Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rose Daphnee Tchonkouang | -- | 2413 | 2023-08-30 11:49:52 | | | |
| 2 | Sirius Huang | Meta information modification | 2413 | 2023-08-31 04:39:12 | | | | |
| 3 | Sirius Huang | Meta information modification | 2413 | 2023-08-31 04:42:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tchonkouang, R.D.; Lima, A.R.; Quintino, A.C.; Cristofoli, N.L.; Vieira, M.C. Applications of UV-C Light in the Food Industry. Encyclopedia. Available online: https://encyclopedia.pub/entry/48639 (accessed on 24 January 2026).
Tchonkouang RD, Lima AR, Quintino AC, Cristofoli NL, Vieira MC. Applications of UV-C Light in the Food Industry. Encyclopedia. Available at: https://encyclopedia.pub/entry/48639. Accessed January 24, 2026.
Tchonkouang, Rose Daphnee, Alexandre R. Lima, Andreia C. Quintino, Nathana L. Cristofoli, Margarida C. Vieira. "Applications of UV-C Light in the Food Industry" Encyclopedia, https://encyclopedia.pub/entry/48639 (accessed January 24, 2026).
Tchonkouang, R.D., Lima, A.R., Quintino, A.C., Cristofoli, N.L., & Vieira, M.C. (2023, August 30). Applications of UV-C Light in the Food Industry. In Encyclopedia. https://encyclopedia.pub/entry/48639
Tchonkouang, Rose Daphnee, et al. "Applications of UV-C Light in the Food Industry." Encyclopedia. Web. 30 August, 2023.
Copy Citation
A variety of bioactive substances present in fruit- and vegetable-processed products have health-promoting properties. The consumption of nutrient-rich plant-based products is essential to address undernutrition and micronutrient deficiencies. Preservation is paramount in manufacturing plant-based nonsolid foods such as juices, purees, and sauces. To prevent the loss of nutrients associated with thermal treatment, alternative technologies are being researched extensively. In studies conducted on nonsolid food, UV-C treatment has been proven to preserve quality and minimize nutrient degradation.
fruits
vegetables
plant-based
minimally processed
UV-C treatment
1. Introduction
Millions of people worldwide are undernourished and affected by “hidden hunger”, which is caused by a lack of essential minerals and micronutrients. Food items need to contain enough nutrients, whether processed or unprocessed, so that these nutrients can be significant contributors to food and nutrition security [1][2]. The majority of consumers view food safety as being of the utmost importance [3]. On the other hand, they are increasingly aware of nutrient uptake and seek to consume more foods that will benefit their health, well-being, and nutritional status. The increased consumption of fruit- and vegetable-based products has been motivated by the potential health benefits based on the significant amounts of vitamins, nutrients, and bioactive compounds contained in these products [4]. Several fruit- and vegetable-based products are preferred in their fresh state. However, they have a high perishability and a short shelf life. This limits the amount of time for which they are available and safe for consumption. Processing techniques can increase food choices while increasing the length of time before a food product becomes unfit for consumption. In the manufacture of processed foods, the use of preservation strategies is unavoidable in suppressing microbial or enzymatic and nonenzymatic spoilage, and therefore achieve an extended shelf life [5].
Thermal processing has historically been one of the most extensively used and approved methods to prevent foodborne illnesses and ensure food safety through the inactivation of spoilage enzymes and the destruction of microbial contaminants (pathogenic and spoilage) in foods and beverages [6]. The intensity of the heat treatment is dependent on the combination of temperature and treatment duration. From a microbiological perspective, intense heat treatment is preferable, but the employment of excessively high temperatures during prolonged times (severe heat treatments) can have deleterious consequences on the flavor, taste, and nutritive quality. Hence, a food product may be free of contaminants, comply with food safety standards, and still be nutritionally poor [7]. For instance, severe heat treatments degrade several heat-labile vitamins (e.g., vitamins A and C, and thiamin) and decrease the biological value (BV) of proteins by denaturing them and reducing their digestibility and bioavailability. The significance of nutrient degradation on nutrition security is determined by the eating habits and consumption frequency of a certain kind of food in the diet. Loss of nutritional value is thus more significant when there is a decrease in nutrients in nutritionally-rich and highly consumed food items that are sources of nutrients for a large share of the population than in foods that are either consumed in low quantities or have low nutritional contents [8][9].
Novel food processing methods are under investigation to address the loss of nutritional value due to thermal preservation [10]. Food processors and scientists have been exploring more effective low-temperature technologies that enable high-quality retention to deliver safe food products with acceptable organoleptic and rich nutritional profiles [7]. Nonthermal processing methods have been employed and among these, ultraviolet irradiation holds great promise as a food preservation technique for pathogen reduction and to minimize nutritional losses observed in heat-processed foods [11][12]. Ultraviolet radiation is divided into four categories in terms of wavelength range: UV-A (315–400 nm), UV-B (280–315 nm), UV-C (200–280 nm), and vacuum-UV (100–200 nm) [13]. The UV-C range possesses great antimicrobial effectiveness, which makes it useful for ensuring the microbial safety of foods. The genetic material (DNA or RNA) of microbes strongly absorbs UV photons within the UV-C range, with a wavelength around 260–265 nm corresponding to maximal UV absorption [14]. The preferred alternative pasteurization and shelf life extension method for beverages for the past two decades has been UV-C radiation at 253.7 nm [15]. UV-C irradiation causes damage to the nucleic acids of microorganisms, mainly due to the formation of dimers of pyrimidine bases between adjacent pyrimidines in a DNA strand, which prevents microbial replication and ultimately leads to cell death [16][17].
UV-C is a nontoxic and noninvasive method with numerous advantages that include the absence of chemical residues, it produces no waste, is cost-effective (low installation and maintenance cost), simple to implement, eco-friendly, has low energy consumption, minimal impact on nutritional quality and organoleptic parameters, and good consumer perception [11][15][18][19]. The primary drawback of this technology is the poor penetration depth of UV-C, which limits its antibacterial efficacy [20]. The microbial inactivation efficiency of UV-C is dependent on several factors like the UV-C dose (UV-C fluence), uniformity of UV-C dose distribution, UV-C sensitivity of the target microbial cells, the ability of the microorganisms to repair UV-induced damage, the physicochemical properties of the treated product (e.g., viscosity, density, soluble and suspended solids), and the optical properties of foods (e.g., transparency, absorption coefficient, scattering) [16][21][22][23]. This poses difficulties in the design of UV-C food treatment devices and for laboratory tests (experiments) that must guarantee a defined and consistent UV-C delivery while ensuring that all of the food surfaces are exposed to the UV-C illumination [22].
2. UV-C Light: Principles and Mechanisms of Germicidal Action
The principle behind UV-C light’s germicidal action is based on its ability to damage the DNA or RNA of microorganisms such as bacteria, viruses, and fungi through interaction between the UV photons and the genetic material of these microorganisms [21]. When UV-C light penetrates the cell wall of a microorganism, it is absorbed by the DNA or RNA inside the cell. This disrupts the genetic material, which can lead to the formation of new bonds or the breakage of existing ones. This alteration results in photodimerization, where two adjacent bases in the DNA/RNA sequence bind together. This genetic damage disrupts the affected cells’ ability to replicate, rendering them unable to cause infection or pose a threat [24].
The mechanism of UV-C germicidal action involves several factors including the light intensity, exposure time, and the type of microorganism being targeted, which can vary depending on the specific application [21][25]. Furthermore, the germicidal effectiveness of UV-C light as a disinfectant is based on the dose–response relationship, microbial susceptibility, and the optical properties of the food matrices or treated surfaces [26][27]. In Figure 1, the main factors that affect the success of UV-C processing are presented as well as a general representation of the reactor chamber.
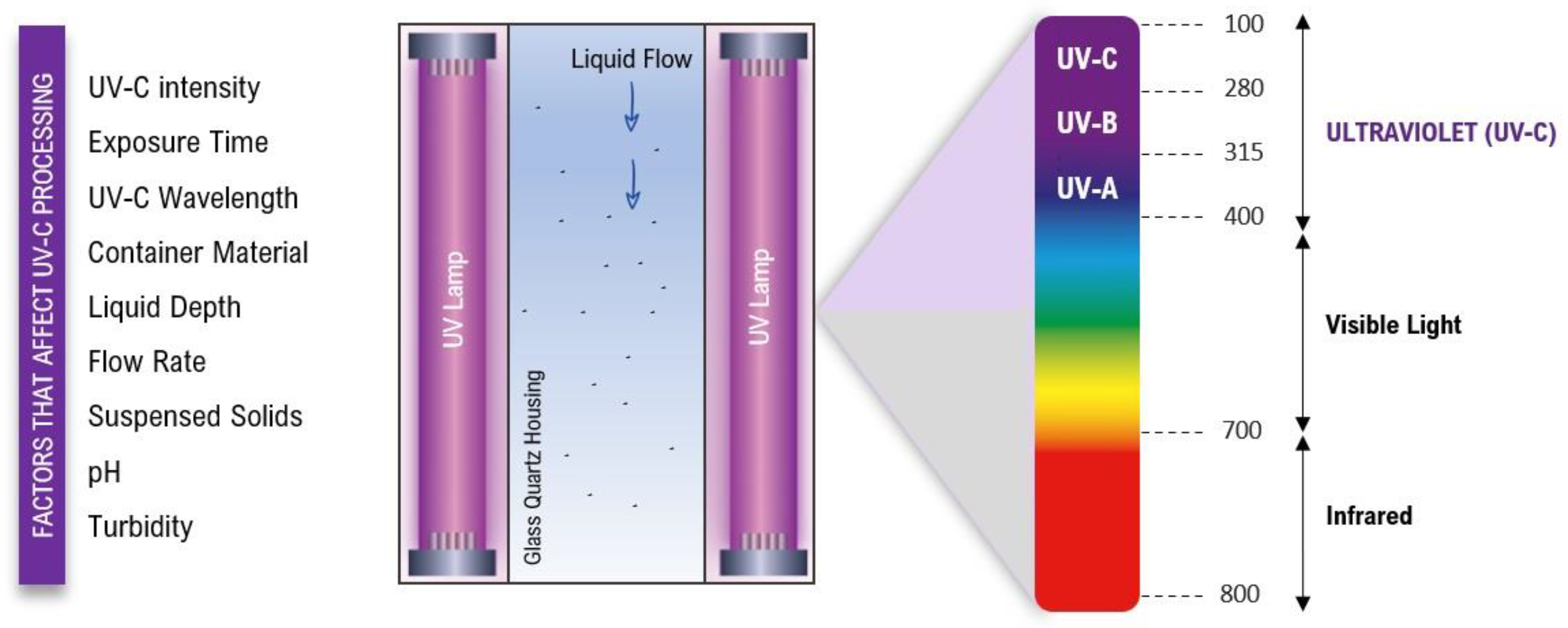
Figure 1. Reactor chamber of UV-C processing for fluid food and the main factors that influence the process.
It is important to note that these factors are interconnected and should be considered collectively during the design and implementation of UV-C treatment processes for developing shelf-stable food. Higher intensity levels of UV-C radiation generally lead to better microbial inactivation [28]. However, the duration of UV-C exposure should be carefully selected to achieve microbial reduction without compromising food composition and quality [29][30]. Furthermore, the choice of the UV-C wavelength should be based on the target microorganisms and the food product [24]. The material of the product’s container can affect UV-C treatment, with transparent materials allowing for better penetration; the depth of the liquid and flow rate through the UV-C system should be considered for uniform exposure [31][32]. At the same time, suspended solids can reduce the effectiveness of UV-C treatment [25][33]. The pH and turbidity of the liquid also impact treatment efficiency, and maintaining optimal ranges enhances the effectiveness of UV-C treatment [25]. From the understanding of these principles, UV-C light technology has been used effectively not only for disinfection and sterilization in various applications such as healthcare settings and water treatment, but also in the food industry and more recently as a neutralizing agent of the infectivity of SARS-CoV-2 [22][34][35][36][37][38]. Some factors that influence UV-C efficacy are described below.
2.1. Dose–Response Relationship
The dose-response relationship of UV-C light germicidal action follows a pattern where the effectiveness of killing microorganisms increases with higher doses or intensities of UV-C light [39][40]. At lower doses, the light exposure may not be sufficient to cause significant damage to the microorganisms, allowing some of them to survive or repair the damage [12][40][41]. As the dose of UV-C light increases, the likelihood of DNA and RNA damage also increases, leading to a higher rate of microorganism inactivation [42].
It is important to note that there is an optimal range of UV-C light intensity for germicidal action. The sensitivity of microbes to UV light varies depending on the wavelength [21]. However, the strong absorption of ultraviolet light by water at wavelengths below 230 nm is a limiting factor for the germicidal effect. Beyond this wavelength, increasing the dose may not significantly enhance the killing efficacy and may even result in diminishing returns. Additionally, excessively high doses of UV-C light can harm human health and damage materials or surfaces [37][43]. In this sense, it is crucial to use UV-C light within safe and recommended exposure limits to balance its germicidal efficacy with potential risks.
2.2. Microbial Susceptibility
The susceptibility of microorganisms to UV-C light varies depending on their structure and genetic makeup [33]. UV susceptibility of microorganisms can differ considerably due to differences in cellular elements like cell wall thickness, composition, nucleic acid structure, type of proteins within the cell, photoproducts, the physiological condition of the microbe, and the cell’s capacity for repairing damage caused by ultraviolet radiation [19]. However, it is worth mentioning that the effectiveness of UV-C light as a microbial inactivation method depends on other factors including the food matrix [44], exposure time, distance from the UV-C source, and the presence of any physical barriers or shadows that may shield microorganisms from direct UV-C exposure [42]. Different microorganisms have varying levels of sensitivity to UV-C-induced DNA/RNA damage. In this sense, viruses with RNA genomes are more susceptible to UV-C light than viruses with DNA genomes [45]. Another important factor is the cell wall structure. Microorganisms with more robust and resistant cell walls may be more resistant to germicidal UV-C light. Viruses and fungi, on the other hand, may be more susceptible to UV-C light due to their fragile cell walls. Gram-negative bacteria, in general, are more sensitive to UV-C light than Gram-positive bacteria due to their thinner cell walls [46]. The efficacy of UV-C microbial inactivation greatly depends on the treated food. Opaque and turbid nonsolid food matrices are more challenging to treat compared to transparent food substrates. This is because the turbidity and presence of suspended solids in nontransparent liquids confer protection to microorganisms by scattering or absorbing the radiation before it reaches them [44].
2.3. Optical Properties of Surfaces
The optical properties of surfaces refer to how they interact with light. These properties can include the reflection, absorption, transmission, and scattering of light [21][32]. When it comes to UV-C light, the optical properties of surfaces that host microorganisms can affect the effectiveness of UV-C light. For example, surfaces that are rough or uneven may scatter UV-C light, potentially reducing the intensity of UV-C radiation in a particular direction [32], and if they are porous, UV-C light can be absorbed. Reflective surfaces can also scatter and absorb UV-C light [22][37]. When compared to smooth surfaces, some of these surfaces require roughly two orders of magnitude greater UV-C doses to adequately inactivate microorganisms [37][47]. Normally, light transmission refers to the passage of UV-C light through materials. Materials like certain types of glass can allow UV-C light to pass through with minimal attenuation, while others may block or attenuate UV-C light, reducing its transmission [31][32].
3. Current Applications of UV-C Light in the Food Industry
The recent consumer demands for safe food with high-quality nutritional (e.g., vitamins, protein) and sensory (mainly color, flavor, and texture) attributes have challenged the scientific community and the food industry to develop and implement nonthermal technologies to process/manufacture foods while minimizing the changes to these attributes [48][49][50]. In this sense, UV-C light has been a promising technology for improving food safety and reducing the risk of foodborne illnesses in the food industry [21][33]. In the last decades, the food industry has used this versatile tool for surface decontamination, air and water treatment, to prevent the spread of microorganisms, and ensure food safety and preservation.
3.1. Air Purification and Surface Disinfection
UV-C light is used to purify air in food processing facilities. UV-C lamps can be installed in air handling units to sterilize the air as it circulates through the facility, reducing the risk of airborne contamination [35]. Air disinfection can be accomplished by irradiating only the upper parts of the room or by irradiating the entire air, either in an empty room or using an air conditioner [51]. UV-C light is also used to disinfect surfaces following routine cleaning procedures in food processing facilities including food preparation areas, packaging areas, and equipment. UV-C light can effectively kill bacteria, viruses, and other microorganisms that may contaminate surfaces and cause foodborne illness [35][52][53]. Low-pressure mercury lamps are ideal for controlling surface microorganisms in the food industry, since 90% of the emitted light is at a 253.7 nm wavelength [54].
3.2. Water Treatment and Food Preservation
UV-C light can be used to sanitize water used in food processing and production as well as help prevent the growth of harmful bacteria and other microorganisms in municipal water supply systems [53][55]. Additionally, UV-C light has been used to extend the shelf life of fresh, minimally processed, and liquid foods by reducing the microbial load and helping to prevent spoilage [12][56][57][58][59][60].
3.3. Retention of Bioactive Compounds
While UV-C light technology is commonly used for its antimicrobial properties in the food industry, there is also research indicating that it can be used to improve and/or preserve the nutritional properties of fruit and vegetables [33][60][61][62][63]. When exposed to UV-C light, certain compounds in foods can be activated or transformed, resulting in the production of bioactive compounds that may have health benefits [64][65][66]. Bhat and Stamminger (2014) reported that exposure to UV-C light has been shown to increase the levels of phenolic compounds and antioxidant activity in strawberry juice [48]. In the same way, UV-C light exposure has been shown to increase the levels of certain phytochemicals in plant produce [67].
Győrfi et al. (2011) identified the capacity of UV-C light to increase the production of vitamin D in mushrooms. When exposed to UV-C light, the ergosterol in mushrooms is converted to vitamin D2, increasing the vitamin D content [68]. Overall, UV-C light can be a useful tool for producing bioactive compounds in foods, which can enhance their nutritional value and potential health benefits. However, it is important to carefully evaluate the safety and efficacy of these compounds before incorporating them into food products.
References
- Ingram, J. Nutrition Security Is More than Food Security. Nat. Food 2020, 1, 2.
- Talens, C.; Garcia-Fontanals, L.; Fabregat, P.; Ibargüen, M. Rational Food Design Targeting Micronutrient Deficiencies in Adolescents: Nutritional, Acoustic-Mechanical and Sensory Properties of Chickpea-Rice Biscuits. Foods 2023, 12, 952.
- Orlowska, M.; Koutchma, T.; Grapperhaus, M.; Gallagher, J.; Schaefer, R.; Defelice, C. Continuous and Pulsed Ultraviolet Light for Nonthermal Treatment of Liquid Foods. Part 1: Effects on Quality of Fructose Solution, Apple Juice, and Milk. Food Bioprocess Technol. 2013, 6, 1580–1592.
- Koren, M.; Livne, D. Novel Industrial UV-C System for Preservation of Fruit and Vegetable Juices. IUVA News 2018, 20, 8–12.
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical Changes of Bioactive Phytochemicals during Thermal Processing. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-08-100596-5.
- Soni, A.; Bremer, P.; Brightwell, G. A Comprehensive Review of Variability in the Thermal Resistance (D-Values) of Food-Borne Pathogens—A Challenge for Thermal Validation Trials. Foods 2022, 11, 4117.
- Tadini, C.C.; Gut, J.A.W. The Importance of Heating Unit Operations in the Food Industry to Obtain Safe and High-Quality Products. Front. Nutr. 2022, 9, 853638.
- Fellows, P.J. 1—Properties of Food and Principles of Processing. In Food Processing Technology (Fourth Edition); Fellows, P.J., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–200. ISBN 978-0-08-101907-8.
- Lešková, E.; Kubíková, J.; Kováčiková, E.; Košická, M.; Porubská, J.; Holčíková, K. Vitamin Losses: Retention during Heat Treatment and Continual Changes Expressed by Mathematical Models. J. Food Compost. Anal. 2006, 19, 252–276.
- Cruz, R.M.S.; Vieira, M.C.; Silva, C.L.M. Effect of Heat and Thermosonication Treatments on Watercress (Nasturtium officinale) Vitamin C Degradation Kinetics. Innov. Food Sci. Emerg. Technol. 2008, 9, 483–488.
- Fundo, J.F.; Miller, F.A.; Mandro, G.F.; Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. UV-C Light Processing of Cantaloupe Melon Juice: Evaluation of the Impact on Microbiological, and Some Quality Characteristics, during Refrigerated Storage. LWT 2019, 103, 247–252.
- Neves, F.I.G.; Silva, C.L.M.; Vieira, M.C. Combined Pre-Treatments Effects on Zucchini (Cucurbita pepo L.) Squash Microbial Load Reduction. Int. J. Food Microbiol. 2019, 305, 108257.
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Adv. Wound Care 2013, 2, 422.
- Green, A.; Popović, V.; Warriner, K.; Koutchma, T. The Efficacy of UVC LEDs and Low Pressure Mercury Lamps for the Reduction of Escherichia coli O157:H7 and Listeria Monocytogenes on Produce. Innov. Food Sci. Emerg. Technol. 2020, 64, 102410.
- Abdul Karim Shah, N.; Shamsudin, R.; Abdul Rahman, R.; Adzahan, N. Fruit Juice Production Using Ultraviolet Pasteurization: A Review. Beverages 2016, 2, 22.
- Taze, B.H.; Akgun, M.P.; Yildiz, S.; Kaya, Z.; Unluturk, S. 2.18—UV Processing and Storage of Liquid and Solid Foods: Quality, Microbial, Enzymatic, Nutritional, Organoleptic, Composition and Properties Effects. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 277–305. ISBN 978-0-12-815782-4.
- Banaś, A.K.; Zgłobicki, P.; Kowalska, E.; Bażant, A.; Dziga, D.; Strzałka, W. All You Need Is Light. Photorepair of UV-Induced Pyrimidine Dimers. Genes 2020, 11, 1304.
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet Radiation: An Interesting Technology to Preserve Quality and Safety of Milk and Dairy Foods. Trends Food Sci. Technol. 2020, 102, 146–154.
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of Ultraviolet Light Treatment on Microbiological Safety and Quality of Fresh Produce: An Overview. Front. Nutr. 2022, 9, 871243.
- Ruiz-Hernández, K.; Ramírez-Rojas, N.Z.; Meza-Plaza, E.F.; García-Mosqueda, C.; Jauregui-Vázquez, D.; Rojas-Laguna, R.; Sosa-Morales, M.E. UV-C Treatments against Salmonella Typhimurium ATCC 14028 in Inoculated Peanuts and Almonds. Food Eng. Rev. 2021, 13, 706–712.
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods. Food Bioprocess Technol. 2009, 2, 138–155.
- Gunter-Ward, D.M.; Patras, A.S.; Bhullar, M.; Kilonzo-Nthenge, A.; Pokharel, B.; Sasges, M. Efficacy of Ultraviolet (UV-C) Light in Reducing Foodborne Pathogens and Model Viruses in Skim Milk. J. Food Process. Preserv. 2018, 42, e13485.
- Atilgan, M.R.; Yildiz, S.; Kaya, Z.; Unluturk, S. Kinetic and Process Modeling of UV-C Irradiation of Foods. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 227–255. ISBN 978-0-12-815782-4.
- Allai, F.M.; Azad, Z.R.A.A.; Mir, N.A.; Gul, K. Recent Advances in Non-Thermal Processing Technologies for Enhancing Shelf Life and Improving Food Safety. Appl. Food Res. 2023, 3, 100258.
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC Radiation for Food Safety: An Emerging Technology for the Microbial Disinfection of Food Products. Chem. Eng. J. 2021, 417, 128084.
- Gouma, M.; Gayán, E.; Raso, J.; Condón, S.; Álvarez, I. UV-Heat Treatments for the Control of Foodborne Microbial Pathogens in Chicken Broth. BioMed Res. Int. 2015, 2015, e436030.
- Ben Said, M.; Khefacha, S.; Maalej, L.; Daly, I.; Hassen, A. Effect of Ultraviolet, Electromagnetic Radiation Subtype C (UV-C) Dose on Biofilm Formation by Pseudomonas Aeruginosa. Afr. J. Microbiol. Res. 2011, 5, 4353–4358.
- Liltved, H.; Landfald, B. Effects of High Intensity Light on Ultraviolet-Irradiated and Non-Irradiated Fish Pathogenic Bacteria. Water Res. 2000, 34, 481–486.
- Gopisetty, V.V.S.; Patras, A.; Kilonzo-Nthenge, A.; Yannam, S.; Bansode, R.R.; Sasges, M.; Burns, S.M.; Vergne, M.J.; Pan, C.; Xiao, H. Impact of UV-C Irradiation on the Quality, Safety, and Cytotoxicity of Cranberry-Flavored Water Using a Novel Continuous Flow UV System. LWT 2018, 95, 230–239.
- Koutchma, T. Advances in UV-C Light Technology Improve Safety and Quality Attributes of Juices, Beverages, and Milk Products. Food Saf. Mag. 2019. Available online: https://www.food-safety.com/articles/6125-advances-in-uv-c-light-technology-improve-safety-and-quality-attributes-of-juices-beverages-and-milk-products#References (accessed on 15 May 2023).
- Duarte, I.; Rotter, A.; Malvestiti, A.; Silva, M. The Role of Glass as a Barrier against the Transmission of Ultraviolet Radiation: An Experimental Study. Photodermatol. Photoimmunol. Photomed. 2009, 25, 181–184.
- Woodling, S.E.; Moraru, C. Influence of Surface Topography on the Effectiveness of Pulsed Light Treatment for the Reduction of Listeria Innocua on Stainless Steel Surfaces. J. Food Sci. 2005, 70, 245–351.
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62.
- Biasin, M.; Strizzi, S.; Bianco, A.; Macchi, A.; Utyro, O.; Pareschi, G.; Loffreda, A.; Cavalleri, A.; Lualdi, M.; Trabattoni, D.; et al. UV and Violet Light Can Neutralize SARS-CoV-2 Infectivity. J. Photochem. Photobiol. 2022, 10, 100107.
- Chawla, A.; Lobacz, A.; Tarapata, J.; Zulewska, J. UV Light Application as a Mean for Disinfection Applied in the Dairy Industry. Appl. Sci. 2021, 11, 7285.
- Lah, E.F.C.; Musa, R.N.A.R.; Ming, H.T. Effect of Germicidal UV-C Light(254 nm) on Eggs and Adult of House Dustmites, Dermatophagoides pteronyssinus and Dermatophagoides farinae (Astigmata: Pyroglyhidae). Asian Pac. J. Trop. Biomed. 2012, 2, 679–683.
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics 2020, 7, 2941–2951.
- Woo, H.; Beck, S.; Boczek, L.; Carlson, K.; Brinkman, N.; Linden, K.; Lawal, O.; Hayes, S.; Ryu, H. Efficacy of Inactivation of Human Enteroviruses by Dual-Wavelength Germicidal Ultraviolet (UV-C) Light Emitting Diodes (LEDs). Water 2019, 11, 1131.
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-642-01998-2.
- Templeton, M.R.; Antonakaki, M.; Rogers, M. UV Dose–Response of Acinetobacter Baumannii in Water. Environ. Eng. Sci. 2009, 26, 697–701.
- Chen, P.-F.; Zhang, R.-J.; Huang, S.-B.; Shao, J.-H.; Cui, B.; Du, Z.-L.; Xue, L.; Zhou, N.; Hou, B.; Lin, C. UV Dose Effects on the Revival Characteristics of Microorganisms in Darkness after UV Disinfection: Evidence from a Pilot Study. Sci. Total Environ. 2020, 713, 136582.
- Sinha, R.P.; Häder, D.-P. UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci. 2002, 1, 225–236.
- Hessling, M.; Haag, R.; Sieber, N.; Vatter, P. The Impact of Far-UVC Radiation (200–230 nm) on Pathogens, Cells, Skin, and Eyes—A Collection and Analysis of a Hundred Years of Data. GMS Hyg. Infect. Control 2021, 16, Doc07.
- Gómez-López, V.M.; Jubinville, E.; Rodríguez-López, M.I.; Trudel-Ferland, M.; Bouchard, S.; Jean, J. Inactivation of Foodborne Viruses by UV Light: A Review. Foods 2021, 10, 3141.
- Zhao, Y.; Dong, J. Effect of Inactivating RNA Viruses by Coupled UVC and UVA LEDs Evaluated by a Viral Surrogate Commonly Used as a Genetic Vector. Biomed. Opt. Express 2022, 13, 4429.
- Koutchma, T. Chapter 2 - Basic Principles of UV Light Generation. In Food Plant Safety; Academic Press: Toronto, ON, Canada, 2014; pp. 3–13. ISBN 9780124166202.
- Cadnum, J.L.; Li, D.; Redmond, S.N.; John, A.R.; Pearlmutter, B.; Donskey, C. Effectiveness of Ultraviolet-C Light and a High-Level Disinfection Cabinet for Decontamination of N95 Respirators. PAI 2020, 5, 52.
- Bhat, R.; Stamminger, R. Impact of Ultraviolet Radiation Treatments on the Physicochemical Properties, Antioxidants, Enzyme Activity and Microbial Load in Freshly Prepared Hand Pressed Strawberry Juice. Food Sci. Technol. Int. 2014, 21, 354–363.
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to Conventional Thermal Treatments in Fruit-Juice Processing. Part 1: Techniques and Applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523.
- Koutchma, T.; Popović, V.; Ros-Polski, V.; Popielarz, A. Effects of Ultraviolet Light and High-Pressure Processing on Quality and Health-Related Constituents of Fresh Juice Products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 844–867.
- Darré, M.; Vicente, A.R.; Cisneros-Zevallos, L.; Artés-Hernández, F. Postharvest Ultraviolet Radiation in Fruit and Vegetables: Applications and Factors Modulating Its Efficacy on Bioactive Compounds and Microbial Growth. Foods 2022, 11, 653.
- Antonio-Gutiérrez, O.T.; López-Díaz, A.S.; López-Malo, A.; Palou, E.; Ramírez-Corona, N. 7—UV-C Light for Processing Beverages: Principles, Applications, and Future Trends. In Processing and Sustainability of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 205–234. ISBN 978-0-12-815259-1.
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and Potential Applications of Ultraviolet Light in the Food Industry—A Critical Review. J. Sci. Food Agric. 2000, 80, 637–645.
- Jildeh, Z.B.; Wagner, P.H.; Schöning, M.J. Sterilization of Objects, Products, and Packaging Surfaces and Their Characterization in Different Fields of Industry: The Status in 2020. Phys. Status Solidi (A) 2021, 218, 2000732.
- Adhikari, A.; Parraga Estrada, K.J.; Chhetri, V.S.; Janes, M.; Fontenot, K.; Beaulieu, J.C. Evaluation of Ultraviolet (UV-C) Light Treatment for Microbial Inactivation in Agricultural Waters with Different Levels of Turbidity. Food Sci. Nutr. 2020, 8, 1237–1243.
- Guneser, O.; Karagul Yuceer, Y. Effect of Ultraviolet Light on Water- and Fat-Soluble Vitamins in Cow and Goat Milk. JDS 2012, 95, 6230–6241.
- Hinojosa, A.; Gatica, I.; Bustamante, A.; Cárdenas, D.; Escalona, V. Effect of the Combined Treatment of UV-C Light and Modified Atmosphere Packaging on the Inactivation of Escherichia coli Inoculated Watercress. J. Food Process. Preserv. 2015, 39, 1525–1533.
- Ortiz-Solà, J.; Valero, A.; Abadias, M.; Nicolau-Lapeña, I.; Viñas, I. Evaluation of Water-Assisted UV-C Light and Its Additive Effect with Peracetic Acid for the Inactivation of Listeria Monocytogenes, Salmonella Enterica and Murine Norovirus on Whole and Fresh-Cut Strawberries during Shelf-Life. J. Sci. Food Agric. 2022, 102, 5660–5669.
- Cruz, R.M.S.; Godinho, A.I.A.; Aslan, D.; Koçak, N.F.; Vieira, M.C. Modeling the Kinetics of Peroxidase Inactivation, Colour and Texture Changes of Portuguese Cabbage (Brassica oleracea L. Var. Costata DC) during UV-C Light and Heat Blanching. Int. J. Food Stud. 2016, 5, 180–192.
- Lima, A.R.; Cristofoli, N.L.; Veneral, J.G.; Fritz, A.R.M.; Vieira, M.C. Optimization Conditions of UV-C Radiation Combined with Ultrasound-Assisted Extraction of Cherry Tomato (Lycopersicon esculentum) Lycopene Extract. Int. J. Food Stud. 2019, 8, 65–80.
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.A.; Gardea, A.A.; Ayala-Zavala, J.F. Improving Antioxidant Capacity of Fresh-Cut Mangoes Treated with UV-C. J. Food Sci. 2007, 72, S197–S202.
- Modesto Junior, E.N.; Martins, M.G.; Pereira, G.A.; Chisté, R.C.; Pena, R.D.S. Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments. Foods 2023, 12, 565.
- Sheng, K.; Shui, S.S.; Yan, L.; Liu, C.; Zheng, L. Effect of Postharvest UV-B or UV-C Irradiation on Phenolic Compounds and Their Transcription of Phenolic Biosynthetic Genes of Table Grapes. J. Food Sci. Technol. 2018, 55, 3292–3302.
- Freitas, A.; Moldão-martins, M.; Costa, H.S.; Tânia, G.; Sanches-silva, A. Effect of UV-C Radiation on Bioactive Compounds of Pineapple (Ananas comosus L. Merr.) by-Products. J. Sci. Food Agric. 2014, 95, 44–52.
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.A.; Aparicio-Fernández, X.; Ávila-Sosa, R.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C.E. Effect of Blue and Ultraviolet-C Light Irradiation on Bioactive Compounds and Antioxidant Capacity of Habanero Pepper (Capsicum chinense) during Refrigeration Storage. Postharvest Biol. Technol. 2018, 135, 19–26.
- Yıkmış, S.; Barut Gök, S.; Levent, O.; Kombak, E. Moderate Temperature and UV-C Light Processing of Uruset Apple Juice: Optimization of Bioactive Components and Evaluation of the Impact on Volatile Profile, HMF and Color. J. Food Process Eng. 2021, 44, e13893.
- Alothman, M.; Bhat, R.; Karim, A.A. Effects of Radiation Processing on Phytochemicals and Antioxidants in Plant Produce. Trends Food Sci. Technol. 2009, 20, 201–212.
- Győrfi, J.; Kovács, A.; Szabó, A. Increasing the Vitamin D Level of Oyster Mushrooms by UV Light. Int. J. Hortic. 2011, 17, 119–123.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
3 times
(View History)
Update Date:
31 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




