
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovanni Cimmino | -- | 4855 | 2023-08-29 13:37:39 | | | |
| 2 | Rita Xu | Meta information modification | 4855 | 2023-08-30 03:52:34 | | | | |
| 3 | Rita Xu | Meta information modification | 4855 | 2023-08-30 03:57:50 | | |
Video Upload Options
Cardiovascular diseases (CVDs), such as arterial hypertension, myocardial infarction, stroke, heart failure, atrial fibrillation, etc., still represent the main cause of morbidity and mortality worldwide. They significantly modify the patients’ quality of life with a tremendous economic impact. It is well established that cardiovascular risk factors increase the probability of fatal and non-fatal cardiac events. These risk factors are classified into modifiable (smoking, arterial hypertension, hypercholesterolemia, low HDL cholesterol, diabetes, excessive alcohol consumption, high-fat and high-calorie diet, reduced physical activity) and non-modifiable (sex, age, family history, of previous cardiovascular disease).
1. Introduction
2. Metabolic Risk Factors
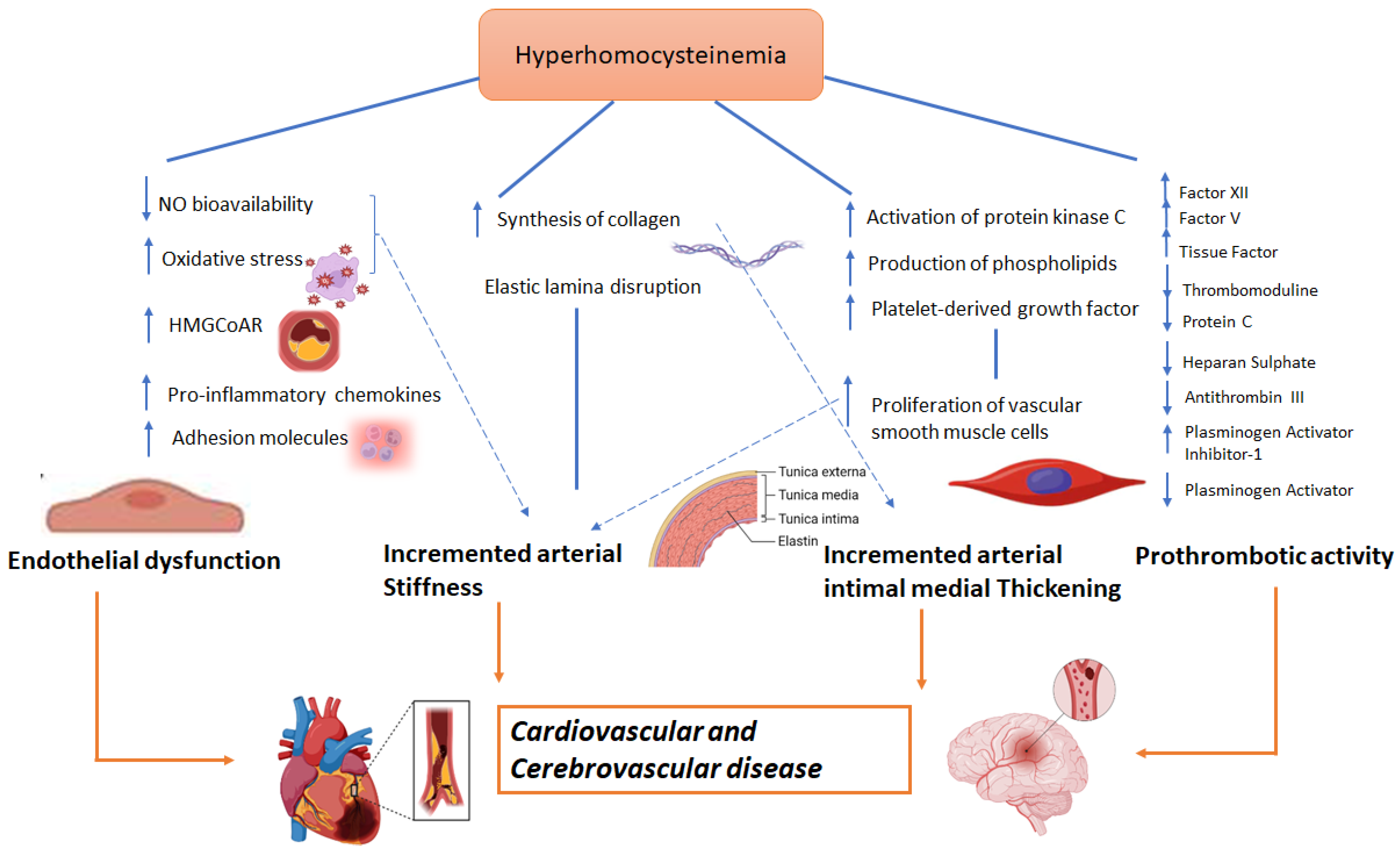
2.1. Homocysteine: The Never-Ending Debate in Cardiovascular Prevention
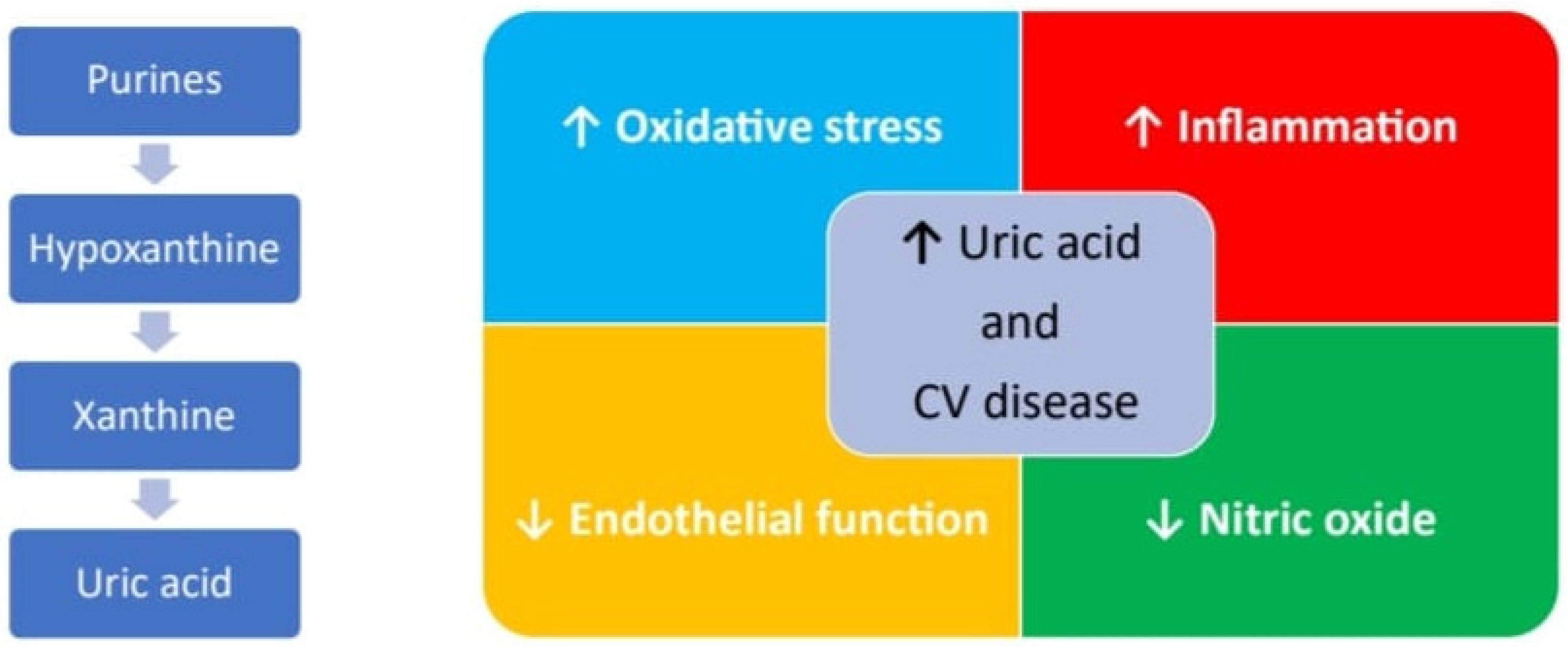
2.2. Uric Acid: Still a Controversial Cardiovascular Risk Factor?
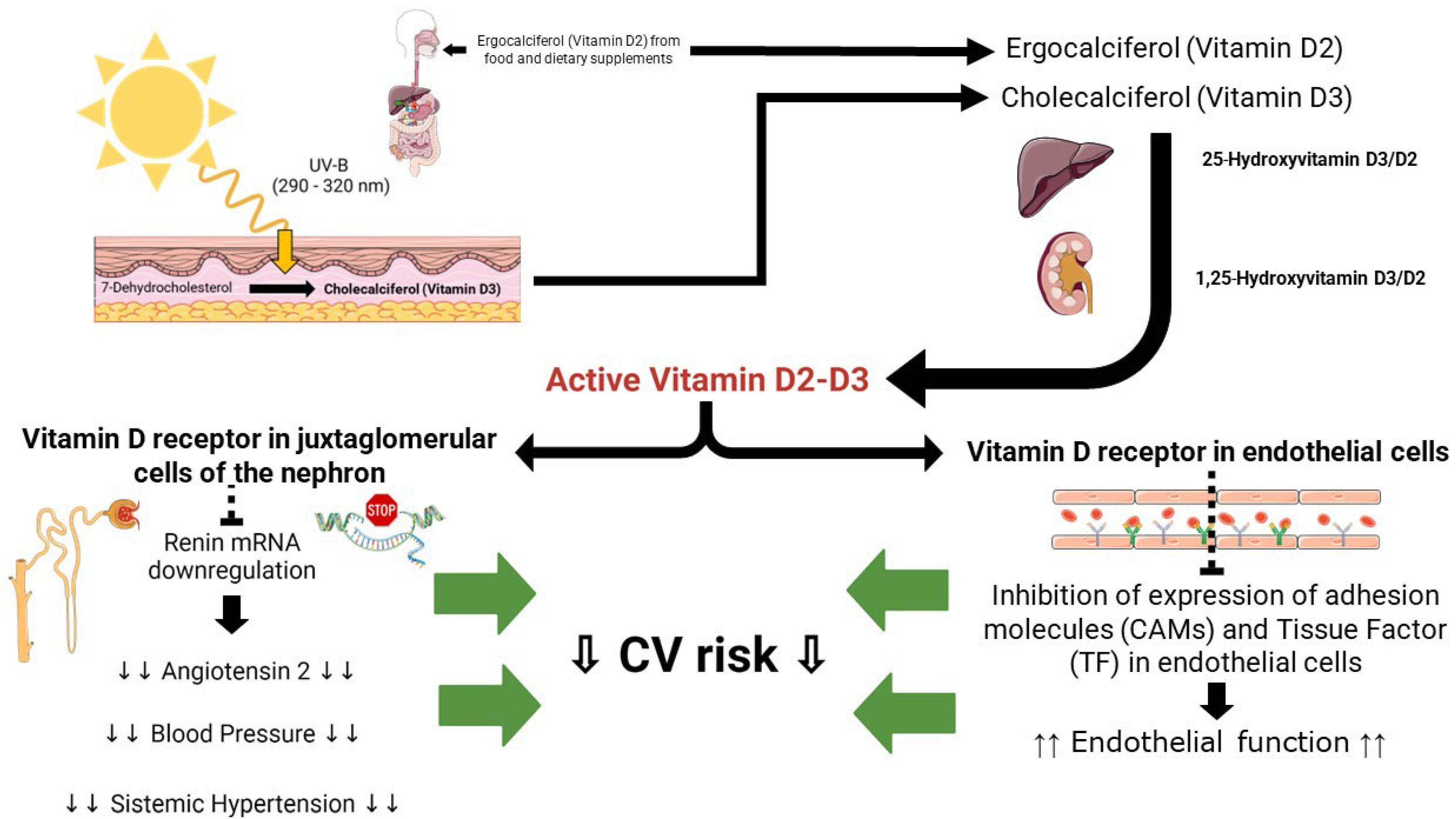
2.3. Vitamin D: Light and Shadow in Cardiovascular Prevention
3. Non-Metabolic Risk Factors and Surrogates
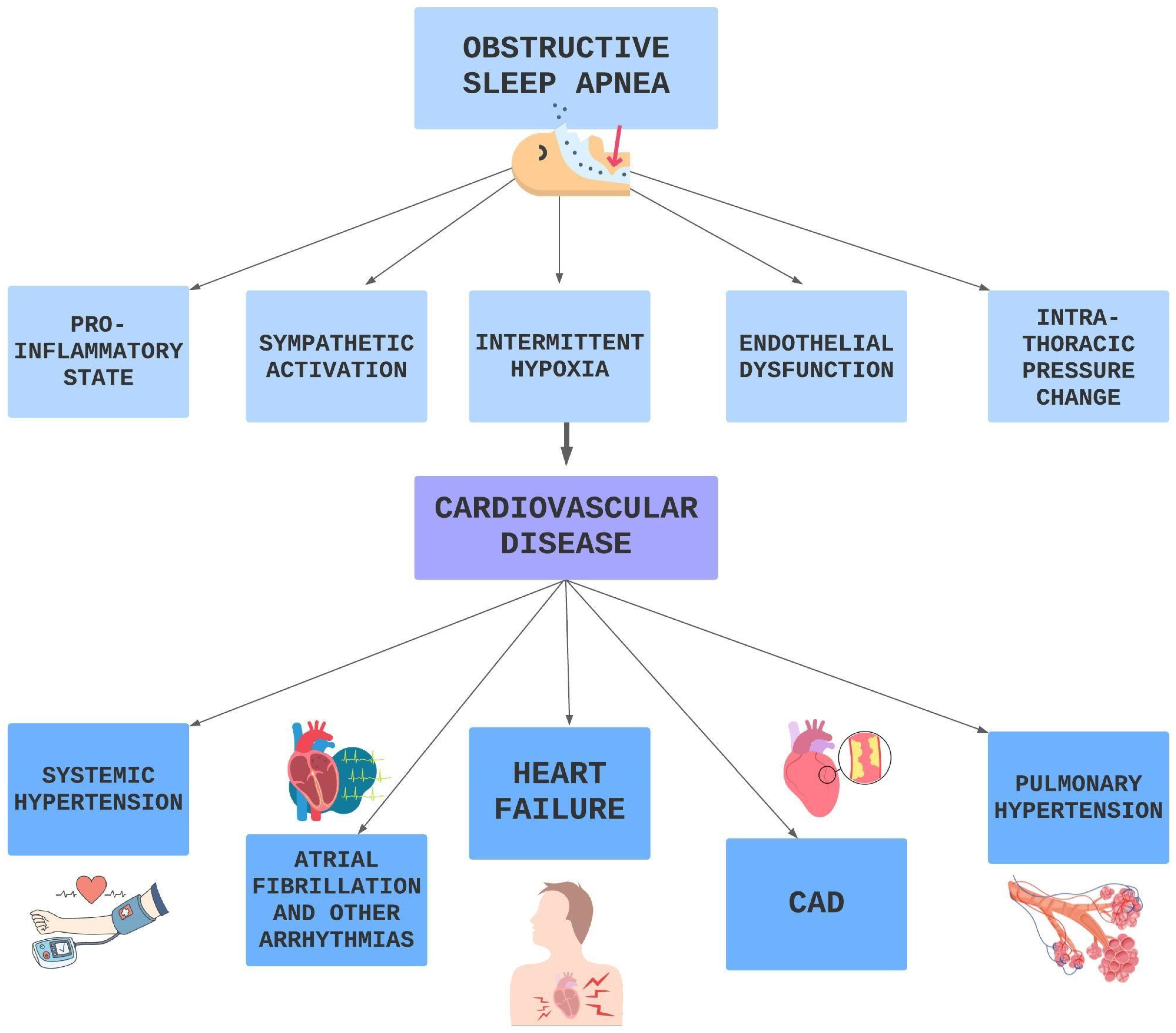
3.1. Obstructive Sleep Apnea Syndrome: The Diving Board to CVDs
3.2. Air Pollution: Health Breath as Part of Prevention
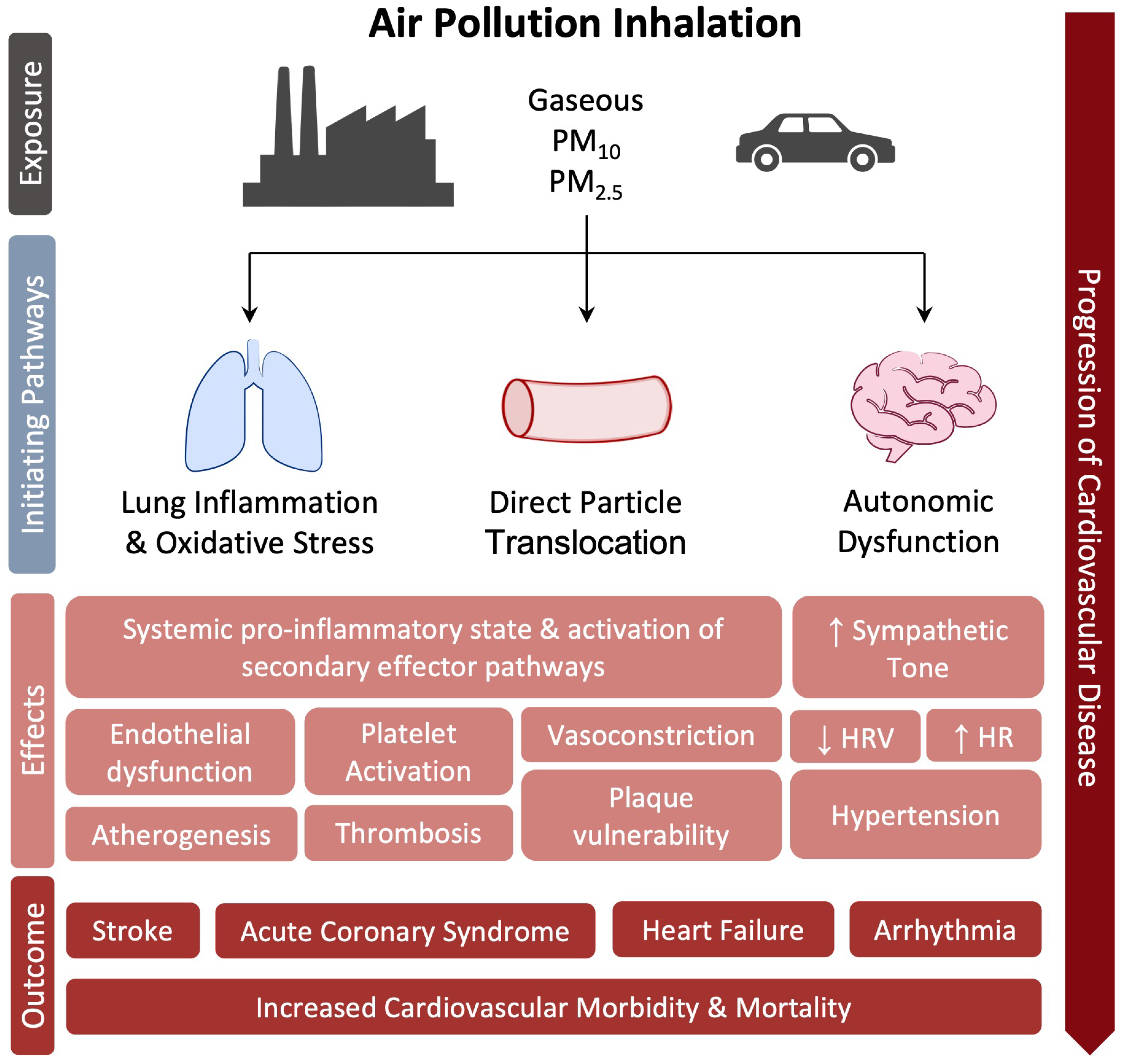
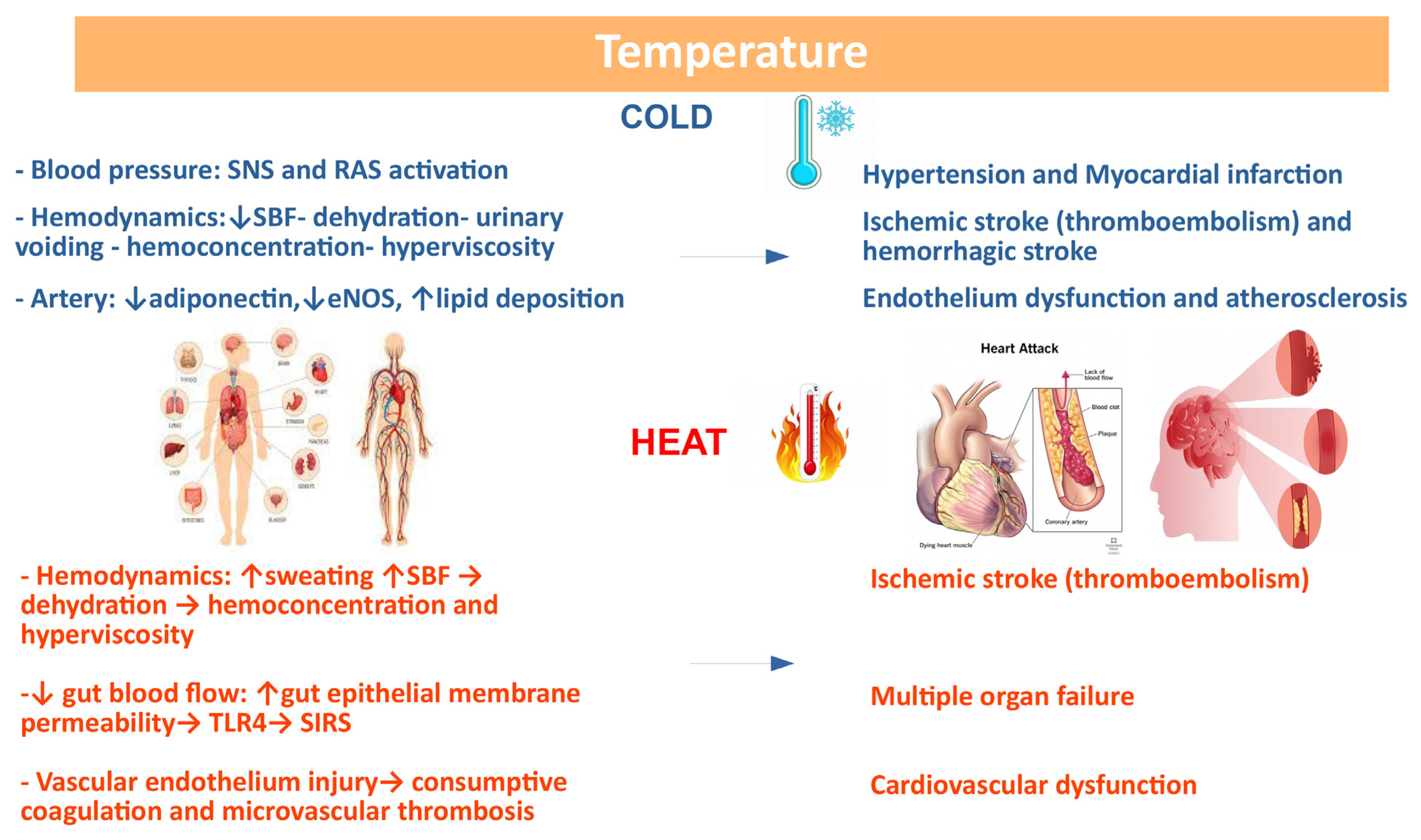
3.3. Climate Change: The Impact of Temperature
References
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008.
- Dzau, V.J.; Antman, E.M.; Black, H.R.; Hayes, D.L.; Manson, J.E.; Plutzky, J.; Popma, J.J.; Stevenson, W. The Cardiovascular Disease Continuum Validated: Clinical Evidence of Improved Patient Outcomes. Circulation 2006, 114, 2850–2870.
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337.
- Townsend, N.; Kazakiewicz, D.; Wright, F.L.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143.
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596.
- Hackam, D.G. The Changing Epidemiology of Cardiovascular Disease: Two Steps Forward, One Step Back. Can. J. Cardiol. 2020, 36, 995–996.
- Noale, M.; Limongi, F.; Maggi, S. Epidemiology of Cardiovascular Diseases in the Elderly. Frailty Cardiovasc. Dis. Res. Elder. Popul. 2020, 1216, 29–38.
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425.
- Lacey, B.; Herrington, W.G.; Preiss, D.; Lewington, S.; Armitage, J. The Role of Emerging Risk Factors in Cardiovascular Outcomes. Curr. Atheroscler. Rep. 2017, 19, 28.
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell Biol. 2000, 32, 385–389.
- Tchantchou, F. Homocysteine metabolism and various consequences of folate deficiency. J. Alzheimer’s Dis. JAD 2006, 9, 421–427.
- Finkelstein, J.D. The metabolism of homocysteine: Pathways and regulation. Eur. J. Pediatr. 1998, 157 (Suppl. S2), S40–S44.
- Kang, S.S.; Wong, P.W.; Malinow, M.R. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992, 12, 279–298.
- Ubbink, J.B.; Vermaak, W.J.; van der Merwe, A.; Becker, P.J. The effect of blood sample aging and food consumption on plasma total homocysteine levels. Clin. Chim. Act Int. J. Clin. Chem. 1992, 207, 119–128.
- Singal, R.; Ferdinand, L.; Das, P.M.; Reis, I.M.; Schlesselman, J.J. Polymorphisms in the methylenetetrahydrofolate reductase gene and prostate cancer risk. Int. J. Oncol. 2004, 25, 1465–1471.
- Sharma, P.; Senthilkumar, R.D.; Brahmachari, V.; Sundaramoorthy, E.; Mahajan, A.; Sharma, A.; Sengupta, S. Mining literature for a comprehensive pathway analysis: A case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis. 2006, 5, 1.
- Summers, C.M.; Hammons, A.L.; Mitchell, L.E.; Woodside, J.V.; Yarnell, J.W.; Young, I.S.; Evans, A.; Whitehead, A.S. Influence of the cystathionine beta-synthase 844ins68 and methylenetetrahydrofolate reductase 677C>T polymorphisms on folate and homocysteine concentrations. Eur. J. Hum. Genet. EJHG 2008, 16, 1010–1013.
- Siri, P.W.; Verhoef, P.; Kok, F.J. Vitamins B6, B12, and folate: Association with plasma total homocysteine and risk of coronary atherosclerosis. J. Am. Coll. Nutr. 1998, 17, 435–441.
- Refsum, H.; Nurk, E.; Smith, A.D.; Ueland, P.M.; Gjesdal, C.G.; Bjelland, I.; Tverdal, A.; Tell, G.S.; Nygard, O.; Vollset, S.E. The Hordaland Homocysteine Study: A community-based study of homocysteine, its determinants, and associations with disease. J. Nutr. 2006, 136, 1731S–1740S.
- Bostom, A.G.; Lathrop, L. Hyperhomocysteinemia in end-stage renal disease: Prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997, 52, 10–20.
- Sengul, E.; Cetinarslan, B.; Tarkun, I.; Canturk, Z.; Turemen, E. Homocysteine concentrations in subclinical hypothyroidism. Endocr. Res. 2004, 30, 351–359.
- Papa, A.; De Stefano, V.; Danese, S.; Chiusolo, P.; Persichilli, S.; Casorelli, I.; Zappacosta, B.; Giardina, B.; Gasbarrini, A.; Leone, G.; et al. Hyperhomocysteinemia and prevalence of polymorphisms of homocysteine metabolism-related enzymes in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2001, 96, 2677–2682.
- Desouza, C.; Keebler, M.; McNamara, D.B.; Fonseca, V. Drugs affecting homocysteine metabolism: Impact on cardiovascular risk. Drugs 2002, 62, 605–616.
- Cybulska, B.; Kłosiewicz-Latoszek, L. Homocysteine—Is it still an important risk factor for cardiovascular disease? Kardiol. Pol. 2015, 73, 1092–1096.
- Tripathi, P. Homocysteine- The Hidden Factor and Cardiovascular Disease: Cause or Effect? Biochem. Anal. Biochem. 2015, 4, 1000237.
- Brattstrom, L.; Wilcken, D.E. Homocysteine and cardiovascular disease: Cause or effect? Am. J. Clin. Nutr. 2000, 72, 315–323.
- Wilcken, D.E.; Wilcken, B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J. Clin. Investig. 1976, 57, 1079–1082.
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212.
- Drewes, Y.M.; Poortvliet, R.K.; Blom, J.W.; de Ruijter, W.; Westendorp, R.G.; Stott, D.J.; Blom, H.J.; Ford, I.; Sattar, N.; Wouter Jukema, J.; et al. Homocysteine levels and treatment effect in the PROspective Study of Pravastatin in the Elderly at Risk. J. Am. Geriatr. Soc. 2014, 62, 213–221.
- Boushey, C.J.; Beresford, S.A.; Omenn, G.S.; Motulsky, A.G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995, 274, 1049–1057.
- Nygard, O.; Nordrehaug, J.E.; Refsum, H.; Ueland, P.M.; Farstad, M.; Vollset, S.E. Plasma homocysteine levels and mortality in patients with coronary artery disease. N. Engl. J. Med. 1997, 337, 230–236.
- Homocysteine Studies, C. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022.
- Peng, H.Y.; Man, C.F.; Xu, J.; Fan, Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: A meta-analysis of prospective studies. J. Zhejiang Univ. Sci. B 2015, 16, 78–86.
- den Heijer, M.; Koster, T.; Blom, H.J.; Bos, G.M.; Briet, E.; Reitsma, P.H.; Vandenbroucke, J.P.; Rosendaal, F.R. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N. Engl. J. Med. 1996, 334, 759–762.
- Ray, J.G. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch. Intern. Med. 1998, 158, 2101–2106.
- Ospina-Romero, M.; Cannegieter, S.C.; den Heijer, M.; Doggen, C.J.M.; Rosendaal, F.R.; Lijfering, W.M. Hyperhomocysteinemia and Risk of First Venous Thrombosis: The Influence of (Unmeasured) Confounding Factors. Am. J. Epidemiol. 2018, 187, 1392–1400.
- Cheng, S.W.; Ting, A.C.; Wong, J. Fasting total plasma homocysteine and atherosclerotic peripheral vascular disease. Ann. Vasc. Surg. 1997, 11, 217–223.
- Vasan, R.S.; Beiser, A.; D’Agostino, R.B.; Levy, D.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; Wilson, P.W. Plasma homocysteine and risk for congestive heart failure in adults without prior myocardial infarction. JAMA 2003, 289, 1251–1257.
- Veeranna, V.; Zalawadiya, S.K.; Niraj, A.; Pradhan, J.; Ference, B.; Burack, R.C.; Jacob, S.; Afonso, L. Homocysteine and reclassification of cardiovascular disease risk. J. Am. Coll. Cardiol. 2011, 58, 1025–1033.
- Yuan, D.; Chu, J.; Lin, H.; Zhu, G.; Qian, J.; Yu, Y.; Yao, T.; Ping, F.; Chen, F.; Liu, X. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 1109445.
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844.
- Nedvetsky, P.I.; Sessa, W.C.; Schmidt, H.H. There’s NO binding like NOS binding: Protein-protein interactions in NO/cGMP signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 16510–16512.
- Poddar, R.; Sivasubramanian, N.; DiBello, P.M.; Robinson, K.; Jacobsen, D.W. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: Implications for vascular disease. Circulation 2001, 103, 2717–2723.
- Lei, W.; Long, Y.; Li, S.; Liu, Z.; Zhu, F.; Hou, F.F.; Nie, J. Homocysteine Induces Collagen I Expression by Downregulating Histone Methyltransferase G9a. PLoS ONE 2015, 10, e0130421.
- Tsai, J.C.; Perrella, M.A.; Yoshizumi, M.; Hsieh, C.M.; Haber, E.; Schlegel, R.; Lee, M.E. Promotion of vascular smooth muscle cell growth by homocysteine: A link to atherosclerosis. Proc. Natl. Acad. Sci. USA 1994, 91, 6369–6373.
- Dalton, M.L.; Gadson, P.F., Jr.; Wrenn, R.W.; Rosenquist, T.H. Homocysteine signal cascade: Production of phospholipids, activation of protein kinase C, and the induction of c-fos and c-myb in smooth muscle cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1997, 11, 703–711.
- Nishio, E.; Watanabe, Y. Homocysteine as a modulator of platelet-derived growth factor action in vascular smooth muscle cells: A possible role for hydrogen peroxide. Br. J. Pharmacol. 1997, 122, 269–274.
- Rolland, P.H.; Friggi, A.; Barlatier, A.; Piquet, P.; Latrille, V.; Faye, M.M.; Guillou, J.; Charpiot, P.; Bodard, H.; Ghiringhelli, O.; et al. Hyperhomocysteinemia-induced vascular damage in the minipig. Captopril-hydrochlorothiazide combination prevents elastic alterations. Circulation 1995, 91, 1161–1174.
- Malinow, M.R.; Nieto, F.J.; Szklo, M.; Chambless, L.E.; Bond, G. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation 1993, 87, 1107–1113.
- Voutilainen, S.; Alfthan, G.; Nyyssonen, K.; Salonen, R.; Salonen, J.T. Association between elevated plasma total homocysteine and increased common carotid artery wall thickness. Ann. Med. 1998, 30, 300–306.
- Arcaro, G.; Fava, C.; Dagradi, R.; Faccini, G.; Gaino, S.; Degan, M.; Lechi, C.; Lechi, A.; Minuz, P. Acute hyperhomocysteinemia induces a reduction in arterial distensibility and compliance. J. Hypertens. 2004, 22, 775–781.
- Upchurch, G.R., Jr.; Welch, G.N.; Fabian, A.J.; Freedman, J.E.; Johnson, J.L.; Keaney, J.F., Jr.; Loscalzo, J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J. Biol. Chem. 1997, 272, 17012–17017.
- Undas, A.; Brozek, J.; Szczeklik, A. Homocysteine and thrombosis: From basic science to clinical evidence. Thromb. Haemost. 2005, 94, 907–915.
- Coppola, A.; Davi, G.; De Stefano, V.; Mancini, F.P.; Cerbone, A.M.; Di Minno, G. Homocysteine, coagulation, platelet function, and thrombosis. Semin. Thromb. Hemost. 2000, 26, 243–254.
- Fryer, R.H.; Wilson, B.D.; Gubler, D.B.; Fitzgerald, L.A.; Rodgers, G.M. Homocysteine, a risk factor for premature vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler. Thromb. A J. Vasc. Biol. 1993, 13, 1327–1333.
- Lentz, S.R.; Sadler, J.E. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J. Clin. Investig. 1991, 88, 1906–1914.
- Rodgers, G.M.; Conn, M.T. Homocysteine, an atherogenic stimulus, reduces protein C activation by arterial and venous endothelial cells. Blood 1990, 75, 895–901.
- Nishinaga, M.; Ozawa, T.; Shimada, K. Homocysteine, a thrombogenic agent, suppresses anticoagulant heparan sulfate expression in cultured porcine aortic endothelial cells. J. Clin. Investig. 1993, 92, 1381–1386.
- Midorikawa, S.; Sanada, H.; Hashimoto, S.; Watanabe, T. Enhancement by homocysteine of plasminogen activator inhibitor-1 gene expression and secretion from vascular endothelial and smooth muscle cells. Biochem. Biophys. Res. Commun. 2000, 272, 182–185.
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14.
- Li, L.; Zhang, Y.; Zeng, C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167–3181.
- Tian, X.; Chen, S.; Zhang, Y.; Zhang, X.; Xu, Q.; Wang, P.; Wu, S.; Luo, Y.; Wang, A. Serum uric acid variation and the risk of cardiovascular disease: A prospective cohort study. Eur. J. Intern. Med. 2023, 112, 37–44.
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update from Molecular Mechanism to Clinical Perspective. Front. Pharmacol. 2020, 11, 582680.
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759–766.
- Zhang, W.; Iso, H.; Murakami, Y.; Miura, K.; Nagai, M.; Sugiyama, D.; Ueshima, H.; Okamura, T.; Epoch-Japan, G. Serum Uric Acid and Mortality Form Cardiovascular Disease: EPOCH-JAPAN Study. J. Atheroscler. Thromb. 2016, 23, 692–703.
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231.
- Kushiyama, A.; Okubo, H.; Sakoda, H.; Kikuchi, T.; Fujishiro, M.; Sato, H.; Kushiyama, S.; Iwashita, M.; Nishimura, F.; Fukushima, T.; et al. Xanthine oxidoreductase is involved in macrophage foam cell formation and atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 291–298.
- Cimmino, G.; Morello, A.; Conte, S.; Pellegrino, G.; Marra, L.; Golino, P.; Cirillo, P. Vitamin D inhibits Tissue Factor and CAMs expression in oxidized low-density lipoproteins-treated human endothelial cells by modulating NF-kappaB pathway. Eur. J. Pharmacol. 2020, 885, 173422.
- Cimmino, G.; Conte, S.; Marra, L.; Morello, A.; Morello, M.; De Rosa, G.; Pepe, M.; Sugralyev, A.; Golino, P.; Cirillo, P. Uric Acid induces a pro-atherothrombotic phenotype in human endothelial cells by imbalancing TF/TFPI pathway. Thromb. Haemost. 2022, 123, 64–75.
- Wang, M.; Lin, X.; Yang, X.; Yang, Y. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren. Fail. 2022, 44, 615–624.
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128.
- Cimmino, G.; Gallinoro, E.; di Serafino, L.; De Rosa, G.; Sugraliyev, A.; Golino, P.; Cirillo, P. Uric acid plasma levels are associated with C-reactive protein concentrations and the extent of coronary artery lesions in patients with acute coronary syndromes. Intern. Emerg. Med. 2023.
- Grayson, P.C.; Kim, S.Y.; LaValley, M.; Choi, H.K. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res. 2011, 63, 102–110.
- Tamariz, L.; Agarwal, S.; Soliman, E.Z.; Chamberlain, A.M.; Prineas, R.; Folsom, A.R.; Ambrose, M.; Alonso, A. Association of serum uric acid with incident atrial fibrillation (from the Atherosclerosis Risk in Communities study). Am. J. Cardiol. 2011, 108, 1272–1276.
- Maharani, N.; Kuwabara, M.; Hisatome, I. Hyperuricemia and Atrial Fibrillation. Int. Heart J. 2016, 57, 395–399.
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281.
- Macdonald, H.M.; Mavroeidi, A.; Fraser, W.D.; Darling, A.L.; Black, A.J.; Aucott, L.; O’Neill, F.; Hart, K.; Berry, J.L.; Lanham-New, S.A.; et al. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: A major cause for concern? Osteoporos. Int. 2011, 22, 2461–2472.
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28.
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch. Intern. Med. 2008, 168, 1629–1637.
- Kilkkinen, A.; Knekt, P.; Aro, A.; Rissanen, H.; Marniemi, J.; Heliovaara, M.; Impivaara, O.; Reunanen, A. Vitamin D status and the risk of cardiovascular disease death. Am. J. Epidemiol. 2009, 170, 1032–1039.
- Skaaby, T.; Thuesen, B.H.; Linneberg, A. Vitamin D, Cardiovascular Disease and Risk Factors. Adv. Exp. Med. Biol. 2017, 996, 221–230.
- Ginde, A.A.; Scragg, R.; Schwartz, R.S.; Camargo, C.A., Jr. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J. Am. Geriatr. Soc. 2009, 57, 1595–1603.
- Pilz, S.; Dobnig, H.; Nijpels, G.; Heine, R.J.; Stehouwer, C.D.; Snijder, M.B.; van Dam, R.M.; Dekker, J.M. Vitamin D and mortality in older men and women. Clin. Endocrinol. 2009, 71, 666–672.
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035.
- Rostand, S.G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension 1997, 30, 150–156.
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776.
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238.
- Cimmino, G.; Conte, S.; Morello, M.; Pellegrino, G.; Marra, L.; Morello, A.; Nicoletti, G.; De Rosa, G.; Golino, P.; Cirillo, P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? J. Cardiovasc. Dev. Dis. 2022, 9, 27.
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: A trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014, 2, 307–320.
- Avenell, A.; MacLennan, G.S.; Jenkinson, D.J.; McPherson, G.C.; McDonald, A.M.; Pant, P.R.; Grant, A.M.; Campbell, M.K.; Anderson, F.H.; Cooper, C.; et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J. Clin. Endocrinol. Metab. 2012, 97, 614–622.
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511.
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440.
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012, 8, 597–619.
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67.
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014.
- Johnson, K.G.; Johnson, D.C. Frequency of sleep apnea in stroke and TIA patients: A meta-analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2010, 6, 131–137.
- Worsnop, C.J.; Naughton, M.T.; Barter, C.E.; Morgan, T.O.; Anderson, A.I.; Pierce, R.J. The prevalence of obstructive sleep apnea in hypertensives. Am. J. Respir. Crit. Care Med. 1998, 157, 111–115.
- Fogel, R.B.; Malhotra, A.; White, D.P. Sleep. 2: Pathophysiology of obstructive sleep apnoea/hypopnoea syndrome. Thorax 2004, 59, 159–163.
- Somers, V.K.; Mark, A.L.; Zavala, D.C.; Abboud, F.M. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J. Appl. Physiol. 1989, 67, 2101–2106.
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904.
- Floras, J.S.; Bradley, T.D. Treating obstructive sleep apnea: Is there more to the story than 2 millimeters of mercury? Hypertension 2007, 50, 289–291.
- Hall, M.J.; Ando, S.; Floras, J.S.; Bradley, T.D. Magnitude and time course of hemodynamic responses to Mueller maneuvers in patients with congestive heart failure. J. Appl. Physiol. 1998, 85, 1476–1484.
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384.
- Suzuki, Y.J.; Jain, V.; Park, A.M.; Day, R.M. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic. Biol. Med. 2006, 40, 1683–1692.
- Ambrosio, G.; Tritto, I. Reperfusion injury: Experimental evidence and clinical implications. Am. Heart J. 1999, 138, S69–S75.
- Ambrosio, G.; Zweier, J.L.; Duilio, C.; Kuppusamy, P.; Santoro, G.; Elia, P.P.; Tritto, I.; Cirillo, P.; Condorelli, M.; Chiariello, M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993, 268, 18532–18541.
- Schulz, R.; Schmidt, D.; Blum, A.; Lopes-Ribeiro, X.; Lucke, C.; Mayer, K.; Olschewski, H.; Seeger, W.; Grimminger, F. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: Response to CPAP therapy. Thorax 2000, 55, 1046–1051.
- Schulz, R.; Seeger, W.; Grimminger, F. Serum nitrite/nitrate levels in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001, 164, 1997–1998.
- Logan, A.G.; Perlikowski, S.M.; Mente, A.; Tisler, A.; Tkacova, R.; Niroumand, M.; Leung, R.S.; Bradley, T.D. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J. Hypertens. 2001, 19, 2271–2277.
- Hoffstein, V.; Mateika, J. Evening-to-morning blood pressure variations in snoring patients with and without obstructive sleep apnea. Chest 1992, 101, 379–384.
- Leung, R.S.; Bradley, T.D. Sleep apnea and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2001, 164, 2147–2165.
- Loredo, J.S.; Ancoli-Israel, S.; Dimsdale, J.E. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am. J. Hypertens. 2001, 14, 887–892.
- Portaluppi, F.; Provini, F.; Cortelli, P.; Plazzi, G.; Bertozzi, N.; Manfredini, R.; Fersini, C.; Lugaresi, E. Undiagnosed sleep-disordered breathing among male nondippers with essential hypertension. J. Hypertens. 1997, 15, 1227–1233.
- Liu, L.; Cao, Q.; Guo, Z.; Dai, Q. Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnea and Resistant Hypertension: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Hypertens. 2016, 18, 153–158.
- Patel, N.; Donahue, C.; Shenoy, A.; Patel, A.; El-Sherif, N. Obstructive sleep apnea and arrhythmia: A systemic review. Int. J. Cardiol. 2017, 228, 967–970.
- Roche, F.; Xuong, A.N.; Court-Fortune, I.; Costes, F.; Pichot, V.; Duverney, D.; Vergnon, J.M.; Gaspoz, J.M.; Barthelemy, J.C. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin. Electrophysiol. PACE 2003, 26, 669–677.
- Shamsuzzaman, A.S.; Winnicki, M.; Lanfranchi, P.; Wolk, R.; Kara, T.; Accurso, V.; Somers, V.K. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002, 105, 2462–2464.
- Kuniyoshi, F.H.; Garcia-Touchard, A.; Gami, A.S.; Romero-Corral, A.; van der Walt, C.; Pusalavidyasagar, S.; Kara, T.; Caples, S.M.; Pressman, G.S.; Vasquez, E.C.; et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J. Am. Coll. Cardiol. 2008, 52, 343–346.
- Zhao, Q.; Liu, Z.H.; Zhao, Z.H.; Luo, Q.; McEvoy, R.D.; Zhang, H.L.; Wang, Y. Effects of obstructive sleep apnea and its treatment on cardiovascular risk in CAD patients. Respir. Med. 2011, 105, 1557–1564.
- Brown, D.L.; Shafie-Khorassani, F.; Kim, S.; Chervin, R.D.; Case, E.; Morgenstern, L.B.; Yadollahi, A.; Tower, S.; Lisabeth, L.D. Sleep-Disordered Breathing Is Associated with Recurrent Ischemic Stroke. Stroke 2019, 50, 571–576.
- Lisabeth, L.D.; Sanchez, B.N.; Lim, D.; Chervin, R.D.; Case, E.; Morgenstern, L.B.; Tower, S.; Brown, D.L. Sleep-disordered breathing and poststroke outcomes. Ann. Neurol. 2019, 86, 241–250.
- Kholdani, C.; Fares, W.H.; Mohsenin, V. Pulmonary hypertension in obstructive sleep apnea: Is it clinically significant? A critical analysis of the association and pathophysiology. Pulm. Circ. 2015, 5, 220–227.
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Topfer, V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 2007, 9, 251–257.
- Phillips, B.G.; Kato, M.; Narkiewicz, K.; Choe, I.; Somers, V.K. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H234–H237.
- Wolk, R.; Somers, V.K. Sleep and the metabolic syndrome. Exp. Physiol. 2007, 92, 67–78.
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Hopper, K.; Lotsikas, A.; Lin, H.M.; Kales, A.; Chrousos, G.P. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. J. Clin. Endocrinol. Metab. 2000, 85, 1151–1158.
- Perez Velasco, R.; Jarosinska, D. Update of the WHO global air quality guidelines: Systematic reviews—An introduction. Environ. Int. 2022, 170, 107556.
- Collaborators, G.B.D.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249.
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715.
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808.
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Diez Roux, A.V.; Gassett, A.J.; Jacobs, D.R., Jr.; et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016, 388, 696–704.
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378.
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93.
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070.
- Shah, A.S.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295.
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019, 155, 417–426.
- Stafoggia, M.; Cesaroni, G.; Peters, A.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; Cyrys, J.; de Faire, U.; de Hoogh, K.; et al. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: Results from 11 European cohorts within the ESCAPE project. Environ. Health Perspect. 2014, 122, 919–925.
- Baldauf, R.W.; Devlin, R.B.; Gehr, P.; Giannelli, R.; Hassett-Sipple, B.; Jung, H.; Martini, G.; McDonald, J.; Sacks, J.D.; Walker, K. Ultrafine Particle Metrics and Research Considerations: Review of the 2015 UFP Workshop. Int. J. Environ. Res. Public Health 2016, 13, 1054.
- Shukla, A.; Timblin, C.; BeruBe, K.; Gordon, T.; McKinney, W.; Driscoll, K.; Vacek, P.; Mossman, B.T. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am. J. Respir. Cell Mol. Biol. 2000, 23, 182–187.
- Roy, A.; Gong, J.; Thomas, D.C.; Zhang, J.; Kipen, H.M.; Rich, D.Q.; Zhu, T.; Huang, W.; Hu, M.; Wang, G.; et al. The cardiopulmonary effects of ambient air pollution and mechanistic pathways: A comparative hierarchical pathway analysis. PLoS ONE 2014, 9, e114913.
- Daiber, A.; Oelze, M.; Steven, S.; Kroller-Schon, S.; Munzel, T. Taking up the cudgels for the traditional reactive oxygen and nitrogen species detection assays and their use in the cardiovascular system. Redox Biol. 2017, 12, 35–49.
- Haberzettl, P.; Lee, J.; Duggineni, D.; McCracken, J.; Bolanowski, D.; O’Toole, T.E.; Bhatnagar, A.; Conklin, D.J. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ. Health Perspect. 2012, 120, 848–856.
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668.
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdorster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health Part A 2002, 65, 1513–1530.
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Lunts, A.; Kreyling, W.; Cox, C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health Part A 2002, 65, 1531–1543.
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552.
- Nemmar, A.; Hoet, P.H.; Dinsdale, D.; Vermylen, J.; Hoylaerts, M.F.; Nemery, B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation 2003, 107, 1202–1208.
- Jacobs, L.; Emmerechts, J.; Mathieu, C.; Hoylaerts, M.F.; Fierens, F.; Hoet, P.H.; Nemery, B.; Nawrot, T.S. Air pollution related prothrombotic changes in persons with diabetes. Environ. Health Perspect. 2010, 118, 191–196.
- Ying, Z.; Xu, X.; Bai, Y.; Zhong, J.; Chen, M.; Liang, Y.; Zhao, J.; Liu, D.; Morishita, M.; Sun, Q.; et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: A role for hypothalamic inflammation. Environ. Health Perspect. 2014, 122, 79–86.
- Bartoli, C.R.; Wellenius, G.A.; Coull, B.A.; Akiyama, I.; Diaz, E.A.; Lawrence, J.; Okabe, K.; Verrier, R.L.; Godleski, J.J. Concentrated ambient particles alter myocardial blood flow during acute ischemia in conscious canines. Environ. Health Perspect. 2009, 117, 333–337.
- Niu, Z.; Liu, F.; Li, B.; Li, N.; Yu, H.; Wang, Y.; Tang, H.; Chen, X.; Lu, Y.; Cheng, Z.; et al. Acute effect of ambient fine particulate matter on heart rate variability: An updated systematic review and meta-analysis of panel studies. Environ. Health Prev. Med. 2020, 25, 77.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065.
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Perier, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721.
- Pope, C.A.; Muhlestein, J.B.; Anderson, J.L.; Cannon, J.B.; Hales, N.M.; Meredith, K.G.; Le, V.; Horne, B.D. Short-Term Exposure to Fine Particulate Matter Air Pollution Is Preferentially Associated with the Risk of ST-Segment Elevation Acute Coronary Events. J. Am. Heart Assoc. 2015, 4, e002506.
- Baneras, J.; Ferreira-Gonzalez, I.; Marsal, J.R.; Barrabes, J.A.; Ribera, A.; Lidon, R.M.; Domingo, E.; Marti, G.; Garcia-Dorado, D.; Codi, I.A.M.R.i. Short-term exposure to air pollutants increases the risk of ST elevation myocardial infarction and of infarct-related ventricular arrhythmias and mortality. Int. J. Cardiol. 2018, 250, 35–42.
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014, 348, f7412.
- Jilani, M.H.; Simon-Friedt, B.; Yahya, T.; Khan, A.Y.; Hassan, S.Z.; Kash, B.; Blankstein, R.; Blaha, M.J.; Virani, S.S.; Rajagopalan, S.; et al. Associations between particulate matter air pollution, presence and progression of subclinical coronary and carotid atherosclerosis: A systematic review. Atherosclerosis 2020, 306, 22–32.
- Provost, E.B.; Madhloum, N.; Int Panis, L.; De Boever, P.; Nawrot, T.S. Carotid intima-media thickness, a marker of subclinical atherosclerosis, and particulate air pollution exposure: The meta-analytical evidence. PLoS ONE 2015, 10, e0127014.
- Khraishah, H.; Alahmad, B.; Ostergard, R.L., Jr.; AlAshqar, A.; Albaghdadi, M.; Vellanki, N.; Chowdhury, M.M.; Al-Kindi, S.G.; Zanobetti, A.; Gasparrini, A.; et al. Climate change and cardiovascular disease: Implications for global health. Nat. Rev. Cardiol. 2022, 19, 798–812.
- Jacobsen, A.P.; Khiew, Y.C.; Duffy, E.; O’Connell, J.; Brown, E.; Auwaerter, P.G.; Blumenthal, R.S.; Schwartz, B.S.; McEvoy, J.W. Climate change and the prevention of cardiovascular disease. Am. J. Prev. Cardiol. 2022, 12, 100391.
- Alahmad, B.; Khraishah, H.; Royé, D.; Vicedo-Cabrera, A.M.; Guo, Y.; Papatheodorou, S.I.; Achilleos, S.; Acquaotta, F.; Armstrong, B.; Bell, M.L.; et al. Associations Between Extreme Temperatures and Cardiovascular Cause-Specific Mortality: Results From 27 Countries. Circulation 2023, 147, 35–46.
- Kysely, J.; Pokorna, L.; Kyncl, J.; Kriz, B. Excess cardiovascular mortality associated with cold spells in the Czech Republic. BMC Public Health 2009, 9, 19.
- Weerasinghe, D.P.; MacIntyre, C.R.; Rubin, G.L. Seasonality of coronary artery deaths in New South Wales, Australia. Heart 2002, 88, 30–34.
- Rogot, E.; Padgett, S.J. Associations of coronary and stroke mortality with temperature and snowfall in selected areas of the United States, 1962–1966. Am. J. Epidemiol. 1976, 103, 565–575.
- Kan, H.D.; Jia, J.; Chen, B.H. Temperature and daily mortality in Shanghai: A time-series study. Biomed. Environ. Sci. BES 2003, 16, 133–139.
- De Lorenzo, F.; Kadziola, Z.; Mukherjee, M.; Saba, N.; Kakkar, V.V. Haemodynamic responses and changes of haemostatic risk factors in cold-adapted humans. QJM Mon. J. Assoc. Physicians 1999, 92, 509–513.
- Neild, P.J.; Syndercombe-Court, D.; Keatinge, W.R.; Donaldson, G.C.; Mattock, M.; Caunce, M. Cold-induced increases in erythrocyte count, plasma cholesterol and plasma fibrinogen of elderly people without a comparable rise in protein C or factor X. Clin. Sci. 1994, 86, 43–48.
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklov, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375.




