
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barbara Speranza | -- | 4161 | 2023-08-30 12:09:32 | | | |
| 2 | Rita Xu | Meta information modification | 4161 | 2023-08-31 03:16:35 | | |
Video Upload Options
Modern society is becoming more and more reluctant to use antibiotic or chemical compounds in food production and is demanding foods without what they perceive as artificial and harmful chemicals, including many used as antimicrobials and preservatives in food. Another big problem is the improper use of antibiotics, especially broad-spectrum ones, which has significantly contributed to increased antibiotic resistance in many microorganisms. As a consequence, the whole scientific world has recently concentrated numerous studies on the research of natural remedies capable of counteracting multidrug-resistant strains and fighting infections: the use of aromatic plants and their essential oils (EOs) as potential alternatives to conventional antimicrobials to extend shelf life and combat foodborne pathogens has heightened.
1. Introduction
2. Salvia officinalis Essential Oils
2.1. Chemical Composition and Biological Properties
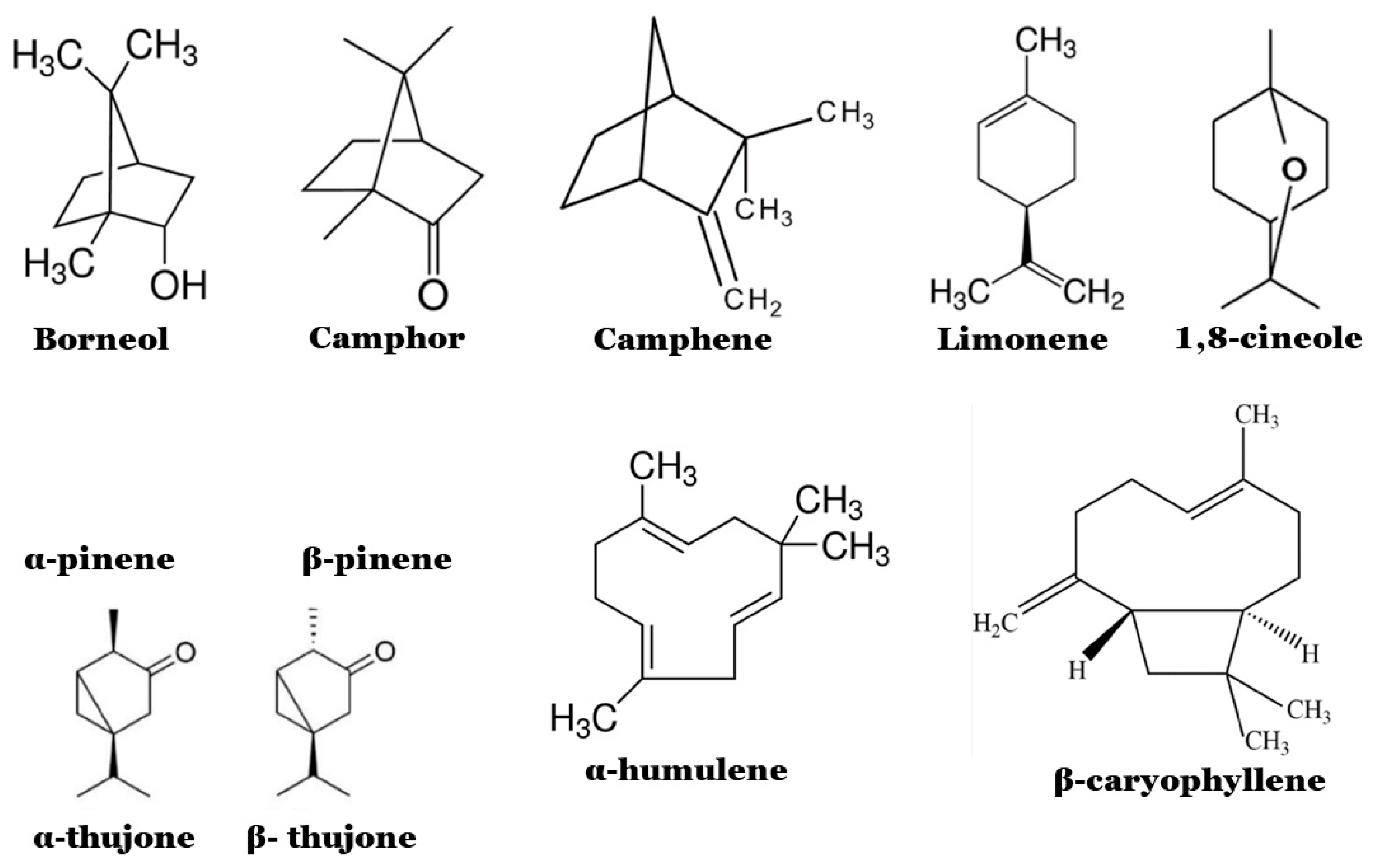
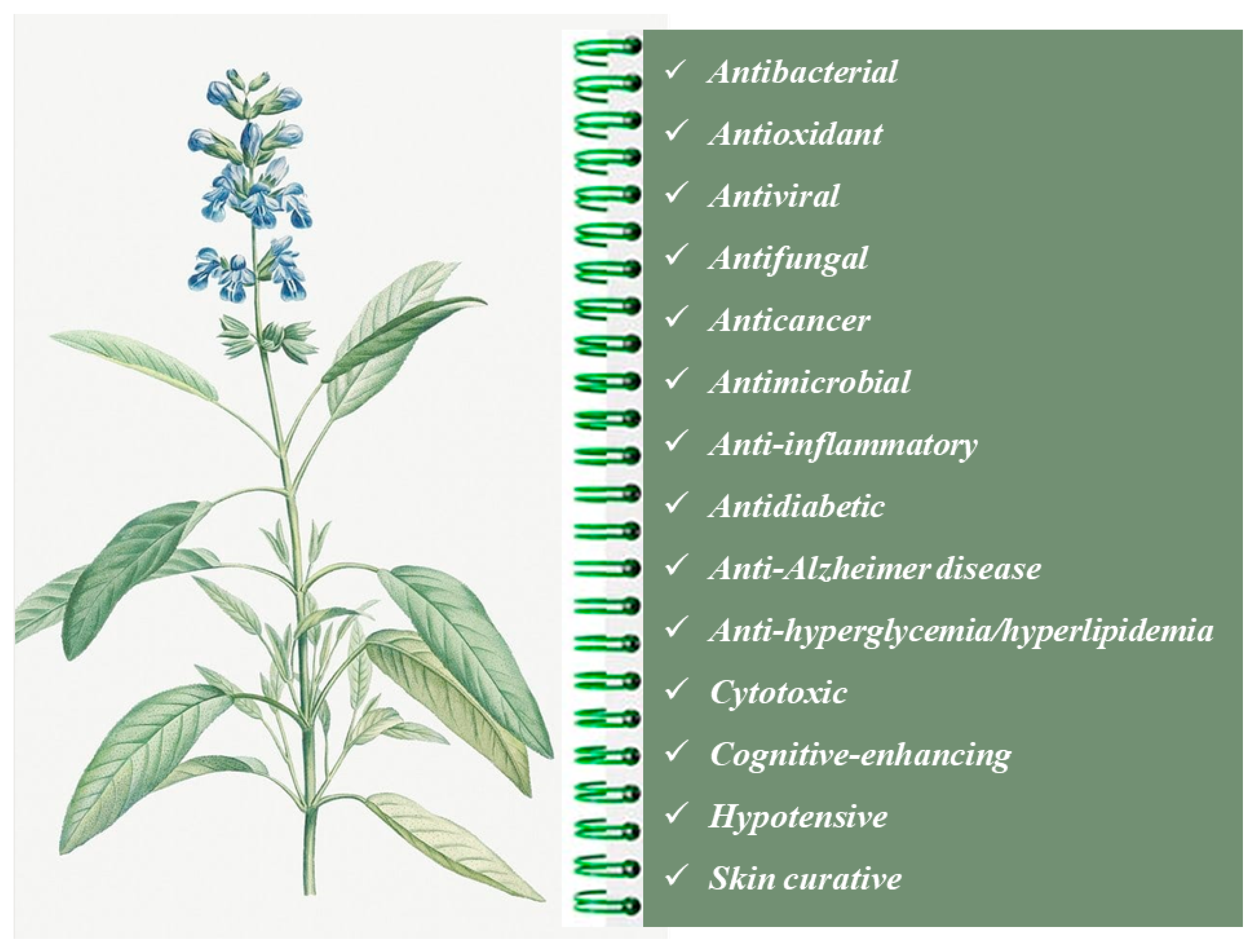
2.2. Antimicrobial Properties
3. Lavandula Essential Oils
3.1. Chemical Composition and Biological Properties
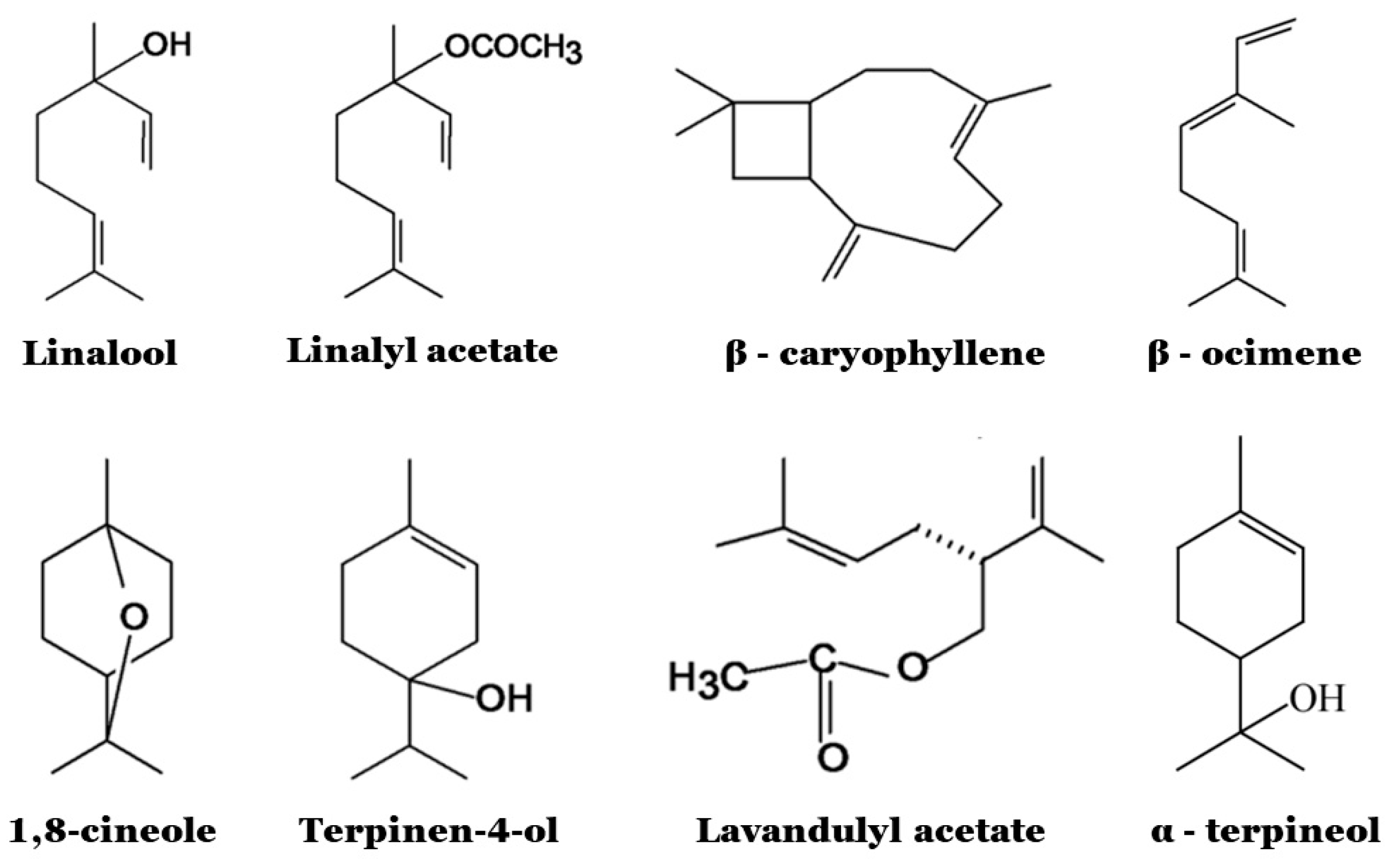

3.2. Antimicrobial Properties
References
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some Essential Oils-present status and future perspectives. Medicines 2017, 4, 58.
- Mérillon, J.-M.; Rivière, C. Natural Antimicrobial Agents; Springer International Publishing AG: Cham, Switzerland, 2018.
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/essential-oil-market-119674487.html (accessed on 20 May 2023).
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind. Crops Prod. 2016, 87, 161–176.
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Borges Leal, A.L.A.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263.
- Shariatifar, N.; Mostaghim, T.; Afshar, A.; Mohammadpourfard, I.; Sayadi, M.; Rezaei, M. Antibacterial properties of essential oil of Heracleum per-sicum (Golpar) and food-borne pathogens. Int. J. Enteric Pathog. 2017, 5, 41–44.
- Hashempour-Baltork, F.; Hosseini, H.; Shojaee-Aliabadi, S.; Torbati, M.; Alizadeh, A.M.; Alizadeh, M. Drug Resistance and the Prevention Strategies in Food Borne Bacteria: An Update Review. Adv. Pharm. Bull. 2019, 9, 335–347.
- Stan, D.; Enciu, A.-M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233.
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella Typhimurium in broth system and on celery. J. Food Process Preserv. 2019, 43, e13888.
- Krasniewska, K.; Gniewosz, M.; Kosakowska, O.; Pobiega, K. Chemical composition and antimicrobial properties of essential oil from lavender (Lavandula angustifolia L.) in commercial available preparation. Postępy Fitoter. 2017, 18, 113–118.
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462.21.
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440.
- Carrasco, A.; Thomas, V.; Tudela, J.; Miguel, M.G. Comparative study of GC-MS characterization, antioxidant activity and hyaluronidase inhibition of different species of Lavandula and Thymus essential oils. Flavour Fragr. J. 2016, 31, 57–69.
- de Rapper, S.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid. Based Complement. Alternat. Med. 2016, 2016, 2752739.
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential oils from wild populations of Algerian Lavandula stoechas L.: Composition, chemical variability, and in vitro biological properties. Chem. Biodivers. 2011, 8, 937–953.
- Turgut, A.C.; Emen, F.M.; Canbay, H.S.; Demirdöğen, R.E.; Çam, N.; Kılıç, D.; Yeşilkaynak, T. Chemical characterization of Lavandula angustifolia Mill. as a phytocosmetic species and investigation of its antimicrobia in cosmetic products. J. Turk. Chem. Soc. Sect. Chem. 2017, 4, 283–298.
- Lis-Balchin, M. Lavender. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 2, pp. 329–347.
- Fattahi, B.; Nazeri, V.; Kalantari, S.; Bonfill, M.; Fattahi, M. Essential oil variation in wild-growing populations of Salvia reuterana Boiss. collected from Iran, using GC–MS and multivariate analysis. Ind. Crops Prod. 2016, 81, 180–190.
- Beheshti-Rouy, M.; Azarsina, M.; Rezaie-Soufi, L.; Alikhani, M.Y.; Roshanaie, G.; Komaki, S. The antibacterial effect of sage extract (Salvia officinalis) mouthwash against Streptococcus mutans in dental plaque: A randomized clinical trial. Iran. J. Microbiol. 2015, 7, 173–177.
- Mokhtari, R.; Fard, M.K.; Rezaei, M.; Moftakharzadeh, S.A.; Mohseni, A. Antioxidant, antimicrobial activities, and characterization of phenolic compounds of Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Thyme–Sage mixture extracts. J. Food. Qual. 2023, 2023, 2602454.
- Sulaiman, A.M.; Abdulaziz, A.S.; Almutawea, A.M.; Alansari, S.A.; Aldoseri, F.A.; Bekhit, S.A.; Al-Thawadi, S.M.; Alqallaf, S.M.; Bekh, A.A. Evaluation of the antibacterial effect of Salvia officinalis Essential Oil and its synergistic effect with Meropenem. Lett. Appl. NanoBioScience 2023, 12, 44.
- ISO 9909:1997; Oil of Dalmatian Sage (Salvia officinalis L.). International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Baricevic, D.; Bartol, T. The biological/pharmacological activity of the Salvia genus. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Singapore, 2005; p. 290.
- Yilar, M.; Kadioǧlu, I.; Telci, I. Chemical composition and antifungal activity of Salvia officinalis (L.), S. cryptantha (montbret et aucher ex benth.), S. tomentosa (mill.) plant essential oils and extracts. Fresenius Environ. Bull. 2018, 27, 1695–1706.
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Antienzymatic, and Antimicrobial Bioactivities. Chem. Biodivers. 2017, 14, e1700102.
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47.
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424.
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672.
- Dweck, A.C. Introduction. The folklore and cosmetic use of various salvia species. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Singapore, 2005.
- Monsen, R.E.; Herlofson, B.B.; Gay, C.; Fjeld, K.G.; Hove, L.H.; Malterud, K.E.; Saghaug, E.; Slaaen, J.; Sundal, T.; Tollisen, A.; et al. A mouth rinse based on a tea solution of Salvia officinalis for oral discomfort in palliative cancer care: A randomized controlled trial. Support. Care Cancer 2021, 29, 4997–5007.
- Lakhal, H.; Ghorab, H.; Chibani, S.; Kabouche, A.; Semra, Z.; Smati, F.; Abuhamdah, S.; Kabouche, Z. Chemical composition and biological activities of the essential oil of Salvia officinalis from Batna (Algeria). Der Pharm. Lett. 2013, 5, 310–314.
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Albdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordon: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940.
- European Medicines Agency (EMA). Assessment Report on Salvia fruticosa Mill Folium. EMA/HMPC/599992/2014. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-salvia-fruticosa-mill-folium_en.pdf (accessed on 15 May 2023).
- European Medicines Agency (EMA). Assessment Report on Salvia officinalis L., folium and Salvia officinalis L., Aetheroleum EMA/HMPC/150801/2015. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-salvia-officinalis-l-folium-salvia-officinalis-l-aetheroleum_en.pdf (accessed on 15 May 2023).
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130.
- Haziri, A.; Faiku, F.; Mehmeti, A.; Kurteshi, K.; Haziri, I.; Rudhani, I. In vitro antibacterial properties of ethanol extract from Salvia officinalis (L.) plant growing wild in Kosovo. Biomed. J. Sci. Tech. Res. 2018, 2, 2578–2580.
- Mohamed, A.Y.; Mustafa, A.A. In vitro anti-microbial activity of Essential Oils and other extracts from Salvia officinalis against some bacteria. Plant Sci. 2019; 2019040012, preprints.
- Medeiros de Almeida, C.; de Souza Sales Rocha, E.A.L.; Ponchet Alves, É.; de Freitas Lima, R.; Wanderley Cavalcanti, Y.; Barbosa Gomes, R.C.; Vieira Pereira, J.; Melo de Brito Costa, E.M. In vitro evaluation of the antimicrobial potential of Salvia officinalis L. against oral pathogens. J. Health Sci. 2019, 21, 129–133.
- de Oliveira, J.R.; Vilela, P.G.D.F.; Almeida, R.B.A.; de Oliveira, F.E.; Carvalho, C.A.T.; Camargo, S.E.A.; Jorge, A.O.C.; de Oliveira, L.D. Antimicrobial activity of noncytotoxic concentrations of Salvia officinalis extract against bacterial and fungal species from the oral cavity. Gen. Dent. 2019, 67, 22–26.
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621.
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea Essential Oil. Bot. Stud. 2015, 56, 16.
- Divyapriya, G.K.; Veeresh, D.J.; Yavagal, P.C. Evaluation of antibacterial efficacy of Chia (Salvia hispanica) seeds extract against Porphyromonas gingivalis, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans. An in-vitro study. Int. J. Ayurveda Pharm. Res. 2016, 4, 22–24.
- Elshafie, H.S.; Aliberti, L.; Amato, M.; De Feo, V.; Camele, I. Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) Essential Oil. Eur. Food Res. Technol. 2018, 244, 1675–1682.
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Lim, S.H.; Lai, K.S. Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 1–6.
- Moon, K.; Cha, J. Enhancement of antioxidant and antibacterial activities of Salvia miltiorrhiza roots fermented with Aspergillus oryzae. Foods 2020, 9, 34.
- Chen, B.-C.; Ding, Z.-S.; Dai, J.-S.; Chen, N.-P.; Gong, X.-W.; Ma, L.-F.; Qian, C.-D. New insights into the antibacterial mechanism of cryptotanshinone, a representative diterpenoid quinone from Salvia miltiorrhiza Bunge. Front. Microbiol. 2021, 12, 647289.
- Lim Ah Tock, M.; Combrinck, S.; Kamatou, G.; Chen, W.; Van Vuuren, S.; Viljoen, A. Antibacterial screening, biochemometric and bioautographic evaluation of the non-volatile bioactive components of three indigenous South African Salvia species. Antibiotics 2022, 11, 901.
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) Methanolic Extracts. J. Agric. Food Chem. 2012, 60, 9603–9608.
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the Genus Lavandula: From Farm to Pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402.
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799.
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776.
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula × intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182.
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of lavandin and lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44.
- Başer, K.H.C.; Buchbauer, G. Handbook of Essential Oils, Science, Technology and Applications, 2nd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2016.
- Lu, H.; Li, H.; Lu, H.; Li, X.; Zhou, A. Chemical composition of lavender essential oil and its antioxidant activity and inhibition against rhinitis-related bacteria. Afr. J. Microbiol. Res. 2010, 4, 309–313.
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940.
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-beta in a rat model. BMC Complement. Altern. Med. 2016, 16, 144.
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Genotoxicity of lavender oil, linalyl acetate, and linalool on human lymphocytes in vitro. Environ. Mol. Mutagen. 2011, 52, 69–71.
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential Oil Analysis and Antimicrobial Evaluation of Three Aromatic Plant Species Growing in Saudi Arabia. Molecules 2021, 26, 959.
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580.
- Hossain, S.; Heo, H.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.J. Antibacterial activity of essential oil from lavender (Lavandula angustifolia) against pet turtle-borne pathogenic bacteria. Lab. Anim. Res. 2017, 33, 195–201.
- Caprari, C.; Fantasma, F.; Divino, F.; Bucci, A.; Iorizzi, M.; Naclerio, G.; Ranalli, G.; Saviano, G. Chemical Profile, In Vitro Biological Activity and Comparison of Essential Oils from Fresh and Dried Flowers of Lavandula angustifolia L. Molecules 2021, 26, 5317.
- Nafis, A.; Ouedrhiri, W.; Iriti, M.; Mezrioui, N.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Hassani, L. Chemical composition and synergistic effect of three Moroccan lavender EOs with ciprofloxacin against foodborne bacteria: A promising approach to modulate antimicrobial resistance. Lett. Appl. Microbiol. 2021, 72, 698–705.
- El Baaboua, A.; El Maadoudi, M.; Bouyahy, A.; Belmehdi, O.; Kounnoun, A.; Cheyadmi, S.; Ouzakar, S.; Skali Senhaji, N.; Abrini, J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. S. Afr. J. Bot. 2022, 150, 451–465.




