Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Reabal Najjar | -- | 4249 | 2023-08-29 23:34:55 | | | |
| 2 | Camila Xu | Meta information modification | 4249 | 2023-08-30 04:34:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Najjar, R. Artificial Intelligence Integration in Medical Imaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/48614 (accessed on 08 March 2026).
Najjar R. Artificial Intelligence Integration in Medical Imaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/48614. Accessed March 08, 2026.
Najjar, Reabal. "Artificial Intelligence Integration in Medical Imaging" Encyclopedia, https://encyclopedia.pub/entry/48614 (accessed March 08, 2026).
Najjar, R. (2023, August 29). Artificial Intelligence Integration in Medical Imaging. In Encyclopedia. https://encyclopedia.pub/entry/48614
Najjar, Reabal. "Artificial Intelligence Integration in Medical Imaging." Encyclopedia. Web. 29 August, 2023.
Copy Citation
Radiology, since its inception, has experienced a revolutionary journey, punctuating modern medicine with its profound influence. From the discovery of X-rays to the subsequent integration of artificial intelligence (AI) and machine learning (ML), this multifaceted discipline continually evolves, transforming itself and the healthcare ecosystem it underpins.
medical imaging
radiology
artificial intelligence
machine learning
1. Radiology in Modern Medicine
Radiology, the medical discipline centred on the utilisation of imaging modalities to diagnose and treat diseases, has emerged as a cornerstone of contemporary medicine, forming an integral part of clinical practice. It extends beyond mere disease detection to encompass treatment guidance and ongoing disease management. Expertise in diagnostic modalities such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), ultrasound, and X-rays guide immediate clinical interventions, treatment monitoring, and chronicle a visual narrative of a patient’s health. The intricate insights into anatomical, physiological, and molecular disease processes provided by medical imaging have a significant impact on patient care, facilitating the tailoring of treatments, thereby improving therapeutic outcomes and minimising adverse effects [1][2][3].
Radiology serves as a crucial gear in the intricate machinery of interdisciplinary medical teams. Radiologists deliver precise, timely imaging reports, thus enhancing communication among various specialists and shaping crucial decisions, which contributes to a holistic, patient-focused healthcare approach [4]. As valued consultative partners, radiologists offer key insights into the choice and interpretation of suitable imaging studies, playing a significant role in radiation safety and dose management while their expertise elucidates the clinical picture, offering insights that can markedly influence patient management [5][6].
2. The Fundamentals of Artificial Intelligence and Machine Learning
This section offers a journey through complex developments of AI and ML, sketching their historical trajectory and explaining the distinctive yet interconnected terminologies of AI, ML, and Deep Learning (DL). It also sheds light on the key ML algorithms and techniques that have shaped the technological landscape, underscoring the indelible imprint they have left on it.
2.1. Chronicle of Artificial Intelligence: Milestones and Breakthroughs
The compelling development of AI, spanning from antiquity—marked by folklore and tales of artificial beings—to the current era of sophisticated AI systems, has roots in the philosophical depictions of human cognition as a mechanistic process (Figure 1). The inception of modern AI concepts is largely accredited to the development of the programmable digital computer in the 1940s, reaching a milestone with the field’s official establishment at the 1956 Dartmouth Conference [7].
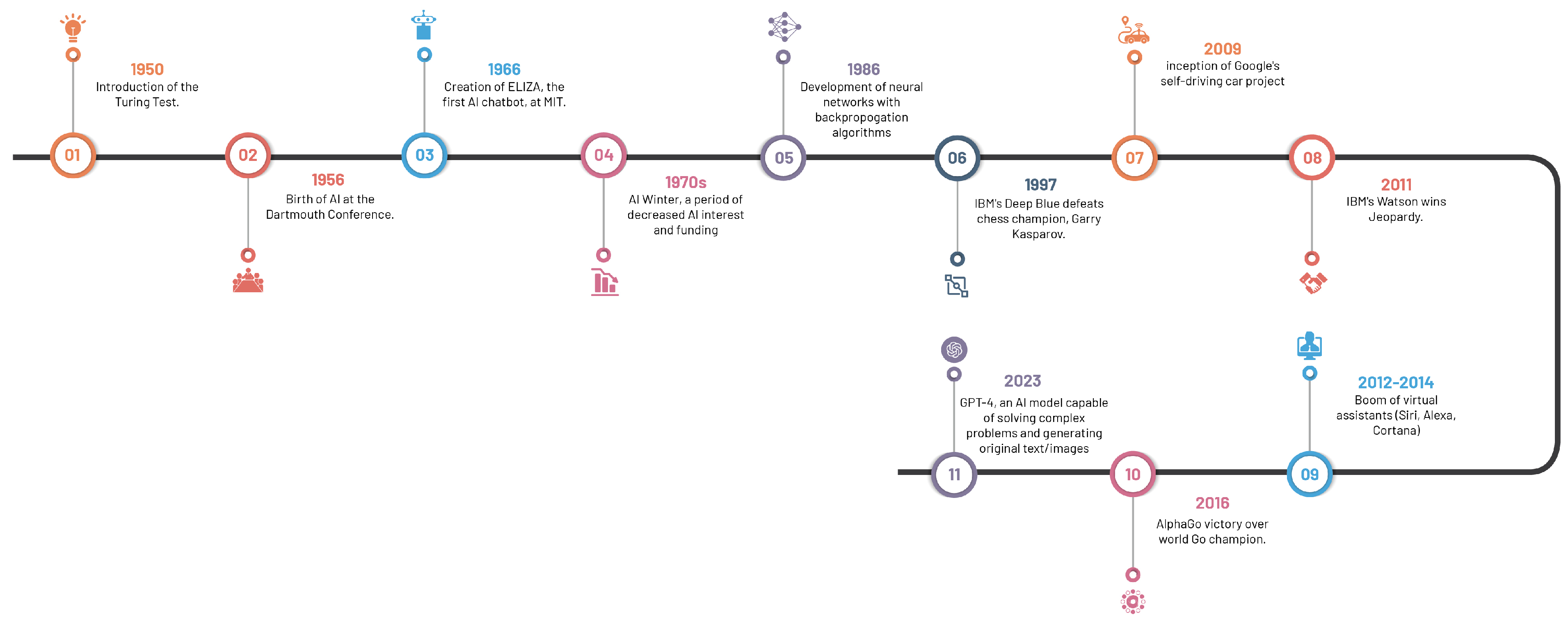
Figure 1. Significant milestones in the evolution of artificial intelligence.
The 1970s saw a significant leap forward with the emergence of rule-based expert systems like MYCIN, a seminal creation by Buchanan and Shortliffe [7][8]. These expert systems, designed to replicate human expertise by leveraging knowledge bases and inference engines, laid the foundation for AI’s influential role in medical diagnosis and clinical decision-making.
Machine learning algorithms, which surfaced in subsequent years, spearheaded a novel paradigm for data-driven predictions and classification. Decision trees introduced in 1986, support vector machines in 1995, and neural networks in 1986, collectively broadened the AI horizon in healthcare [9][10][11]. These algorithms propelled the analysis of extensive datasets, thereby inaugurating a novel epoch of pattern recognition and predictive modelling in healthcare.
The turn of the century witnessed a paradigm shift with the advent of deep learning approaches, particularly convolutional neural networks (CNNs). Surpassing previous methodologies in image recognition tasks, CNNs, architecturally modelled on structure and function of the human brain, precipitated major advancements in medical image classification, segmentation, and detection, due to their capacity to learn hierarchical representations from vast amounts of labelled data [12][13].
The accelerated progression of AI in recent years is a by-product of the synergistic interplay of two factors: the surge in big data and advancements in computational power. The widespread dissemination of electronic health records (EHRs), medical imaging archives, and annotated datasets provide ample training data, while progress in hardware, including graphical processing units (GPUs) and distributed computing, have expedited the deployment of computationally demanding AI algorithms [14].
The rapid advancement of AI language models, such as GPT-4, has led to unique applications and implications across a multitude of discplines, including healthcare and medical imaging [15]. These models, whilst capable of generating human-like text and facilitating communication, have raised salient concerns. The potential for these models to contribute substantially to medical research and patient care is undeniable; however, experts express reservations about their limitations and potential to inadvertently engender inequities or disseminate misinformation, thus emphasising the need for robust strategies to manage these risks responsibly. These strategies include increasing transparency about potential harms, promoting early detection of issues, and implementing regulatory measures and peer reviews [16]. By doing so, the goal is to ensure that AI technologies, including language models, are utilised optimally and ethically, contributing positively to medical imaging and healthcare outcomes.
The intertwining of rule-based systems, traditional machine learning algorithms, and the transformative influence of deep learning techniques has laid the foundation for the current state-of-the-art AI applications in radiology and other medical specialties.
2.2. Decoding the Terminology: AI, ML, and DL
The computational intelligence landscape is an expansive system, constituted of distinct yet interconnected systems, with each sector playing a specific role and interacts uniquely within the data science sphere (Figure 2).
Artificial intelligence is defined as the replication of human intelligence in machines that are programmed to emulate human cognition and actions, encompassing learning, problem-solving, reasoning, and perception. AI can be classified into two major types: narrow AI, which is designed for specific tasks (e.g., facial recognition or voice commands), and general AI, which mimics a broader spectrum of human intellect. AI aims to develop systems with autonomous intelligent functionality, capable of problem-solving, decision-making, and performing tasks typically requiring human intelligence [17].
Machine learning, a subset of AI, is centred around the development of software that learns autonomously from accessed data. The learning process is derived from the analysis of observations or data to identify patterns and make informed future decisions based on these observations. Its fundamental objective is to enable computers to adapt their actions autonomously without human intervention. The primary categories of ML algorithms are supervised learning, which predicts or classifies new data based on examples, and unsupervised learning, which identifies inherent patterns and structures within the data without guidance from pre-established outputs [18].
Deep learning, an ML subset, utilises multilayered artificial neural networks (aptly coined “deep”), enabling DL algorithms to model and understand complex data patterns. These algorithms are especially effective for tasks where manual feature extraction proves challenging, such as image or speech recognition. It is noteworthy that these feature layers are data-learned, not human-engineered, embodying an architecture inspired by the human brain and have proven successful in visual recognition tasks, even outperforming human performance [12].
Recognising the nuanced interrelationships between AI, ML, and DL aids in conceptualising each subfield’s contribution and progression within the broader AI narrative. AI lays the foundation for ML and DL. ML amplifies AI’s potential by enabling machine data learning, and DL further deepens these capabilities with neural networks that decipher complex data patterns. Each field enriches the wider AI domain, culminating in the modern AI landscape where each layer contributes to the evolution of intelligent systems.
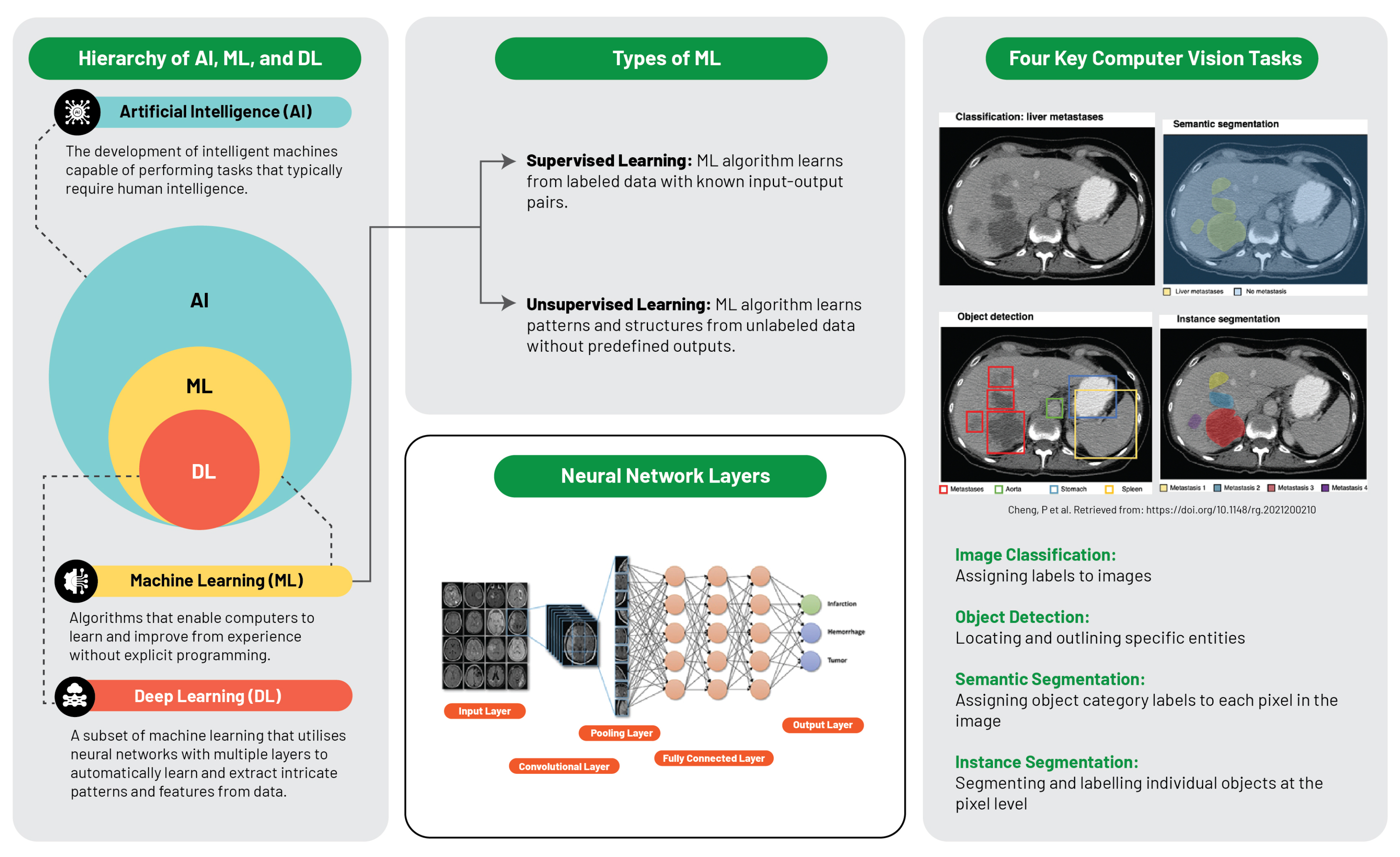
Figure 2. Schematic illustration of artificial intelligence and machine learning frameworks [19].
2.3. Machine Learning Foundations: Algorithms and Techniques
Machine learning is anchored by a plethora of algorithms and techniques that enable computers to learn from data. The two primary types of machine learning, supervised and unsupervised learning, lie at the heart of this field.
Supervised learning, a dominant machine learning variant, harnesses pre-established examples or training data, consisting of input-output pairs. The objective is to formulate a function that maps input data to corresponding outputs, enabling accurate predictions or classifications for unfamiliar data. Central to supervised learning are algorithms such as linear regression, logistic regression, and decision trees [20].
In contrast, unsupervised learning navigates the data space autonomously to uncover inherent patterns, structures, or relationships devoid of predefined outputs. Its focus is on discovering intrinsic data structures, thus offering insights that could potentially solve complex problems. Notable unsupervised learning algorithms include clustering techniques like k-means, hierarchical clustering, and dimensionality reduction methods such as principal component analysis (PCA) [21].
Artificial Neural Networks (ANNs) simulate the operational framework of the human brain, executing intricate tasks via a network of interconnected artificial neurons arranged in layers. The backpropagation algorithm, a vital cog in ANN operations, exhibits high fault tolerance, ensuring the system’s functionality despite occasional neuronal failures [22]. ANNs facilitate feature extraction and sophisticated pattern recognition, crucial for machine learning, which enhance data representation or class differentiation by aiding raw data pre-processing for feature extraction or selection [23].
Developments in ANNs have given rise to complex structures like deep learning models comprising multilayer neurons. Notably, CNNs employ convolution in lieu of standard matrix multiplication in certain layers. Tailored for processing pixel data, CNNs excel in tasks related to pattern recognition in images, audio, or text, substantially contributing to computer vision and Natural Language Processing (NLP) by simplifying complex patterns into abstract representations through layers of features [24].
3. Integrating AI into Medical Imaging: The Dawn of Radiology 2.0
This section delves into the remarkable role of AI within medical imaging, spotlighting the revolutionary shifts it catalyses in the world of radiology. Its multifaceted potential, from revolutionising image acquisition to reshaping radiological analyses, and from streamlining reporting to crafting personalised medical narratives, positions AI at the epicentre of the ongoing healthcare revolution. Beyond radiology, this transformation extends to other areas of healthcare—such as pathology, cardiology, genomics, drug discovery, and healthcare delivery—where the impactful strides of AI are being increasingly recognised. Concluding this exploration is the emergent paradigm of AI-facilitated personalised medicine, underscoring a more proactive, patient-oriented, and holistic patient care approach.
3.1. A Paradigm Shift in Radiology
AI has instigated a profound metamorphosis in the field of radiology, redefining traditional workflows and elevating the radiologist’s role (Figure 3). In the realm of image acquisition, AI augments scanning procedures, optimises image fidelity, and fosters sophisticated image reconstruction across MRI, CT, and PET modalities. Foremost among these advancements, deep learning accelerates MRI scanning, harmonising efficiency and quality, with commensurate progress witnessed in CT and PET image reconstruction [25].
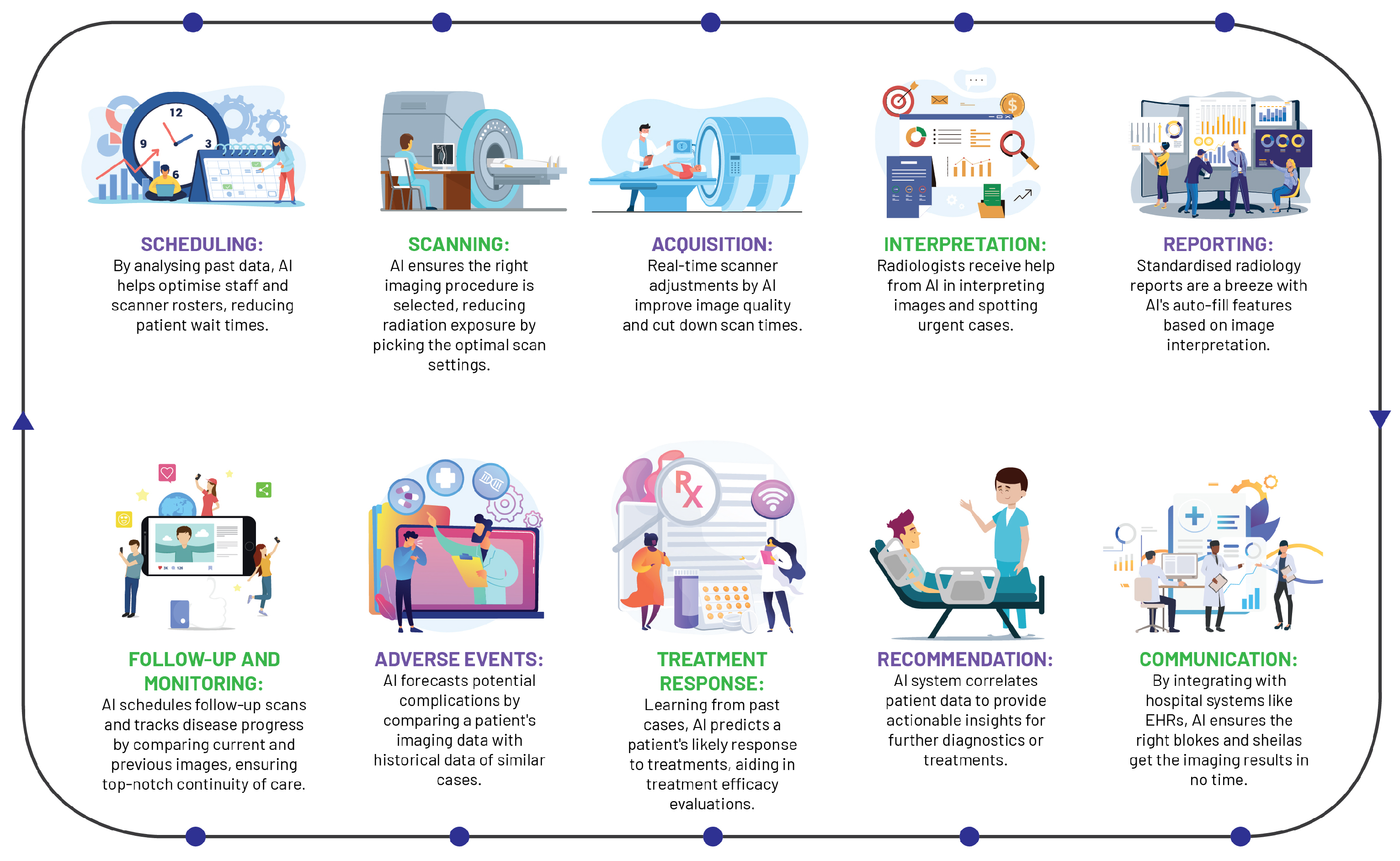
Figure 3. A streamlined workflow diagram illustrating the role of artificial intelligence in radiological practice.
AI significantly streamlines the acquisition of radiologist analyses on chest X-rays, as evidenced by a study wherein an AI system reduced interpretation delivery times from 11.2 days to a mere 2.7 days, reinforcing the potency of automated triaging systems in streamlining healthcare workflows and amplifying patient care standards [26].
As one of the pioneering healthcare specialties to adopt digital technology, radiology has capitalised on machine learning in CAD tools for over two decades, demonstrating robust performance in sensitivity and specificity [27]. Although clinical adoption has been slow due to various challenges, AI is posited as a pivotal tool to surmount these, enhancing CAD performance, streamlining radiology services, and fostering the development of integrated diagnostic services.
The integration of AI into radiology reporting has structured and annotated data to bolster report uniformity and streamlining patient history tracking. These cutting-edge tools generate comprehensive task lists, incorporating pertinent information from the patient’s history into EHRs, with the prime objective of enhancing report accessibility and integration into care pathways [28].
Beyond AI’s transformative influence on reporting and imaging procedures, these state-of-the-art systems play a pivotal role in maintaining continuity in provider communication and patient care, validating correlations between imaging diagnoses, radiological reports, and treatment plans, alerting providers to any discrepancies. Moreover, AI optimises personnel allocation and scanner usage and reduces radiation exposure, thereby boosting efficiency and quality of care [29]. By virtue of its broad-spectrum capabilities, AI is redefining the landscape of radiology, cementing its indispensable status within the discipline.
3.2. Beyond Radiology: Broader Applications of AI in Healthcare
AI has indelibly altered several healthcare areas, demonstrating its capability to enhance clinical practice beyond radiology via improvements in diagnostics, genomics, drug discovery, and healthcare delivery optimisation.
Pathology has witnessed the successful application of AI algorithms in tissue analysis, markedly enhancing diagnostic accuracy and speed. Automated image analysis tools enable pathologists to scrutinise tissues microscopically, identifying subtle histopathological attributes often overlooked by the human eye [30]. AI also accelerates the move towards digital pathology, converting traditional glass slides into digital scans for remote diagnostics and collaborative work—both of which are vital in the digital age of telemedicine [31].
AI shows considerable promise in cardiology, particularly in interpreting electrocardiograms and echocardiograms. Sophisticated ML algorithms detect complex cardiac patterns and abnormalities, accurately predicting conditions such as atrial fibrillation and myocardial infarction [32]. Its exponential growth in echocardiography is evident, with automated algorithms aiding in the interpretation of cardiac structure parameters, mitigating interobserver variability, and enhancing diagnostic precision [33].
The complex nature of genomics renders it an ideal candidate for AI intervention. DL techniques decipher genomic data, aiding in identifying genetic variants linked to disease susceptibility, and opening avenues for tailored treatment strategies based on individual genetic profiles [34].
AI has proven invaluable in the field of drug discovery by expediting the search for potent therapeutic compounds, and subsequently, accelerating the drug development process. For example, AI can predict the pharmacokinetic and pharmacodynamic properties of novel compounds, pinpoint potential drug targets, and simulate clinical trials, substantially reducing both time and costs associated with drug development [35].
Additionally, AI’s ability to optimise healthcare delivery is remarkable. AI-driven predictive analytics can enhance hospital workflows, accurately predicting patient admission rates, and optimising resource allocation [36]. AI applications in cost reduction have also emerged, with machine learning algorithms identifying inefficiencies in healthcare systems, thus enabling cost-effective care (SHAH 2021). The overarching aim of these AI applications is to improve patient outcomes by streamlining diagnostic processes and personalising treatment plans.
3.3. A New Era of Personalised Medicine
AI has catalysed a paradigm shift in the arena of personalised medicine. With its unparalleled capacity for processing vast amounts of complex data, AI is broadening the scope of personalised medicine, venturing into previously uncharted territories.
One of the most significant contributions of AI to personalised medicine is its potential to unlock the treasure trove of information embedded in EHRs. By harnessing the power of advanced machine learning algorithms, AI can discern patterns within EHRs, which can provide critical insights into specific disease states or risk factors. This process enables a proactive approach to patient care, allowing healthcare providers to foresee potential health risks and intervene accordingly [4]. In a landmark study by Rajkomar et al. (2018), AI was successfully utilised to predict medical events using data from EHRs, underlining its instrumental role in preventative medicine [37].
By integrating various sources of patient data—ranging from medical imaging and EHRs to genomic data—AI enables a more holistic approach to healthcare. This unification allows for personalised treatment plans, precise disease risk prediction, and improved monitoring of treatment responses. AI systems also enhance clinical decision-making by harmonising patient-specific data with up-to-date scientific discoveries, generating clinically relevant recommendations [38]. However, it is vital to ensure that AI’s implementation is patient-centric and ethical considerations such as data privacy, security, and algorithmic bias are rigorously adhered to. This vigilance guarantees the equitable application of AI technologies, respecting the rights and needs of all patients [4].
In conclusion, AI is shaping a new era of truly personalised patient care. However, it is paramount to acknowledge the necessity for continuous research and meticulous validation to ensure the safe and effective deployment of AI in healthcare. Multidisciplinary collaborations, involving specialists from AI, radiology, genomics, and clinical practice, are crucial for fine-tuning AI-driven models and technologies. Such partnerships will help maximise the potential benefits of AI, leading to enhanced patient outcomes and propelling the field of medicine into the future.
4. Practical Applications of AI in Radiology Practice
This section critically examines the practical applications of AI in the field of radiology, elaborating on the novel approaches it brings to imaging techniques, diagnosis, and patient care. By navigating through AI-driven methodologies like DL and CNNs, the section illuminates how AI is redefining the way image segmentation and classification, and diagnostics are conducted. Moreover, it explores the prognostic power of radiomics and predictive potential of AI in optimising workflows. Throughout the discourse, the section also confronts the inherent challenges and bottlenecks in the integration of AI within radiology, underpinning the critical need for interpretability, validation, standardisation, and the need to preserve the human element in healthcare.
4.1. Image Segmentation and Classification
DL has ignited a significant shift within radiology that is particularly noticeable in the domains of image segmentation and classification, where substantial strides have been made. The advancements brought about by these AI-centric methods have amplified the precision and speed of diagnosis, thereby amplifying the competency of radiologists and raising the bar of patient care. Nevertheless, the assimilation of AI brings about numerous challenges that need to be overcome to facilitate its optimal integration and application within radiology.
CNNs, given their inherent ability to learn complex patterns through backpropagation, have emerged as formidable tools for computational visual tasks, including various radiological applications. Their distinct architectural layers, from calculations in convolutional layers to the generation of predictions in fully connected layers, all converge to form a proficient object detection system. These networks have demonstrated remarkable proficiency in object detection tasks, thanks to their integrated capabilities of feature extraction, semantic segmentation, and the handling of multi-scale features. The efficiency and effectiveness of CNNs can be further amplified through the application of transfer learning, which allows for the reuse of pre-existing models. This, in turn, enhances accuracy, facilitates efficient training even with limited datasets, and minimises the need for labour-intensive and error-prone manual segmentation [24][39].
Illustrating CNNs’ potential, the segmentation of lung nodules from CT scans using AI has shown superior performance in the early detection and treatment of lung cancer, achieving an area under the receiver operating characteristic curve (AUROC) of 94.4% and outperforming six radiologists in the task [40]. Similarly, CNNs have played a pivotal role in the segmentation of brain tumours from MRI scans and the analysis of retinal images for early symptoms of diabetic retinopathy, further underlining AI’s broad applicability and versatility of AI in the sphere of medical imaging [24][39].
Image classification, an equally critical application of AI in radiology, leverages CNNs to differentiate between normal and pathological findings. AI models have been engineered to distinguish between benign and malignant tumours in mammography, achieving performance metrics comparable to human radiologists, thus facilitating early detection of breast cancer [41].
Notwithstanding these significant strides, integrating AI within radiology invites a suite of challenges that need to be addressed. The construction of robust and reliable AI models necessitates access to extensive datasets and thorough validation, both of which can be challenging due to privacy concerns and the heterogeneous nature of medical imaging data. Second, ensuring the interpretability and explicability of the “black-box” dilemma in deep learning models is paramount to garner trust and adoption from both radiologists and patients. Lastly, integrating AI into existing clinical workflows and fostering a harmonious human-AI collaboration are essential to fully realise the potential of AI in radiology and translate these technological advances into tangible enhancements in patient care.
4.2. Advancing Diagnostics with AI and CAD Systems
The third wave of AI, notably the integration of deep learning technology into CAD systems, has propelled radiology into a digital era. The emergence of AI-integrated CAD (AI-CAD) systems has revolutionised radiology services, underpinning transformative changes in the landscape of diagnostics. These systems have redefined how radiologists interpret images, increasing diagnostic accuracy, reducing false positives, and significantly improving workflow efficiencies.
AI-CAD systems’ merit lies in their substantial reduction of false positives, enhancing dependability in clinical settings. This is corroborated by a study comparing AI-CAD and traditional CAD software, where the AI system outperformed by decreasing the false-positive marks per image (FPPI) by a significant 69%. It specifically excelled in identifying microcalcifications and masses, reducing false positives by 83% and 56% respectively. Such reductions optimise radiologists’ efficiency, potentially cutting their case reading time by an estimated 17%, and mitigate socioeconomic issues like patients’ unnecessary emotional stress and financial burden [42].
Employing ensemble learning methodologies can hone CAD systems further, as evidenced by a recent study deploying a calibrated ensemble of deep learners for the task of detecting abnormalities in musculoskeletal radiographs. This ensemble model excelled over individual models, even outperforming expert radiologists in three out of the seven upper extremity anatomical regions (with an AUC of 0.93, Accuracy of 0.87, and Precision of 0.93). These results lend compelling support to the utility of the calibrated ensemble approach in identifying abnormalities in musculoskeletal X-rays [43].
AI has also shown remarkable promise in equalling, and in some cases surpassing, the performance of radiologists in breast screening through the deployment of AI algorithms for automated patient triage and predicting treatment outcomes—tasks that extend beyond human capabilities [44]. However, to fully exploit the benefits of AI in routine breast imaging fully, there’s a need to ensure sufficient data availability for testing and monitoring AI algorithms pre and post-integration into healthcare systems [44]. Yet, the dawn of a new AI epoch has presented a promising avenue that is anticipated to spur the next major transformation in radiology: the growth of radiomics—a field seeking to unify data from radiology, pathology, and genomics to offer a comprehensive diagnostic service [45].
4.3. Prognostics with Radiomics and Predictive Analytics
Radiomics is an emerging field within medicine, predicated on the extraction of high-dimensional data from radiological images and harbours immense potential for the landscape of medical diagnostics, prognosis, and the evaluation of disease response to treatment. However, radiomics still faces challenges, including the need for standardisation and validation to ensure reliable and reproducible outcomes. The main strength of radiomics lies in its ability to complement traditional clinical practice with precise, quantitative information, thereby revolutionising medical decision-making processes [46].
The rapid exponential growth of medical imaging data has fostered a conducive environment for the application of ML and data-driven science. Radiomics-based decision-support systems for precision diagnosis and treatment are poised to become integral tools in the armamentarium of modern medicine. This, however, is not to say that the journey of radiomics towards full clinical applicability is without challenges. The field currently grapples with a lack of standardisation and validation that are quintessential for ensuring reliable and reproducible outcomes [46][47].
In this milieu, the advent of AI presents promising avenues for overcoming these challenges and unlocking the full potential of radiomics. Through modelling high-dimensional data, AI-driven analytics enable accurate predictions pertaining to disease progression, treatment response, or patient survival. This significant stride offers clinicians an unprecedented wealth of information that vastly transcends the limitations of human perception. Particularly within oncology, radiomics has proven instrumental in identifying molecular phenotypes and lymph node metastases, appraising treatment response, and prognosticating disease survival [46].
It is crucial to acknowledge that the amalgamation of AI into radiomics remains in its infancy. To fully harness its potential in medical imaging, a concerted effort towards research and development is crucial. A key facet of this development will be the facilitation of large-scale data sharing, the establishment of standardised data collection protocols, clear evaluation criteria, and robust reporting guidelines. These elements are fundamental to the maturation and widespread adoption of radiomics as a discipline, opening the door to a new era of precision medicine.
4.4. Workflow Optimisation Using AI
AI is gaining traction in radiology, aiming to optimise workflows and enhance non-interpretative tasks’ efficacy. When coupled with NLP, AI can automate the triage of imaging studies, prioritising urgent cases based on the retrieval and analysis of key data from patients’ EHR. This expedites patient triage, radiology reporting, and managing incidental finding follow-ups [45].
AI significantly enhances the radiology process by automating triage and improving report generation. It swiftly sorts and prioritises radiological studies like CT scans and MRIs based on urgency, highlighting critical cases for immediate review. This automation aids in consistently detecting severe conditions such as stroke, haemorrhage, and malignancy, reducing errors. The use of AI, particularly NLP, in non-interpretative tasks alleviates tedious aspects of the workflow, potentially mitigating radiologist burnout [4].
Furthermore, AI enhances the generation and interpretation of radiology reports. Deep Learning algorithms address the limitations of traditional reporting, including fatigue-induced errors or inconsistencies due to varied expertise levels. They detect and characterise findings to improve consistency, facilitate standardised report creation, and reduce errors. This additional analysis layer streamlines the workflow and augments report clarity, significantly contributing to the quality of radiology services [48].
The incorporation of AI transcends the limits of purely diagnostic capabilities, profoundly amplifying interdisciplinary collaboration and patient-radiologist communication. AI platforms serve as a vital conduit that nurtures a collective understanding of imaging results among disparate healthcare professionals as they possess the potential to demystify the complexity of medical terminologies for patients. This transparency aids in establishing a strong rapport between the patient and the radiologist while also cultivating a higher degree of patient involvement in their personal health [46].
As strides are made towards AI integration in radiology, it’s crucial to recognise that as of 2021, only 30% of radiologists reported clinical AI use, with over 70% expressing reluctance to invest in AI. Many perceived AI as offering negligible benefits, hinting at the field of radiology being in the “trough of disillusionment” phase in the AI adoption process [49]. This disillusionment emanates from factors such as scepticism about AI performance and applicability, a perceived lack of necessity, inadequate workflows for efficient AI utilisation, and a dearth of scalable AI-supporting infrastructure.
To transition into the “slope of enlightenment”, the field must establish infrastructure that supports optimal AI functionality, involving the redefinition and disruption of existing systems such as image management and PACS for intelligent workflow orchestration [50]. Notwithstanding AI’s potential, the importance of preserving the human element in patient care cannot be overstated, underlining that while AI can augment the work of radiologists, it is not a substitute for the nuanced judgement and empathetic communication that are at the heart of patient care.
References
- Brady, A.P.; Bello, J.A.; Derchi, L.E.; Fuchsjäger, M.; Goergen, S.; Krestin, G.P.; Lee, E.J.Y.; Levin, D.C.; Pressacco, J.; Rao, V.M.; et al. Radiology in the era of value-based healthcare: A multi-society expert statement from the ACR, CAR, ESR, IS3R, RANZCR, and RSNA. Insights Imaging 2020, 11, 136.
- Giardino, A.; Gupta, S.; Olson, E.; Sepulveda, K.; Lenchik, L.; Ivanidze, J.; Rakow-Penner, R.; Patel, M.J.; Subramaniam, R.M.; Ganeshan, D. Role of Imaging in the Era of Precision Medicine. Acad. Radiol. 2017, 24, 639–649.
- Jameson, J.L.; Longo, D.L. Precision medicine–personalized, problematic, and promising. N. Engl. J. Med. 2015, 372, 2229–2234.
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510.
- European Society of Radiology (ESR); European Federation of Radiographer Societies (EFRS). Patient Safety in Medical Imaging: A joint paper of the European Society of Radiology (ESR) and the European Federation of Radiographer Societies (EFRS). Insights Imaging 2019, 10, 45.
- Dreyer, K.J.; Geis, J.R. When Machines Think: Radiology’s Next Frontier. Radiology 2017, 285, 713–718.
- Buchanan, B.; Shortliffe, E. Rule-Based Expert Systems: The MYCIN Experiments of the Stanford Heuristic Programming Project; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1984.
- Shortliffe, E. (Ed.) Computer-Based Medical Consultations: MYCIN, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1976.
- Quinlan, J. Induction of decision trees. Mach. Learn. 1986, 1, 81–106.
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297.
- Rumelhart, D.; Hinton, G.; Williams, R. Learning representations by back-propagating errors. Nature 1986, 323, 533–536.
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444.
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90.
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252.
- OpenAI. ChatGPT: Improving Language Understanding by Generative Pre-Training. 2022. Available online: https://openai.com/research/language-unsupervised (accessed on 19 May 2023).
- Brown, T.; Mann, B.; Ryder, N.; Subbiah, M.; Kaplan, J.; Dhariwal, P.; Neelakantan, A.; Shyam, P.; Sastry, G.; Askell, A.; et al. Language Models are Few-Shot Learners. arXiv 2020, arXiv:2005.14165.
- Pennachin, C.; Goertzel, B. (Eds.) Contemporary Approaches to Artificial General Intelligence. In Artificial General Intelligence; Cognitive Technologies; Springer: Berlin/Heidelberg, Germany, 2007.
- Mitchell, T.M. Machine Learning, 1st ed.; McGraw-Hill, Inc.: New York, NY, USA, 1997.
- Cheng, P.M.; Montagnon, E.; Yamashita, R.; Pan, I.; Cadrin-Chênevert, A.; Perdigón Romero, F.; Chartrand, G.; Kadoury, S.; Tang, A. Deep Learning: An Update for Radiologists. Radiographics 2021, 41, 1427–1445.
- Rashidi, H.H.; Tran, N.K.; Betts, E.V.; Howell, L.P.; Green, R. Artificial Intelligence and Machine Learning in Pathology: The Present Landscape of Supervised Methods. Acad. Pathol. 2019, 6, 2374289519873088.
- Glickenstein, D.; Hamm, K.; Huo, X.; Mei, Y.; Stoll, M. Editorial: Mathematical Fundamentals of Machine Learning. Front. Appl. Math. Stat. 2021, 7, 674785.
- Pires, P.B.; Santos, J.D.; Pereira, I.V. Artificial Neural Networks: History and State of the Art. In Encyclopedia of Information Science and Technology, 6th ed.; Khosrow-Pour, M., Ed.; IGI Global: Hershey, PA, USA, 2023.
- Ghojogh, B.; Samad, M.N.; Mashhadi, S.A.; Kapoor, T.; Ali, W.; Karray, F.; Crowley, M. Feature Selection and Feature Extraction in Pattern Analysis: A Literature Review. arXiv 2019, arXiv:1905.02845.
- Salehi, A.W.; Khan, S.; Gupta, G.; Alabduallah, B.I.; Almjally, A.; Alsolai, H.; Siddiqui, T.; Mellit, A. A Study of CNN and Transfer Learning in Medical Imaging: Advantages, Challenges, Future Scope. Sustainability 2023, 15, 5930.
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88.
- Annarumma, M.; Withey, S.J.; Bakewell, R.J.; Pesce, E.; Goh, V.; Montana, G. Automated Triaging of Adult Chest Radiographs with Deep Artificial Neural Networks. Radiology 2019, 291, 196–202.
- Tadavarthi, Y.; Makeeva, V.; Wagstaff, W.; Zhan, H.; Podlasek, A.; Bhatia, N.; Heilbrun, M.; Krupinski, E.; Safdar, N.; Banerjee, I.; et al. Overview of Noninterpretive Artificial Intelligence Models for Safety, Quality, Workflow, and Education Applications in Radiology Practice. Radiol. Artif. Intell. 2022, 4, e210114.
- Bizzo, B.C.; Almeida, R.R.; Alkasab, T.K. Artificial Intelligence Enabling Radiology Reporting. Radiol. Clin. N. Am. 2021, 59, 1045–1052.
- European Society of Radiology (ESR). What the radiologist should know about artificial intelligence—An ESR white paper. Insights Imaging 2019, 10, 44.
- Kumar, N.; Verma, R.; Sharma, S.; Bhargava, S.; Vahadane, A.; Sethi, A. A Dataset and a Technique for Generalized Nuclear Segmentation for Computational Pathology. IEEE Trans. Med. Imaging 2017, 36, 1550–1560.
- Komura, D.; Ishikawa, S. Machine Learning Methods for Histopathological Image Analysis. Comput. Struct. Biotechnol. J. 2018, 16, 34–42.
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867.
- Narula, S.; Shameer, K.; Salem Omar, A.M.; Dudley, J.T.; Sengupta, P.P. Machine-Learning Algorithms to Automate Morphological and Functional Assessments in 2D Echocardiography. J. Am. Coll. Cardiol. 2016, 68, 2287–2295.
- Zou, J.; Huss, M.; Abid, A.; Mohammadi, P.; Torkamani, A.; Telenti, A. A primer on deep learning in genomics. Nat. Genet. 2019, 51, 12–18.
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477.
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98.
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M.; et al. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 2018, 1, 18.
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; De Pristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29.
- Qiang, B.; Chen, R.; Zhou, M.; Pang, Y.; Zhai, Y.; Yang, M. Convolutional Neural Networks-Based Object Detection Algorithm by Jointing Semantic Segmentation for Images. Sensors 2020, 20, 5080.
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961.
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A.; Broeders, M.; Gennaro, G.; Clauser, P.; Helbich, T.H.; Chevalier, M.; Tan, T.; Mertelmeier, T.; et al. Stand-Alone Artificial Intelligence for Breast Cancer Detection in Mammography: Comparison With 101 Radiologists. J. Natl. Cancer Inst. 2019, 111, 916–922.
- Mayo, R.C.; Kent, D.; Sen, L.C.; Kapoor, M.; Leung, J.W.T.; Watanabe, A.T. Reduction of False-Positive Markings on Mammograms: A Retrospective Comparison Study Using an Artificial Intelligence-Based CAD. J. Digit. Imaging 2019, 32, 618–624.
- He, M.; Wang, X.; Zhao, Y. A calibrated deep learning ensemble for abnormality detection in musculoskeletal radiographs. Sci. Rep. 2021, 11, 9097.
- Hickman, S.E.; Baxter, G.C.; Gilbert, F.J. Adoption of artificial intelligence in breast imaging: Evaluation, ethical constraints and limitations. Br. J. Cancer 2021, 125, 15–22.
- Mun, S.K.; Wong, K.H.; Lo, S.B.; Li, Y.; Bayarsaikhan, S. Artificial Intelligence for the Future Radiology Diagnostic Service. Front. Mol. Biosci. 2021, 7, 614258.
- Pesapane, F.; Rotili, A.; Agazzi, G.M.; Botta, F.; Raimondi, S.; Penco, S.; Dominelli, V.; Cremonesi, M.; Jereczek-Fossa, B.A.; Carrafiello, G.; et al. Recent Radiomics Advancements in Breast Cancer: Lessons and Pitfalls for the Next Future. Curr. Oncol. 2021, 28, 2351–2372.
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762.
- Lakhani, P.; Sundaram, B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology 2017, 284, 574–582.
- Allen, B.; Agarwal, S.; Coombs, L.; Wald, C.; Dreyer, K. 2020 ACR Data Science Institute Artificial Intelligence Survey. J. Am. Coll. Radiol. 2021, 18, 1153–1159.
- Makeeva, V. An Essential Roadmap for AI in Radiology. Am. Coll. Radiol. 2022. Available online: https://www.acr.org/Practice-Management-Quality-Informatics/ACR-Bulletin/Articles/September-2022/An-Essential-Roadmap-for-AI-in-Radiology (accessed on 20 May 2023).
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
30 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





