Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hadi Pirasteh-Anosheh | -- | 2557 | 2023-08-29 14:25:08 | | | |
| 2 | Dean Liu | -1 word(s) | 2556 | 2023-08-30 02:42:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pirasteh-Anosheh, H. ROS and Antioxidants in Halophytes. Encyclopedia. Available online: https://encyclopedia.pub/entry/48600 (accessed on 16 January 2026).
Pirasteh-Anosheh H. ROS and Antioxidants in Halophytes. Encyclopedia. Available at: https://encyclopedia.pub/entry/48600. Accessed January 16, 2026.
Pirasteh-Anosheh, Hadi. "ROS and Antioxidants in Halophytes" Encyclopedia, https://encyclopedia.pub/entry/48600 (accessed January 16, 2026).
Pirasteh-Anosheh, H. (2023, August 29). ROS and Antioxidants in Halophytes. In Encyclopedia. https://encyclopedia.pub/entry/48600
Pirasteh-Anosheh, Hadi. "ROS and Antioxidants in Halophytes." Encyclopedia. Web. 29 August, 2023.
Copy Citation
Reactive oxygen species (ROS) are excited or partially reduced forms of atmospheric oxygen, which are continuously produced during aerobic metabolism like many physiochemical processes operating throughout seed life. Previously, it was believed that ROS are merely cytotoxic molecules, however, now it has been established that they perform numerous beneficial functions in plants including many critical roles in seed physiology. ROS facilitate seed germination via cell wall loosening, endosperm weakening, signaling, and decreasing abscisic acid (ABA) levels. Most of the existing knowledge about ROS homeostasis and functions is based on the seeds of common plants or model ones.
oxidative
ROS scavenging
salinity
tolerance
1. Introduction
In an ideal situation, the coordinated activity of various antioxidant enzymes and compounds assists plant cells in balancing their levels of ROS [1]. Nevertheless, when stressors are present, the amount of ROS produced surpasses the intrinsic antioxidant defense system’s capacity to eliminate them, leading to oxidative harm to various cell components such as proteins, membrane lipids, and nucleic acids [2]. These deleterious effects of ROS are often termed oxidative stress.
One of the most common plant responses to salinity and drought stress is to reduce stomatal conductivity, thus minimizing water loss [3]. This response limits the Calvin cycle’s access to CO2 for carbon stabilization; consequently, the absorbed light exceeds the amount required for normal photosynthesis [4]. Combined with the toxic impacts of sodium and chlorine accumulated in the cytosol of plants under salinity stress, this excess excitation light by depleting electron receptors (QA in PSII and NADP in PSI) affects electron transfer through photosystems and also increases ROS production (mainly O2•− and 1O2) by reducing O2 [5]. Limiting CO2 availability increases the rate of photorespiration in C3 plants, which in turn increases ROS production [6]. Therefore, salt-stressed plants use mechanisms to reduce ROS production that can maintain an acceptable level of net photosynthesis under CO2 limitation conditions and/or use alternative electron sinks that can prevent ROS formation from O2 [5].
Oxidative damage and the activity of antioxidant enzymes occur in halophyte plants like all plants. Halophytes are plants with very high tolerance to salinity stress that use different mechanisms for growth and survival in very saline environments. These plants grow in saline habitats in soils with a high salt concentration and with their special capabilities can replace ordinary plants in saline conditions [7]. Despite the differences in the definition of halophytes, in which plants are included, there are about 2500 to 3000 species of halophytes in the world [8].
It seems that halophyte species have a more noteworthy capacity than glycophytes to preserve net photosynthesis and to secure and stabilize both PS I and PS II beneath saline conditions [5][9]. Preserving net photosynthesis and protecting photosystems are the physiological mechanisms by which halophytes prevent the production of ROS in oxidative damage induced by salinity. Another interesting mechanism of halophytes to avoid oxidative damage is the switch from different modes of carbon assimilation; as some halophytes can change the carbon assimilation from C3 or C4 to Crassulacean acid metabolism (CAM) in high-salinity environments [10][11]. This switch is useful in reducing the production of salt-induced ROS in halophytes because CAM plants keep the stomata open at night and closed throughout the day to maintain photosynthesis during the day [5]. Although this mechanism is at first glance to prevent water loss during the day, however, by consuming the absorbed light in the current photosynthesis, it prevents the overproduction of ROS [12].
However, there is generally a dearth of knowledge about the production, scavenging, and roles of ROS in seeds and especially during the germination stage [13]. This information is even scarcer for the seeds of halophytes which naturally are salinity-tolerant plants of saline environments and hold immense potential to become non-conventional crops for arid saline lands in the future [2].
2. Halophytes: Importance, Classification, and Salt Tolerance Mechanisms
Halophytes have the ability to naturally inhabit man-made areas like salt pans, roadside verges, and salt marshes [14]. This makes them ideal for bioenergy production and saline agriculture because they do not compete with crops for arable land or freshwater resources [15][16]. However, researchers must be cautious about the impact of activities on wild halophyte populations as their preservation is at risk due to exploitation. To ensure their long-term survival it is crucial to develop management plans that prevent the gathering of these valuable halophytic species [9][14][16].
Many studies have shown that at high salinity, the amount of halophyte production is much higher than the economic yield of commercial crops and trees [3][17]. However, it should be noted that the growth of the halophytes may be decreased at very high salinities. Therefore, is an inverse relationship between intensifying salinity and the proper halophyte number/abundance of halophytes [17], so only a few halophytes, like some Salicornia species, can grow with the salinity of seawater [18].
In general, the reaction of plants to salinity depends on their tolerance to soil salinity. Therefore, plants are divided into four categories based on the amount of dry matter production under saline conditions [19][20]:
- (1)
-
Eu-halophytes: Growth of eu-halophytes is stimulated even in moderate salinities (such as Salicornia europaea and Suaeda maritima).
- (2)
-
Facultative halophytes: Growth of these halophytes is slightly stimulated at low salinity (such as Plantago maritima and Aster tripolium).
- (3)
-
Non-halophytes with low salinity tolerance: These plants are not halophytes and often include salt-tolerant crops and orchards. This group includes a wide range of economic plants such as barley (Hordeum vulgare), sorghum (Sorghum bicolor), cotton (Gossypium spp.), and pistachios (Pistacia vera).
- (4)
-
Halophobic: Plants that are sensitive to salinity and even at low salinity levels there is a significant reduction in their growth and yield, such as saffron (Crocus sativus), common bean (Phaseolus vulgaris), and most vegetables.
Halophytes are categorized according to the mechanism of salt extrusion [21]:
-
Recretohalophytes: Include halophytes that excrete salts on the outer surface (Exo-recretohalophytes) or the inside (Endo-recretohalophytes) of plant tissue.
-
Euhalophytes: Or true halophytes with succulent leaves or stems.
-
Pseudo-halophytes: Unreal halophytes that store salts in the parenchymal organs of the root.
By all categories, halophytes are plants with special capabilities that are good choices for the current global situation where severely limited freshwater resources, poor soil quality, and climate change have restricted the production of conventional plants [22]. Therefore, understanding the physiology of these species and elucidating their mechanisms for their high salinity tolerance is essential. Although good research has been carried out on the production of ROS, oxidative damage, and antioxidant enzymes in halophytes, more research is required to emphasize the role of ROS and antioxidants in the germination of halophytes.
Halophytes have various important mechanisms for salt tolerance, which work together to help them maintain ion balance and protect their cells from the effects of high salinity. The main important mechanisms of salt tolerance in halophytes include:
-
Some halophytes have evolved salt glands or bladders that actively excrete sodium ions from their tissues (excretion of sodium ions.) By removing these ions from their cells, they effectively regulate salt concentration and protect themselves against saline stress [24].
These mechanisms collectively allow halophytes to tolerate and thrive in environments with levels of salt. By minimizing sodium influx compartmentalizing ions within structures and actively excreting them when necessary, halophytes ensure ion homeostasis and safeguard their cells from the damaging effects of elevated salinity [25].
3. ROS and Antioxidants in Halophytes and Their Role in Salinity Tolerance
Plants primarily generate ROS in their chloroplasts during photosynthesis, resulting in the production of O2•, H2O2, and O21. Meanwhile, mitochondria produce O2• and H2O2 as a byproduct of respiration, and peroxisomes generate H2O2 during the process of photorespiration [1] as illustrated in Figure 1. Plant antioxidant defense systems include non-enzymatic and enzymatic systems involving compounds and enzymes that are distributed in different cellular compartments. The synergic action of both systems is responsible for the enhancement of the antioxidative response under salinity stress for many halophytes [26][27]. Enzymatic components of antioxidant defense include superoxide dismutases (SOD), catalases (CAT), peroxidases (POX) (glutathione peroxidase—GPX, ascorbate peroxidase—APX), and reductases (dehydroascorbate reductase—DHAR and monodehydroascorbate reductase—MDHAR). Certain antioxidant enzymes, including SOD and CAT, are speculated to have emerged as early as 3.6–4.1 billion years ago, prior to the great oxidation event that enabled organisms to cope with reactive oxygen species (ROS). These ROS emerged on Earth around 2.5 billion years ago, along with atmospheric oxygen [28]. The key non-enzymatic components are ascorbate, glutathione, tocopherol, phenolic, flavonoid, and carotenoid compounds [29][30].
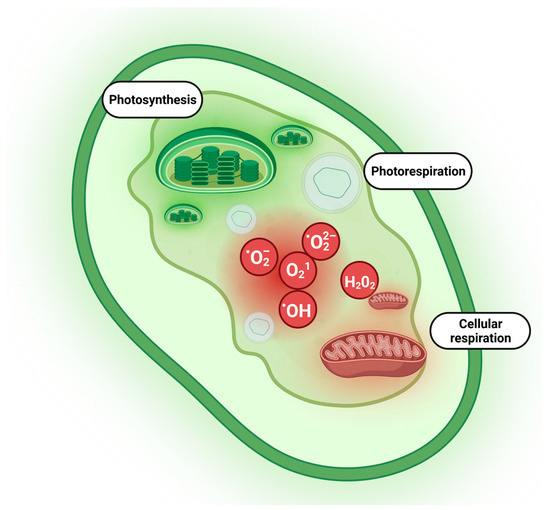
Figure 1. Major compartments of plant cells responsible for ROS generation. Photosynthesis, photorespiration, and cellular respiration as crucial processes responsible for ROS production were highlighted in the plant cell. ROS are constantly synthesized in chloroplasts, mitochondria, and peroxisomes as part of normal plant cell metabolism. The leak out in the electron transport chain in chloroplasts and mitochondria leads to the formation of superoxide (O2−) or hydrogen peroxide (H2O2). Oxidative metabolism in peroxisome produces H2O2, O2−, and singlet oxygen (1O2). Created with BioRender.com.
By transforming O2• into H2O2, superoxide dismutase acts as “the first line of defense against ROSs”. Three primary varieties of SOD, namely cytosolic Cu-Zn SOD, mitochondrial Mn-SOD, and chloroplastic Fe-SOD, have been identified in plants [31]. Both glycophytes and halophytes exhibit a positive association between SOD activity and tolerance to salinity [5]. Nevertheless, halophytes are recognized to have relatively higher levels of SOD activity than glycophytes. For instance, when exposed to salt, Cakile maritima as a halophyte exhibits greater SOD activity than Arabidopsis thaliana as a glycophyte [32]. Yildiztugay et al. [33] indicated high SOD activity under toxic salt concentrations for Salsola crassa. However, after 30 days of salinity exposure, the activity of antioxidant enzymes in S. crassa was decreased. Therefore, the fast and stronger enhancement in SOD activity in halophytes could play an important role in stress signaling in halophytes. For example, SOD activity for halophyte Tripolium pannonicum can be dependent on salinity level and also on organs [34]. However, in Salicornia europaea, both ROS production and SOD activity are not growing at high NaCl concentrations i.e., 800 mM NaCl [35].
Catalases are enzymes consisting of 4 haem-containing subunits that help convert H2O2 into oxygen and water (H2O), effectively detoxifying it [36]. The CAT performs a range of functions, such as participating in photorespiration, eliminating H2O2 during the β-oxidation of fatty acids in germinating seeds, and promoting stress tolerance. Multiple isoforms of CAT are frequently present in plants, primarily located in the mitochondria or peroxisomes [37]. Catalases play a less significant role than SOD as was demonstrated for obligatory halophyte S. europaea [35]. Many other antioxidant enzymes such as glutathione peroxidase (GPX), glutathione S-transferases (GST), thiol peroxidase type II peroxiredoxin (Prx), and guaiacol peroxidase (GPOX) have also been reported from plants and contribute toward ROS homeostasis [5].
Peroxidases (POX) are glycoproteins catalyzing the oxidation of substrates by degradation of H2O2 analogical to CAT activity. The increase in the activity of POX and SOD at 300 mM NaCl in S. europaea was activated by the peroxidation of lipid membranes [38]. The highest peak intensities of POX activity were observed for S. europaea compared with A. macrostachyum and S. fruticosa from the same ecological habitat indicating the importance of POD activity in salinity tolerance strategy [39]. Kumar et al. [40] documented also that APX and POX are one of the main strategies for halophytes to control ion fluxes under high salinity for ten halophytic species.
Dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR) are essential enzymes in the ascorbate-glutathione cycle, a key part of plant antioxidant defense mechanisms. DHAR and MDHAR regenerate strong antioxidant ascorbate utilizing dehydroascorbate (DHA) and monodehydroascorbate (MDHA) to maintain the pool of ascorbate in the cell [41]. The activity of both APX and MDHAR are enhanced in salt-stressed plants compared with unstressed plants. The addition of external ascorbic acid and tocopherol to plants affected by salt further heightened the activities of these enzymes compared with untreated salt-stressed controls [42]. DHAR and MDHAR activity was increased after long-term salinity stress for S. crassa. Additionally, the expression of these enzymes was found to increase 2–3 fold with increasing salinity in the halophytes Urochondra setulosa and Dichanthium annulatum [43].
To keep ROS levels within the tolerable range, plants also utilize low molecular weight non-enzymatic antioxidants such as ascorbate (AsA), glutathione (GSH), tocopherols, and flavonoids (Figure 2). Studies Anjum et al. [36] indicate a direct correlation between a plant’s tolerance to salinity and the levels of these antioxidants. AsA and GSH, two frequently occurring non-enzymatic antioxidants in plants, are present in all major compartments of plant cells, including the cytoplasm, apoplast, and chloroplast, with the ability to scavenge free radicals [44]. Their significance in enhancing salinity tolerance has been well documented. Ascorbate is a multifunctional cellular compound with an antioxidant and cofactor function. AsA is thought to play a role in mitigating the effects of salt stress by maintaining the osmotic balance in cells [45]. Sphaerophysa kotschyana as reported by Yildiztugay et al. [46] and Limonium stocksii as reported by Hameed et al. [47], both halophytic species, demonstrated an increase in AsA and GSH levels in response to salinity. Tocopherols, also known as vitamin E, are lipid-soluble compounds found in four distinct forms and are known to serve as an effective antioxidant defense for biological membranes [4].
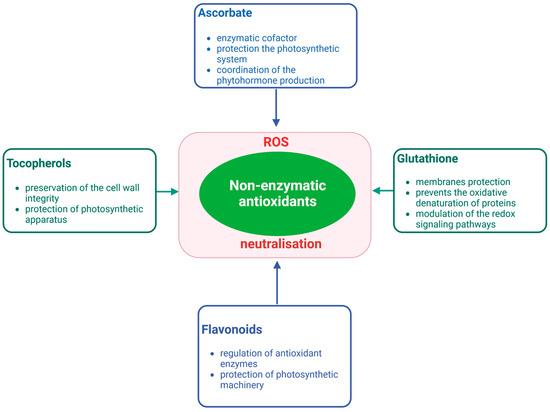
Figure 2. Essential non-enzymatic antioxidants and their multifunctional role. Ascorbate, glutathione, tocopherols, and flavonoids function as protection molecules against oxidative stress in the cell were marked. As non-enzymatic antioxidants cooperate to neutralize ROS and their negative impact on cells and by additional function (listed in the frame) mitigate abiotic stress. Created with BioRender.com.
The α-tocopherol, as a dominant form of vitamin E in the green tissues of plants, plays a crucial role in reducing the production of reactive oxygen species in the chloroplast under environmental stress conditions [48]. Ellouzi et al. [32] demonstrated an impressive antioxidant capacity of Cakile maritima correlated with the retention of a significant amount of α-tocopherol [38]. However, the level of α-tocopherol did not change significantly under salt stress, which implies that Crithmum maritima might neutralize the ROS production through direct quenching. The antioxidant role of phenolic compounds, including the subgroup flavonoids is particularly important in halophytes exposed to high salt conditions generating oxidative stress. The high content of flavonoids was noticed for Salicornia europaea, Crithmum maritimum L., Mesembryanthemum edule, and Juncus acutus [49].
The collaborative efforts of enzymatic and non-enzymatic antioxidants help maintain the levels of various ROS within a critical range necessary for regulating a variety of plant processes [5]. For example, ROSs are involved in the regulation of seed germination and dormancy [4], growth and development [50], stress acclimation, and programmed cell death [50]. However, these benefits are strictly dose-dependent. During periods of environmental stress, the production of ROS surpasses the cell’s ability to eliminate them, leading to elevated levels of ROS in the cell. This, in turn, results in oxidative damage to various cellular components such as nucleic acids, membrane lipids, and proteins [5]. Therefore, an effective antioxidant system is crucial for plants to cope with salinity stress, maintaining a crucial balance between the production of harmful ROS and their removal protects plant cells from potential oxidative damage. Furthermore, in response to salinity stress, some plants may even boost the production or activity of specific antioxidants to establish their ability to effectively manage this challenging condition [15][30].
References
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19.
- Shabala, S. Signalling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007.
- Pirasteh-Anosheh, H.; Ranjbar, G.; Akram, N.A.; Ghafar, M.A.; Panico, A. Forage potential of several halophytic species grown on saline soil in arid environments. Environ. Res. 2023, 219, 114954.
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847.
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 241–1257.
- Saed-Moucheshi, A.; Pakniyat, H.; Pirasteh-Anosheh, H.; Azooz, M.M. Role of ROS as signaling molecules in plants. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 585–620.
- Öztürk, M.; Altay, V.; Güvensen, A. Sustainable Use of Halophytic Taxa as Food and Fodder: An Important Genetic Resource in Southwest Asia. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019.
- Guvensen, A.; Gork, G.; Ozturk, M. An overview of the halophytes in Turkey. In Sabkha Ecosystems; Khan, M.A., Böer, B., Kust, G.S., Barth, H.J., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 2006; Volume 42.
- Pirasteh-Anosheh, H.; Parvizi, H.; Parnian, A.; Esfahan, E.Z.; Ranjbar, G.; Bhardwaj, A.K. Relationship between soil salinity and alkalinity with Alhagi camelorum growth in hypersaline and hyperarid environments. J. Arid Environ. 2022, 206, 104830.
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845.
- Shah, W.H.; Saleem, S.; Mushtaq, N.U.; Rasool, A.; Tahir, I.; Rehman, R.U. C4 and CAM Plants with Better Resilience to Environmental Stresses. In Photosynthesis and Respiratory Cycles during Environmental Stress Response in Plants; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 163–191.
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; John Wiley & Sons: London, UK, 2016; pp. 24–40.
- Pehlivan, F.E. Free radicals and antioxidant system in seed biology. In Advances in Seed Biology; InTech: London, UK, 2017; pp. 167–175.
- Polo-Ávila, A.; Infante-Izquierdo, M.D.; Sánchez-Gullón, E.; Castillo, J.M.; Muñoz-Rodríguez, A.F. Population Dynamic of the Annual Halophyte Salicornia ramosissima in Salt Pans: Towards a Sustainable Exploitation of Its Wild Populations. Plants 2022, 11, 1676.
- Hedayati-Firoozabadi, A.; Kazemeini, S.A.; Pirasteh-Anosheh, H.; Ghadiri, H.; Pessarakli, M. Forage yield and quality as affected by salt stress in different ratios of Sorghum bicolor-Bassia indica intercropping. J. Plant Nutr. 2020, 43, 2579–2589.
- Fekete, R.; Bak, H.; Vincze, O.; Süveges, K.; Molnár, V.A. Road traffic and landscape characteristics predict the occurrence of native halophytes on roadside verges. Sci. Rep. 2022, 12, 1298.
- Ranjbar, G.; Pirasteh-Anosheh, H.; Banakar, M.H.; Miri, H.R. Review on halophytes researches in Iran: Explanation of Challenges and Offer Approaches. J. Plant Ecophysiol. 2018, 10, 117–129.
- Ranjbar, G.; Pirasteh-Anosheh, H.; Dehghanie, F.; Keshtkar, S.; Race, M. Feasibility of growing Salicornia species in a coastal environment through planting date and density management in a direct seawater irrigation system. Environ. Sci. Pollut. Res. 2022, 29, 47800–47809.
- Prasad, M.N.V. Trace Elements as Contaminants and Nutrients: Consequences in Ecosystems and Human Health; John Wiley & Sons: Hoboken, NJ, USA, 2008.
- Kue Foka, I.C.; Ketehouli, T.; Zhou, Y.; Li, X.W.; Wang, F.W.; Li, H. The emerging roles of diacylglycerol kinase (DGK) in plant stress tolerance, growth, and development. Agronomy 2020, 10, 1375.
- Kefu, Z.; Hai, F.; Ungar, I.A. Survey of halophyte species in China. Plant Sci. 2002, 163, 491–498.
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2020, 72, 842–862.
- Mishra, A.; Tanna, B. Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front. Plant Sci. 2017, 8, 829.
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of Salt Tolerance in Halophytes: Current Understanding and Recent Advances. Open Life Sci. 2018, 13, 149–154.
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331.
- Hafsi, C.; Romero-Puertas, M.C.; Gupta, D.K.; del Río, L.A.; Sandalio, L.M.; Abdelly, C. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environ. Exp. Bot. 2010, 69, 129–136.
- Reginato, M.; Cenzano, A.M.; Arslan, I.; Furlan, A.; Varela, C.; Cavallin, V.; Papenbrock, J.; Luna, V. Na2SO4 and NaCl salts differentially modulate the antioxidant systems in the highly stress tolerant halophyte Prosopis strombulifera. Plant Physiol. Biochem. 2021, 167, 748–762.
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943.
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells. Apoptosis 2012, 17, 852–870.
- Pakar, N.; Pirasteh-Anosheh, H.; Emam, Y.; Pessarakli, M. Barley growth, yield, antioxidant enzymes, and ion accumulation affected by PGRs under salinity stress conditions. J. Plant Nutr. 2016, 39, 1372–1379.
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341.
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143.
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. The role of antioxidant responses on the tolerance range of extreme halophyte Salsola crassa grown under toxic salt concentrations. Ecotoxicol. Environ. Saf. 2014, 110, 21–30.
- Ludwiczak, A.; Ciarkowska, A.; Rajabi Dehnavi, A.; Cárdenas-Pérez, S.; Piernik, A. Growth Stage-, Organ- and Time-Dependent Salt Tolerance of Halophyte Tripolium pannonicum (Jacq.) Dobrocz. Life 2023, 13, 462.
- Cárdenas-Pérez, S.; Rajabi Dehnavi, A.; Leszczynski, K.; Lubinska-Mielinska, S.; Ludwiczak, A.; Piernik, A. Salicornia europaea L. Functional Traits Indicate Its Optimum Growth. Plants 2022, 11, 1051.
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Sofo, A. Catalase and ascorbate peroxidase representative H2O2 detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029.
- Shugaev, A.G.; Lashtabega, D.A.; Shugaeva, N.A.; Vyskrebentseva, E.I. Activities of antioxidant enzymes in mitochondria of growing and dormant sugar beet roots. Russ. J. Plant Physiol. 2011, 58, 387–393.
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195.
- Ghanem, A.-M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100.
- Kumar, A.; Mann, A.; Kumar, A.; Kumar, N.; Meena, B.L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Int. J. Phytoremediat. 2021, 23, 1041–1051.
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384.
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ros and no metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641.
- Mann, A.; Kumar, N.; Lata, C.; Kumar, A.; Meena, B.L.; Kumar, A. Physiological and differential gene expression reveals a trade-off between antioxidant capacity and salt tolerance in halophytes Urochondra setulosa and Dichanthium annulatum. Plant Growth Regul. 2023. preprint.
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613.
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173.
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. Sphaerophysa kotschyana, an endemic species from Central Anatolia: Antioxidant system responses under salt stress. J. Plant Res. 2013, 126, 729–742.
- Hameed, A.; Rasheed, A.; Gul, B.; Khan, M.A. Salinity inhibits seed germination of perennial halophytes Limonium stocksii and Suaeda fruticosa by reducing water uptake and ascorbate dependent antioxidant system. Environ. Exp. Bot. 2014, 107, 32–38.
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The ascorbate–glutathione–α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483.
- Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Magné, C.; Foggi, G.; Conte, G.; Santin, M.; et al. Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils. Horticulturae 2022, 8, 828.
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
932
Revisions:
2 times
(View History)
Update Date:
30 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




