughout its life cycle rely on the tight regulation of stage-specific gene expression.
1. Introduction
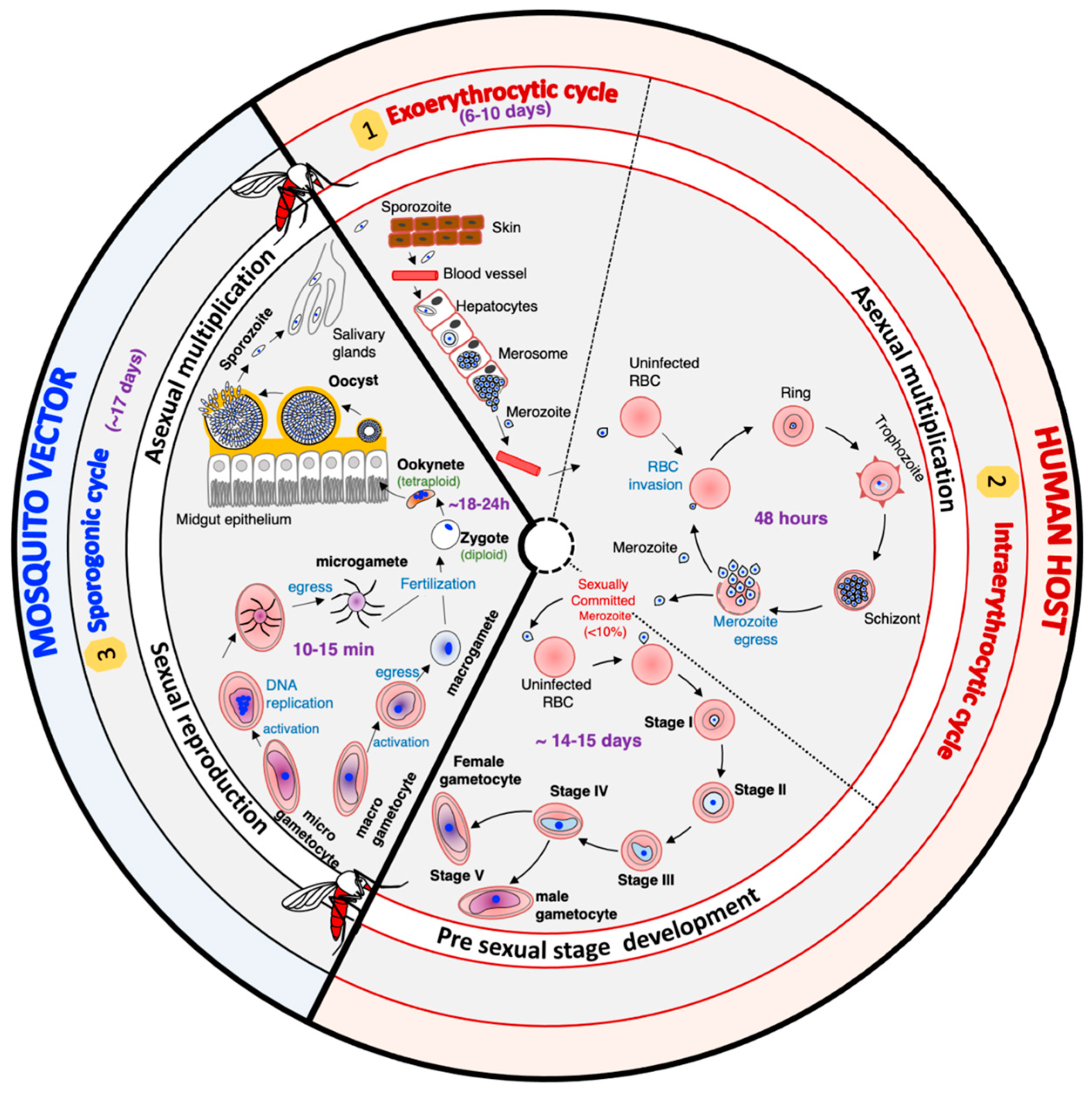
2. Gametocyte Development
3. Gamete Development
3.1. Gene Expression Regulation Controlling Gametogenesis
3.2. Signaling Cascade Controlling Gametogenesis
4. Zygote to Ookinete Development
4.1. Gamete Fusion and Zygote Formation
4.2. Molecular and Genetic Mechanisms of Fertilization
4.3. Formation and Maturation of Ookinete
5. Ookinete to Oocyst Development
References
- World Health Organization. World Malaria Report 2022; 9789240064898; World Health Organization: Geneva, Switzerland, 2022.
- Alonso, P.L.; Brown, G.; Arevalo-Herrera, M.; Binka, F.; Chitnis, C.; Collins, F.; Doumbo, O.K.; Greenwood, B.; Hall, B.F.; Levine, M.M.; et al. A research agenda to underpin malaria eradication. PLoS Med. 2011, 8, e1000406.
- Kamiya, T.; Paton, D.G.; Catteruccia, F.; Reece, S.E. Targeting malaria parasites inside mosquitoes: Ecoevolutionary consequences. Trends Parasitol. 2022, 38, 1031–1040.
- Aingaran, M.; Zhang, R.; Law, S.K.; Peng, Z.; Undisz, A.; Meyer, E.; Diez-Silva, M.; Burke, T.A.; Spielmann, T.; Lim, C.T.; et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell. Microbiol. 2012, 14, 983–993.
- Kono, M.; Herrmann, S.; Loughran, N.B.; Cabrera, A.; Engelberg, K.; Lehmann, C.; Sinha, D.; Prinz, B.; Ruch, U.; Heussler, V.; et al. Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol. Biol. Evol. 2012, 29, 2113–2132.
- Aikawa, M. Plasmodium: The fine structure of malarial parasites. Exp. Parasitol. 1971, 30, 284–320.
- Beri, D.; Balan, B.; Tatu, U. Commit, hide and escape: The story of Plasmodium gametocytes. Parasitology 2018, 145, 1772–1782.
- Lasonder, E.; Rijpma, S.R.; van Schaijk, B.C.; Hoeijmakers, W.A.; Kensche, P.R.; Gresnigt, M.S.; Italiaander, A.; Vos, M.W.; Woestenenk, R.; Bousema, T.; et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: Molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016, 44, 6087–6101.
- Silvestrini, F.; Bozdech, Z.; Lanfrancotti, A.; Di Giulio, E.; Bultrini, E.; Picci, L.; Derisi, J.L.; Pizzi, E.; Alano, P. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 2005, 143, 100–110.
- Yahiya, S.; Jordan, S.; Smith, H.X.; Gaboriau, D.C.A.; Famodimu, M.T.; Dahalan, F.A.; Churchyard, A.; Ashdown, G.W.; Baum, J. Live-cell fluorescence imaging of microgametogenesis in the human malaria parasite Plasmodium falciparum. PLoS Pathog. 2022, 18, e1010276.
- Bannister, L.H.; Sinden, R.E. New knowledge of parasite morphology. Br. Med. Bull. 1982, 38, 141–145.
- Sinden, R.E.; Strong, K. An ultrastructural study of the sporogonic development of Plasmodium falciparum in Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 477–491.
- Vlachou, D.; Zimmermann, T.; Cantera, R.; Janse, C.J.; Waters, A.P.; Kafatos, F.C. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell. Microbiol. 2004, 6, 671–685.
- Yoshikawa, Y.; Kimura, S.; Soga, A.; Sugiyama, M.; Ueno, A.; Kondo, H.; Zhu, Z.; Ochiai, K.; Nakayama, K.; Hakozaki, J.; et al. Plasmodium berghei Brca2 is required for normal development and differentiation in mice and mosquitoes. Parasites Vectors 2022, 15, 244.
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C.; et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re245.
- Josling, G.A.; Llinas, M. Sexual development in Plasmodium parasites: Knowing when it’s time to commit. Nat. Rev. Microbiol. 2015, 13, 573–587.
- Nilsson, S.K.; Childs, L.M.; Buckee, C.; Marti, M. Targeting Human Transmission Biology for Malaria Elimination. PLoS Pathog. 2015, 11, e1004871.
- Meibalan, E.; Marti, M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. Med. 2017, 7, a025452.
- Buckling, A.; Ranford-Cartwright, L.C.; Miles, A.; Read, A.F. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology 1999, 118, 339–346.
- Barkakaty, B.N.; Sharma, G.K.; Chakravorty, N.K. Studies on efficacy of treatment with sulfamethoxazole + trimethoprim and sulfalene + pyrimethamine combinations in Plasmodium falciparum malaria of known and unknown resistant status. J. Commun. Dis. 1988, 20, 165–174.
- Brancucci, N.M.B.; Gerdt, J.P.; Wang, C.; De Niz, M.; Philip, N.; Adapa, S.R.; Zhang, M.; Hitz, E.; Niederwieser, I.; Boltryk, S.D.; et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell 2017, 171, 1532–1544.e15.
- Abdi, A.I.; Achcar, F.; Sollelis, L.; Silva-Filho, J.L.; Mwikali, K.; Muthui, M.; Mwangi, S.; Kimingi, H.W.; Orindi, B.; Andisi Kivisi, C.; et al. Plasmodium falciparum adapts its investment into replication versus transmission according to the host environment. Elife 2023, 12, e85140.
- Bruce, M.C.; Alano, P.; Duthie, S.; Carter, R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 1990, 100 Pt 2, 191–200.
- Nixon, C.P.; Nixon, C.E.; Michelow, I.C.; Silva-Viera, R.A.; Colantuono, B.; Obeidallah, A.S.; Jha, A.; Dockery, D.; Raj, D.; Park, S.; et al. Antibodies to PfsEGXP, an Early Gametocyte-Enriched Phosphoprotein, Predict Decreased Plasmodium falciparum Gametocyte Density in Humans. J. Infect. Dis. 2018, 218, 1792–1801.
- Ayanful-Torgby, R.; Oppong, A.; Abankwa, J.; Acquah, F.; Williamson, K.C.; Amoah, L.E. Plasmodium falciparum genotype and gametocyte prevalence in children with uncomplicated malaria in coastal Ghana. Malar. J. 2016, 15, 592.
- Chawla, J.; Goldowitz, I.; Oberstaller, J.; Zhang, M.; Pires, C.V.; Navarro, F.; Sollelis, L.; Wang, C.C.Q.; Seyfang, A.; Dvorin, J.; et al. Phenotypic Screens Identify Genetic Factors Associated with Gametocyte Development in the Human Malaria Parasite Plasmodium falciparum. Microbiol. Spectr. 2023, 11, e0416422.
- Kafsack, B.F.; Rovira-Graells, N.; Clark, T.G.; Bancells, C.; Crowley, V.M.; Campino, S.G.; Williams, A.E.; Drought, L.G.; Kwiatkowski, D.P.; Baker, D.A.; et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 2014, 507, 248–252.
- Sinha, A.; Hughes, K.R.; Modrzynska, K.K.; Otto, T.D.; Pfander, C.; Dickens, N.J.; Religa, A.A.; Bushell, E.; Graham, A.L.; Cameron, R.; et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 2014, 507, 253–257.
- Josling, G.A.; Russell, T.J.; Venezia, J.; Orchard, L.; van Biljon, R.; Painter, H.J.; Llinas, M. Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nat. Commun. 2020, 11, 1503.
- von Gruning, H.; Coradin, M.; Mendoza, M.R.; Reader, J.; Sidoli, S.; Garcia, B.A.; Birkholtz, L.M. A Dynamic and Combinatorial Histone Code Drives Malaria Parasite Asexual and Sexual Development. Mol. Cell. Proteom. 2022, 21, 100199.
- Filarsky, M.; Fraschka, S.A.; Niederwieser, I.; Brancucci, N.M.B.; Carrington, E.; Carrio, E.; Moes, S.; Jenoe, P.; Bartfai, R.; Voss, T.S. GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science 2018, 359, 1259–1263.
- Shang, X.; Shen, S.; Tang, J.; He, X.; Zhao, Y.; Wang, C.; He, X.; Guo, G.; Liu, M.; Wang, L.; et al. A cascade of transcriptional repression determines sexual commitment and development in Plasmodium falciparum. Nucleic Acids Res. 2021, 49, 9264–9279.
- Bancells, C.; Llora-Batlle, O.; Poran, A.; Notzel, C.; Rovira-Graells, N.; Elemento, O.; Kafsack, B.F.C.; Cortes, A. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol. 2019, 4, 144–154.
- Li, Z.; Cui, H.; Guan, J.; Liu, C.; Yang, Z.; Yuan, J. Plasmodium transcription repressor AP2-O3 regulates sex-specific identity of gene expression in female gametocytes. EMBO Rep. 2021, 22, e51660.
- Coetzee, N.; Sidoli, S.; van Biljon, R.; Painter, H.; Llinas, M.; Garcia, B.A.; Birkholtz, L.M. Quantitative chromatin proteomics reveals a dynamic histone post-translational modification landscape that defines asexual and sexual Plasmodium falciparum parasites. Sci. Rep. 2017, 7, 607.
- Bunnik, E.M.; Cook, K.B.; Varoquaux, N.; Batugedara, G.; Prudhomme, J.; Cort, A.; Shi, L.; Andolina, C.; Ross, L.S.; Brady, D.; et al. Changes in genome organization of parasite-specific gene families during the Plasmodium transmission stages. Nat. Commun. 2018, 9, 1910.
- Stenzel, K.; Chua, M.J.; Duffy, S.; Antonova-Koch, Y.; Meister, S.; Hamacher, A.; Kassack, M.U.; Winzeler, E.; Avery, V.M.; Kurz, T.; et al. Design and synthesis of terephthalic acid-based histone deacetylase inhibitors with dual-stage anti-Plasmodium activity. Chem. Med. Chem. 2017, 12, 1627–1636.
- Vaughan, J.A.; Noden, B.H.; Beier, J.C. Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. Am. J. Trop. Med. Hyg. 1994, 51, 233–243.
- Sauerwein, R.W.; Bousema, T. Transmission blocking malaria vaccines: Assays and candidates in clinical development. Vaccine 2015, 33, 7476–7482.
- Wirth, C.C.; Pradel, G. Molecular mechanisms of host cell egress by malaria parasites. Int. J. Med. Microbiol. 2012, 302, 172–178.
- Ngwa, C.J.; Scheuermayer, M.; Mair, G.R.; Kern, S.; Brügl, T.; Wirth, C.C.; Aminake, M.N.; Wiesner, J.; Fischer, R.; Vilcinskas, A.; et al. Changes in the transcriptome of the malaria parasite Plasmodium falciparum during the initial phase of transmission from the human to the mosquito. BMC Genom. 2013, 14, 256.
- Mair, G.R.; Braks, J.A.M.; Garver, L.S.; Wiegant, J.C.A.G.; Hall, N.; Dirks, R.W.; Khan, S.M.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Regulation of sexual development of Plasmodium by translational repression. Science 2006, 313, 667–669.
- Mair, G.R.; Lasonder, E.; Garver, L.S.; Franke-Fayard, B.M.; Carret, C.K.; Wiegant, J.C.; Dirks, R.W.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010, 6, e1000767.
- Guerreiro, A.; Deligianni, E.; Santos, J.M.; Silva, P.A.G.C.; Louis, C.; Pain, A.; Janse, C.J.; Franke-Fayard, B.; Carret, C.K.; Siden-Kiamos, I.; et al. Genome-wide RIP-Chip analysis of translational repressor-bound mRNAs in the Plasmodium gametocyte. Genome Biol. 2014, 15, 493.
- Tarique, M.; Ahmad, M.; Ansari, A.; Tuteja, R. Plasmodium falciparum DOZI, an RNA helicase interacts with eIF4E. Gene 2013, 522, 46–59.
- Invergo, B.M.; Brochet, M.; Yu, L.; Choudhary, J.; Beltrao, P.; Billker, O. sub-minute phosphoregulation of cell cycle systems during Plasmodium gamete formation. Cell. Rep. 2017, 21, 2017–2029.
- Billker, O.; Dechamps, S.; Tewari, R.; Wenig, G.; Franke-Fayard, B.; Brinkmann, V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 2004, 117, 503–514.
- Garcia, C.H.S.; Depoix, D.; Queiroz, R.M.L.; Souza, J.M.F.; Fontes, W.; de Sousa, M.V.; Santos, M.D.M.; Carvalho, P.C.; Grellier, P.; Charneau, S. Dynamic molecular events associated to Plasmodium berghei gametogenesis through proteomic approach. J. Proteom. 2018, 180, 88–98.
- Alonso-Morales, A.; Gonzalez-Lopez, L.; Cazares-Raga, F.E.; Cortes-Martinez, L.; Torres-Monzon, J.A.; Gallegos-Perez, J.L.; Rodriguez, M.H.; James, A.A.; Hernandez-Hernandez Fde, L. Protein phosphorylation during Plasmodium berghei gametogenesis. Exp. Parasitol. 2015, 156, 49–60.
- Guttery, D.S.; Poulin, B.; Ramaprasad, A.; Wall, R.J.; Ferguson, D.J.; Brady, D.; Patzewitz, E.M.; Whipple, S.; Straschil, U.; Wright, M.H.; et al. Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe 2014, 16, 128–140.
- Billker, O.; Shaw, M.K.; Margos, G.; Sinden, R.E. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology 1997, 115 Pt 1, 1–7.
- Garcia, G.E.; Wirtz, R.A.; Barr, J.R.; Woolfitt, A.; Rosenberg, R. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. J. Biol. Chem. 1998, 273, 12003–12005.
- Kawamoto, F.; Alejo-Blanco, R.; Fleck, S.L.; Sinden, R.E. Plasmodium berghei: Ionic regulation and the induction of gametogenesis. Exp. Parasitol. 1991, 72, 33–42.
- McRobert, L.; Taylor, C.J.; Deng, W.; Fivelman, Q.L.; Cummings, R.M.; Polley, S.D.; Billker, O.; Baker, D.A. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008, 6, e139.
- Brochet, M.; Balestra, A.C.; Brusini, L. cGMP homeostasis in malaria parasites-The key to perceiving and integrating environmental changes during transmission to the mosquito. Mol. Microbiol. 2021, 115, 829–838.
- Muhia, D.K.; Swales, C.A.; Deng, W.; Kelly, J.M.; Baker, D.A. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 2001, 42, 553–560.
- Brochet, M.; Collins, M.O.; Smith, T.K.; Thompson, E.; Sebastian, S.; Volkmann, K.; Schwach, F.; Chappell, L.; Gomes, A.R.; Berriman, M.; et al. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites. PLoS Biol. 2014, 12, e1001806.
- Carucci, D.J.; Witney, A.A.; Muhia, D.K.; Warhurst, D.C.; Schaap, P.; Meima, M.; Li, J.L.; Taylor, M.C.; Kelly, J.M.; Baker, D.A. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. J. Biol. Chem. 2000, 275, 22147–22156.
- Wang, P.P.; Jiang, X.; Zhu, L.; Zhou, D.; Hong, M.; He, L.; Chen, L.; Yao, S.; Zhao, Y.; Chen, G.; et al. A G-Protein-coupled receptor modulates gametogenesis via PKG-mediated signaling cascade in Plasmodium berghei. Microbiol. Spectr. 2022, 10, e0015022.
- Taylor, C.J.; McRobert, L.; Baker, D.A. Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol. Microbiol. 2008, 69, 110–118.
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell. Microbiol. 2016, 18, 905–918.
- Martin, S.K.; Jett, M.; Schneider, I. Correlation of phosphoinositide hydrolysis with exflagellation in the malaria microgametocyte. J. Parasitol. 1994, 80, 371–378.
- Alves, E.; Nakaya, H.; Guimarães, E.; Garcia, C.R.S. Combining IP3 affinity chromatography and bioinformatics reveals a novel protein-IP3 binding site on Plasmodium falciparum MDR1 transporter. Curr. Res. Microb. Sci. 2022, 4, 100179.
- Holder, A.A.; Mohd Ridzuan, M.A.; Green, J.L. Calcium dependent protein kinase 1 and calcium fluxes in the malaria parasite. Microbes Infect. 2012, 14, 825–830.
- Sebastian, S.; Brochet, M.; Collins, M.O.; Schwach, F.; Jones, M.L.; Goulding, D.; Rayner, J.C.; Choudhary, J.S.; Billker, O. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe 2012, 12, 9–19.
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Liu, P.; Luo, Y.; Gunalan, K.; Li, Y.; Ribeiro, J.M.C.; Miller, L.H. PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc. Natl. Acad. Sci. USA 2018, 115, 774–779.
- Kumar, S.; Haile, M.T.; Hoopmann, M.R.; Tran, L.T.; Michaels, S.A.; Morrone, S.R.; Ojo, K.K.; Reynolds, L.M.; Kusebauch, U.; Vaughan, A.M.; et al. Plasmodium falciparum Calcium-Dependent Protein Kinase 4 is Critical for Male Gametogenesis and Transmission to the Mosquito Vector. mBio 2021, 12, e0257521.
- Guttery, D.S.; Ferguson, D.J.; Poulin, B.; Xu, Z.; Straschil, U.; Klop, O.; Solyakov, L.; Sandrini, S.M.; Brady, D.; Nieduszynski, C.A.; et al. A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development. PLoS Pathog. 2012, 8, e1002554.
- Wall, R.J.; Ferguson, D.J.P.; Freville, A.; Franke-Fayard, B.; Brady, D.; Zeeshan, M.; Bottrill, A.R.; Wheatley, S.; Fry, A.M.; Janse, C.J.; et al. Plasmodium APC3 mediates chromosome condensation and cytokinesis during atypical mitosis in male gametogenesis. Sci. Rep. 2018, 8, 5610.
- Laurentino, E.C.; Taylor, S.; Mair, G.R.; Lasonder, E.; Bartfai, R.; Stunnenberg, H.G.; Kroeze, H.; Ramesar, J.; Franke-Fayard, B.; Khan, S.M.; et al. Experimentally controlled downregulation of the histone chaperone FACT in Plasmodium berghei reveals that it is critical to male gamete fertility. Cell Microbiol. 2011, 13, 1956–1974.
- Deligianni, E.; Morgan, R.N.; Bertuccini, L.; Kooij, T.W.; Laforge, A.; Nahar, C.; Poulakakis, N.; Schuler, H.; Louis, C.; Matuschewski, K.; et al. Critical role for a stage-specific actin in male exflagellation of the malaria parasite. Cell. Microbiol. 2011, 13, 1714–1730.
- Straschil, U.; Talman, A.M.; Ferguson, D.J.; Bunting, K.A.; Xu, Z.; Bailes, E.; Sinden, R.E.; Holder, A.A.; Smith, E.F.; Coates, J.C.; et al. The Armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes. PLoS ONE 2010, 5, e12901.
- Marques, S.R.; Ramakrishnan, C.; Carzaniga, R.; Blagborough, A.M.; Delves, M.J.; Talman, A.M.; Sinden, R.E. An essential role of the basal body protein SAS-6 in Plasmodium male gamete development and malaria transmission. Cell Microbiol. 2015, 17, 191–206.
- Tewari, R.; Straschil, U.; Bateman, A.; Bohme, U.; Cherevach, I.; Gong, P.; Pain, A.; Billker, O. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 2010, 8, 377–387.
- Rangarajan, R.; Bei, A.K.; Jethwaney, D.; Maldonado, P.; Dorin, D.; Sultan, A.A.; Doerig, C. A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Rep. 2005, 6, 464–469.
- Tewari, R.; Dorin, D.; Moon, R.; Doerig, C.; Billker, O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol. Microbiol. 2005, 58, 1253–1263.
- Dorin, D.; Le Roch, K.; Sallicandro, P.; Alano, P.; Parzy, D.; Poullet, P.; Meijer, L.; Doerig, C. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum. Eur. J. Biochem. 2001, 268, 2600–2608.
- Lye, Y.M.; Chan, M.; Sim, T.S. Pfnek3: An atypical activator of a MAP kinase in Plasmodium falciparum. FEBS Lett. 2006, 580, 6083–6092.
- Reininger, L.; Tewari, R.; Fennell, C.; Holland, Z.; Goldring, D.; Ranford-Cartwright, L.; Billker, O.; Doerig, C. An essential role for the Plasmodium Nek-2 Nima-related protein kinase in the sexual development of malaria parasites. J. Biol. Chem. 2009, 284, 20858–20868.
- Reininger, L.; Billker, O.; Tewari, R.; Mukhopadhyay, A.; Fennell, C.; Dorin-Semblat, D.; Doerig, C.; Goldring, D.; Harmse, L.; Ranford-Cartwright, L.; et al. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J. Biol. Chem. 2005, 280, 31957–31964.
- Penzo, M.; de Las Heras-Duena, L.; Mata-Cantero, L.; Diaz-Hernandez, B.; Vazquez-Muniz, M.J.; Ghidelli-Disse, S.; Drewes, G.; Fernandez-Alvaro, E.; Baker, D.A. High-throughput screening of the Plasmodium falciparum cGMP-dependent protein kinase identified a thiazole scaffold which kills erythrocytic and sexual stage parasites. Sci. Rep. 2019, 9, 7005.
- Jia, X.; Liu, F.; Bai, J.; Zhang, Y.; Cui, L.; Cao, Y.; Luo, E. Phosphatase inhibitors BVT-948 and alexidine dihydrochloride inhibit sexual development of the malaria parasite Plasmodium berghei. Int. J. Parasitol. Drugs Drug Resist. 2022, 19, 81–88.
- Williamson, K.C. Pfs230: From malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 2003, 25, 351–359.
- Rener, J.; Graves, P.M.; Carter, R.; Williams, J.L.; Burkot, T.R. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 1983, 158, 976–981.
- van Dijk, M.R.; Janse, C.J.; Thompson, J.; Waters, A.P.; Braks, J.A.; Dodemont, H.J.; Stunnenberg, H.G.; van Gemert, G.J.; Sauerwein, R.W.; Eling, W. A central role for P48/45 in malaria parasite male gamete fertility. Cell 2001, 104, 153–164.
- Ko, K.T.; Lennartz, F.; Mekhaiel, D.; Guloglu, B.; Marini, A.; Deuker, D.J.; Long, C.A.; Jore, M.M.; Miura, K.; Biswas, S.; et al. Structure of the malaria vaccine candidate Pfs48/45 and its recognition by transmission blocking antibodies. Nat. Commun. 2022, 13, 5603.
- van Schaijk, B.C.; van Dijk, M.R.; van de Vegte-Bolmer, M.; van Gemert, G.J.; van Dooren, M.W.; Eksi, S.; Roeffen, W.F.; Janse, C.J.; Waters, A.P.; Sauerwein, R.W. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 2006, 149, 216–222.
- Liu, Y.; Tewari, R.; Ning, J.; Blagborough, A.M.; Garbom, S.; Pei, J.; Grishin, N.V.; Steele, R.E.; Sinden, R.E.; Snell, W.J.; et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008, 22, 1051–1068.
- Patil, H.; Hughes, K.R.; Lemgruber, L.; Philip, N.; Dickens, N.; Starnes, G.L.; Waters, A.P. Zygote morphogenesis but not the establishment of cell polarity in Plasmodium berghei is controlled by the small GTPase, RAB11A. PLoS Pathog. 2020, 16, e1008091.
- Tremp, A.Z.; Al-Khattaf, F.S.; Dessens, J.T. Palmitoylation of Plasmodium alveolins promotes cytoskeletal function. Mol. Biochem. Parasitol. 2017, 213, 16–21.
- Santos, J.M.; Kehrer, J.; Franke-Fayard, B.; Frischknecht, F.; Janse, C.J.; Mair, G.R. The Plasmodium palmitoyl-S-acyl-transferase DHHC2 is essential for ookinete morphogenesis and malaria transmission. Sci. Rep. 2015, 5, 16034.
- Frenal, K.; Tay, C.L.; Mueller, C.; Bushell, E.S.; Jia, Y.; Graindorge, A.; Billker, O.; Rayner, J.C.; Soldati-Favre, D. Global analysis of apicomplexan protein S-acyl transferases reveals an enzyme essential for invasion. Traffic 2013, 14, 895–911.
- Tay, C.L.; Jones, M.L.; Hodson, N.; Theron, M.; Choudhary, J.S.; Rayner, J.C. Study of Plasmodium falciparum DHHC palmitoyl transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell. Microbiol. 2016, 18, 1596–1610.
- Yadav, P.; Ayana, R.; Garg, S.; Jain, R.; Sah, R.; Joshi, N.; Pati, S.; Singh, S. Plasmodium palmitoylation machinery engineered in E. coli for high-throughput screening of palmitoyl acyl-transferase inhibitors. FEBS Open Bio 2019, 9, 248–264.
- Akinosoglou, K.A.; Bushell, E.S.; Ukegbu, C.V.; Schlegelmilch, T.; Cho, J.S.; Redmond, S.; Sala, K.; Christophides, G.K.; Vlachou, D. Characterization of Plasmodium developmental transcriptomes in Anopheles gambiae midgut reveals novel regulators of malaria transmission. Cell. Microbiol. 2015, 17, 254–268.
- Ukegbu, C.V.; Cho, J.-S.; Christophides, G.K.; Vlachou, D. Transcriptional silencing and activation of paternal DNA during Plasmodium berghei zygotic development and transformation to oocyst. Cell. Microbiol. 2015, 17, 1230–1240.
- Volkmann, K.; Pfander, C.; Burstroem, C.; Ahras, M.; Goulding, D.; Rayner, J.C.; Frischknecht, F.; Billker, O.; Brochet, M. The alveolin IMC1h is required for normal ookinete and sporozoite motility behaviour and host colonisation in Plasmodium berghei. PLoS ONE 2012, 7, e41409.
- Poulin, B.; Patzewitz, E.M.; Brady, D.; Silvie, O.; Wright, M.H.; Ferguson, D.J.; Wall, R.J.; Whipple, S.; Guttery, D.S.; Tate, E.W.; et al. Unique apicomplexan IMC sub-compartment proteins are early markers for apical polarity in the malaria parasite. Biol. Open 2013, 2, 1160–1170.
- Dorin-Semblat, D.; Sicard, A.; Doerig, C.; Ranford-Cartwright, L.; Doerig, C. Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum. Eukaryot. Cell 2008, 7, 279–285.
- Wetzel, J.; Herrmann, S.; Swapna, L.S.; Prusty, D.; John Peter, A.T.; Kono, M.; Saini, S.; Nellimarla, S.; Wong, T.W.; Wilcke, L.; et al. The role of palmitoylation for protein recruitment to the inner membrane complex of the malaria parasite. J. Biol. Chem. 2015, 290, 1712–1728.
- Guttery, D.S.; Poulin, B.; Ferguson, D.J.; Szoor, B.; Wickstead, B.; Carroll, P.L.; Ramakrishnan, C.; Brady, D.; Patzewitz, E.M.; Straschil, U.; et al. A unique protein phosphatase with kelch-like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion. PLoS Pathog. 2012, 8, e1002948.
- Yuda, M.; Iwanaga, S.; Shigenobu, S.; Mair, G.R.; Janse, C.J.; Waters, A.P.; Kato, T.; Kaneko, I. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol. Microbiol. 2009, 71, 1402–1414.
- Kaneko, I.; Iwanaga, S.; Kato, T.; Kobayashi, I.; Yuda, M. Genome-Wide Identification of the Target Genes of AP2-O, a Plasmodium AP2-Family Transcription Factor. PLoS Pathog. 2015, 11, e1004905.
- Janse, C.J.; Van der Klooster, P.F.; Van der Kaay, H.J.; Van der Ploeg, M.; Overdulve, J.P. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 154–157.
- Janse, C.J.; van der Klooster, P.F.; van der Kaay, H.J.; van der Ploeg, M.; Overdulve, J.P. DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol. Biochem. Parasitol. 1986, 20, 173–182.
- Zhang, V.M.; Chavchich, M.; Waters, N.C. Targeting protein kinases in the malaria parasite: Update of an antimalarial drug target. Curr. Top. Med. Chem. 2012, 12, 456–472.
- Moolman, C.; Sluis, R.V.; Beteck, R.M.; Legoabe, L.J. An Update on Development of Small-Molecule Plasmodial Kinase Inhibitors. Molecules 2020, 25, 5182.
- Baton, L.A.; Ranford-Cartwright, L.C. How do malaria ookinetes cross the mosquito midgut wall? Trends Parasitol. 2005, 21, 22–28.
- Moon, R.W.; Taylor, C.J.; Bex, C.; Schepers, R.; Goulding, D.; Janse, C.J.; Waters, A.P.; Baker, D.A.; Billker, O. A cyclic GMP signalling module that regulates gliding motility in a malaria parasite. PLoS Pathog. 2009, 5, e1000599.
- Ishino, T.; Orito, Y.; Chinzei, Y.; Yuda, M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol. Microbiol. 2006, 59, 1175–1184.
- Lal, K.; Prieto, J.H.; Bromley, E.; Sanderson, S.J.; Yates, J.R., 3rd; Wastling, J.M.; Tomley, F.M.; Sinden, R.E. Characterisation of Plasmodium invasive organelles; an ookinete microneme proteome. Proteomics 2009, 9, 1142–1151.
- Dessens, J.T.; Beetsma, A.L.; Dimopoulos, G.; Wengelnik, K.; Crisanti, A.; Kafatos, F.C.; SINDEN, R.E. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999, 18, 6221–6227.
- Kadota, K.; Ishino, T.; Matsuyama, T.; Chinzei, Y.; Yuda, M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc. Natl. Acad. Sci. USA 2004, 101, 16310–16315.
- Dessens, J.T.; Siden-Kiamos, I.; Mendoza, J.; Mahairaki, V.; Khater, E.; Vlachou, D.; Xu, X.J.; Kafatos, F.C.; Louis, C.; Dimopoulos, G.; et al. SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol. Microbiol. 2003, 49, 319–329.
- Yuda, M.; Yano, K.; Tsuboi, T.; Torii, M.; Chinzei, Y. von Willebrand Factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol. Biochem. Parasitol. 2001, 116, 65–72.
- Kariu, T.; Ishino, T.; Yano, K.; Chinzei, Y.; Yuda, M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol. Microbiol. 2006, 59, 1369–1379.
- Vinetz, J.M.; Valenzuela, J.G.; Specht, C.A.; Aravind, L.; Langer, R.C.; Ribeiro, J.M.; Kaslow, D.C. Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J. Biol. Chem. 2000, 275, 10331–10341.
- Viswanath, V.K.; Gore, S.T.; Valiyaparambil, A.; Mukherjee, S.; Lakshminarasimhan, A. Plasmodium chitinases: Revisiting a target of transmission-blockade against malaria. Protein. Sci. 2021, 30, 1493–1501.
- Hoermann, A.; Habtewold, T.; Selvaraj, P.; Del Corsano, G.; Capriotti, P.; Inghilterra, M.G.; Kebede, T.M.; Christophides, G.K.; Windbichler, N. Gene drive mosquitoes can aid malaria elimination by retarding Plasmodium sporogonic development. Sci. Adv. 2022, 8, eabo1733.
- Burrows, J.N.; Duparc, S.; Gutteridge, W.E.; Hooft van Huijsduijnen, R.; Kaszubska, W.; Macintyre, F.; Mazzuri, S.; Mohrle, J.J.; Wells, T.N.C. New developments in anti-malarial target candidate and product profiles. Malar. J. 2017, 16, 26.
- Dinglasan, R.R.; Kalume, D.E.; Kanzok, S.M.; Ghosh, A.K.; Muratova, O.; Pandey, A.; Jacobs-Lorena, M. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc. Natl. Acad. Sci. USA 2007, 104, 13461–13466.
- Kaslow, D.C.; Nussenzweig, V.; Miller, L. Meeting on Parasites and the invertebrate vector. John D and Catherine T MacArthur Foundation, 18–21 November, 1993. Mem. Inst. Oswaldo. Cruz. 1994, 89, 279–295.
- López-Barragán, M.J.; Lemieux, J.; Quiñones, M.; Williamson, K.C.; Molina-Cruz, A.; Cui, K.; Barillas-Mury, C.; Zhao, K.; Su, X.-Z. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genom. 2011, 12, 587.




