
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paul Chazot | -- | 2456 | 2023-08-29 09:59:38 | | | |
| 2 | Lindsay Dong | Meta information modification | 2456 | 2023-08-29 10:05:48 | | |
Video Upload Options
Chronic Traumatic Encephalopathy (CTE) is a neurodegenerative disease consistently associated with repetitive traumatic brain injuries (TBIs), which makes multiple professions, such as contact sports athletes and the military, especially susceptible to its onset. There are currently no approved biomarkers to diagnose CTE, thus it can only be confirmed through a post-mortem brain autopsy. Several imaging and cerebrospinal fluid biomarkers have shown promise in the diagnosis. However, blood-based biomarkers can be more easily obtained and quantified, increasing their clinical feasibility and potential for prophylactic use.
1. Introduction
2. Pathology of CTE and Rationale for Blood-Based Biomarkers
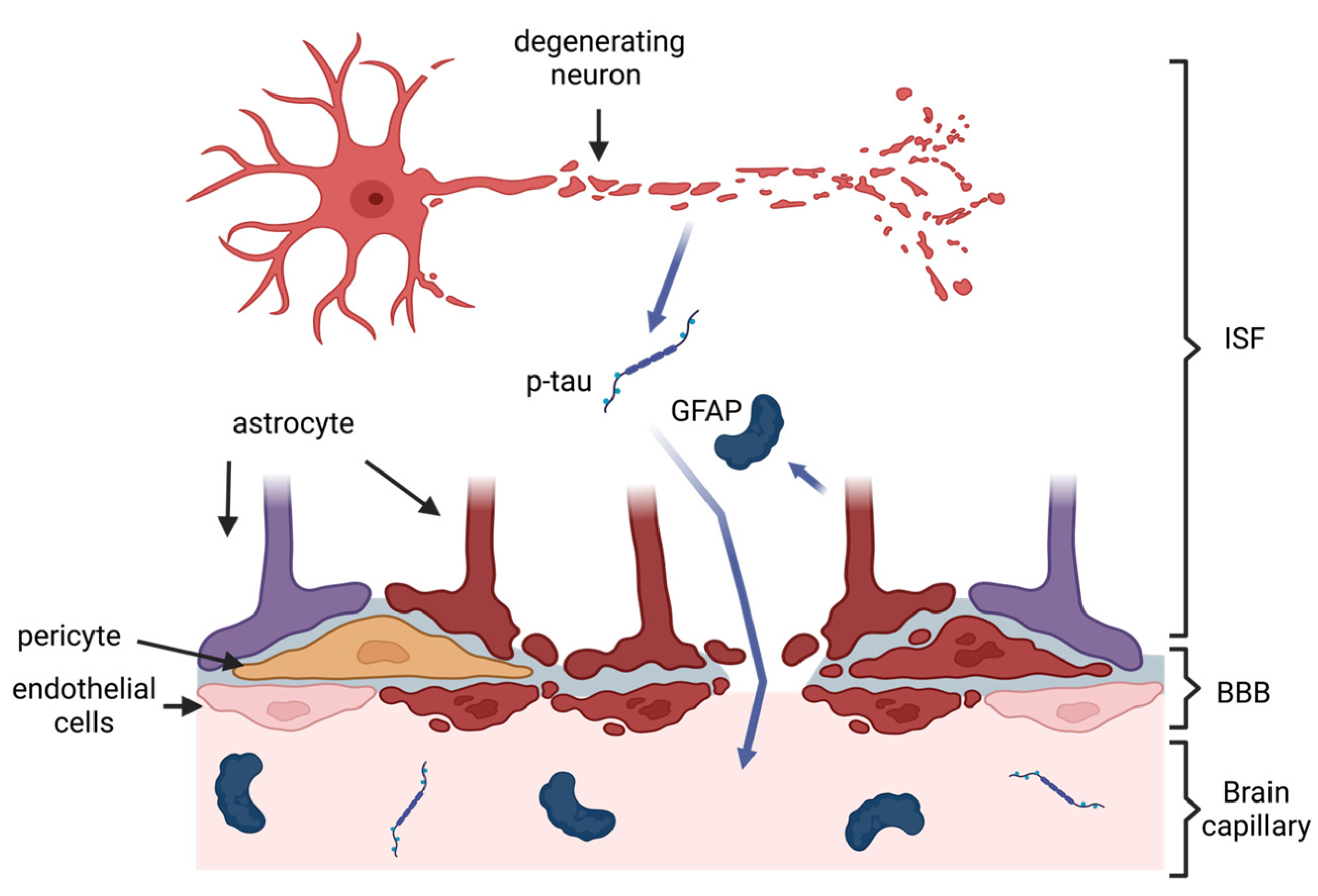
3. Biomarkers of Neurodegeneration in CTE
3.1. Total Tau and Phosphorylated Tau
3.2. Amyloid Beta
3.3. Neurofilament Light
3.4. Other Biomarkers of Neurodegeneration
4. Biomarkers of Neuroinflammation in CTE
4.1. Glial Fibrillary Acidic Protein
4.2. Inflammatory Cytokines
5. Micro RNA Biomarkers in CTE
6. Conclusions
There are currently no approved biomarkers to diagnose CTE, thus it can only be confirmed through a post-mortem brain autopsy. Several imaging and cerebrospinal fluid biomarkers have shown promise in the diagnosis. However, blood-based biomarkers can be more easily obtained and quantified, increasing their clinical feasibility and potential for prophylactic use. This entry comprehensively summarizes the studies into potential blood-based biomarkers of CTE, discussing common themes and limitations, as well as suggesting future research directions. several molecules, such as different phosphorylated tau isoforms, were able to discern CTE from different neurodegenerative diseases. Further, the results from studies on exosomal biomarkers suggest that exosomes are a promising source of biomarkers, reflective of the internal environment of the brain. Nonetheless, more longitudinal studies combining imaging, neurobehavioral, and biochemical approaches are warranted to establish robust biomarkers for CTE.
References
- World Health Organization. Global Status Report on the Public Health Response to Dementia; WHO: Geneva, Switzerland, 2021.
- Martland, H.S. Punch Drunk. JAMA 1928, 91, 1103–1107.
- McKee, A.C.; Stein, T.D.; Nowinski, C.J.; Stern, R.A.; Daneshvar, D.H.; Alvarez, V.E.; Lee, H.-S.; Hall, G.; Wojtowicz, S.M.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136, 43–64.
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599.
- Robinson, L.; Tang, E.; Taylor, J.-P. Dementia: Timely diagnosis and early intervention. BMJ 2015, 350, h3029.
- Schwab, N.; Wennberg, R.; Grenier, K.; Tartaglia, C.; Tator, C.; Hazrati, L.-N. Association of Position Played and Career Duration and Chronic Traumatic Encephalopathy at Autopsy in Elite Football and Hockey Players. Neurology 2021, 96, e1835–e1843.
- Omalu, B.I.; DeKosky, S.T.; Minster, R.L.; Kamboh, M.I.; Hamilton, R.L.; Wecht, C.H. Chronic Traumatic Encephalopathy in a National Football League Player. Neurosurgery 2005, 57, 128–134.
- Omalu, B.I.; DeKosky, S.T.; Hamilton, R.L.; Minster, R.L.; Kamboh, M.I.; Shakir, A.M.; Wecht, C.H. Chronic Traumatic Encephalopathy in a National Football League Player: Part II. Neurosurgery 2006, 59, 1086–1093.
- Stein, T.D.; Montenigro, P.H.; Alvarez, V.E.; Xia, W.; Crary, J.F.; Tripodis, Y.; Daneshvar, D.H.; Mez, J.; Solomon, T.; Meng, G.; et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015, 130, 21–34.
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; Alosco, M.L.; Solomon, T.M.; Nowinski, C.J.; McHale, L.; et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA 2017, 318, 360–370.
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.-L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic Traumatic Encephalopathy in Blast-Exposed Military Veterans and a Blast Neurotrauma Mouse Model. Sci. Transl. Med. 2012, 4, 134.
- Maroon, J.C.; Winkelman, R.; Bost, J.; Amos, A.; Mathyssek, C.; Miele, V. Chronic Traumatic Encephalopathy in Contact Sports: A Systematic Review of All Reported Pathological Cases. PLoS ONE 2015, 10, e0117338.
- McKee, A.C.; Stein, T.D.; Huber, B.R.; Crary, J.F.; Bieniek, K.; Dickson, D.; Alvarez, V.E.; Cherry, J.D.; Farrell, K.; Butler, M.; et al. Chronic traumatic encephalopathy (CTE): Criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta Neuropathol. 2023, 145, 371–394.
- Stern, R.A.; Daneshvar, D.H.; Baugh, C.M.; Seichepine, D.R.; Montenigro, P.H.; Riley, D.O.; Fritts, N.G.; Stamm, J.M.; Robbins, C.A.; McHale, L.; et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013, 81, 1122–1129.
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80.
- Guskiewicz, K.M.; Marshall, S.W.; Bailes, J.; McCrea, M.; Cantu, R.C.; Randolph, C.; Jordan, B.D. Association between Recurrent Concussion and Late-Life Cognitive Impairment in Retired Professional Football Players. Neurosurgery 2005, 57, 719–726.
- Perry, D.C.; Sturm, V.E.; Peterson, M.J.; Pieper, C.F.; Bullock, T.; Boeve, B.F.; Miller, B.L.; Guskiewicz, K.M.; Berger, M.S.; Kramer, J.H.; et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. JNS 2016, 124, 511–526.
- Barnes, D.E.; Byers, A.L.; Gardner, R.C.; Seal, K.H.; Boscardin, W.J.; Yaffe, K. Association of Mild Traumatic Brain Injury with and without Loss of Consciousness with Dementia in US Military Veterans. JAMA Neurol. 2018, 75, 1055–1061.
- Hind, K.; Konerth, N.; Entwistle, I.; Hume, P.; Theadom, A.; Lewis, G.; King, D.; Goodbourn, T.; Bottiglieri, M.; Ferraces-Riegas, P.; et al. Mental Health and Wellbeing of Retired Elite and Amateur Rugby Players and Non-contact Athletes and Associations with Sports-Related Concussion: The UK Rugby Health Project. Sports. Med. 2022, 52, 1419–1431.
- Alosco, M.L.; Culhane, J.; Mez, J. Neuroimaging Biomarkers of Chronic Traumatic Encephalopathy: Targets for the Academic Memory Disorders Clinic. Neurotherapeutics 2021, 18, 772–791.
- Alosco, M.L.; Tripodis, Y.; Fritts, N.G.; Heslegrave, A.; Baugh, C.M.; Conneely, S.; Mariani, M.; Martin, B.M.; Frank, S.; Mez, J.; et al. Cerebrospinal fluid tau, Aβ, and sTREM2 in Former National Football League Players: Modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimer’s Dement. 2018, 14, 1159–1170.
- Turk, K.W.; Geada, A.; Alvarez, V.E.; Xia, W.; Cherry, J.D.; Nicks, R.; Meng, G.; Daley, S.; Tripodis, Y.; Huber, B.R. A comparison between tau and amyloid-β cerebrospinal fluid biomarkers in chronic traumatic encephalopathy and Alzheimer disease. Alzheimer’s Res. Ther. 2022, 14, 28.
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77.
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66.
- Edwards, G.; Zhao, J.; Dash, P.K.; Soto, C.; Moreno-Gonzalez, I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J. Neurotrauma 2020, 37, 80–92.
- Butler, M.L.M.D.; Dixon, E.; Stein, T.D.; Alvarez, V.E.; Huber, B.; Buckland, M.E.; McKee, A.C.; Cherry, J.D. Tau Pathology in Chronic Traumatic Encephalopathy is Primarily Neuronal. J. Neuropathol. Exp. Neurol. 2022, 81, 773–780.
- Gendron, T.F.; Petrucelli, L. The role of tau in neurodegeneration. Mol. Neurodegener. 2009, 4, 13.
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy After Repetitive Head Injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735.
- McKee, A.C.; Stein, T.D.; Kiernan, P.T.; Alvarez, V.E. The Neuropathology of Chronic Traumatic Encephalopathy: CTE Neuropathology. Brain Pathol. 2015, 25, 350–364.
- Smith, D.H.; Meaney, D.F.; Shull, W.H. Diffuse Axonal Injury in Head Trauma. J. Head Trauma Rehabil. 2003, 18, 307–316.
- Inglese, M.; Makani, S.; Johnson, G.; Cohen, B.A.; Silver, J.A.; Gonen, O.; Grossman, R.I. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J. Neurosurg. 2005, 103, 298–303.
- Petzold, A. Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 2005, 233, 183–198.
- Siedler, D.G.; Chuah, M.I.; Kirkcaldie, M.T.K.; Vickers, J.C.; King, A.E. Diffuse axonal injury in brain trauma: Insights from alterations in neurofilaments. Front. Cell. Neurosci. 2014, 8, 429.
- Hay, J.R.; Johnson, V.E.; Young, A.M.H.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157.
- Kornguth, S.; Rutledge, N.; Perlaza, G.; Bray, J.; Hardin, A. A Proposed Mechanism for Development of CTE Following Concussive Events: Head Impact, Water Hammer Injury, Neurofilament Release, and Autoimmune Processes. Brain Sci. 2017, 7, 164.
- Mayeux, R. Biomarkers: Potential uses and limitations. Neurotherapeutics 2004, 1, 182–188.
- Ray, P.; Manach, Y.L.; Riou, B.; Houle, T.T.; Warner, D.S. Statistical Evaluation of a Biomarker. Anesthesiology 2010, 112, 1023–1040.
- Shahim, P.; Gill, J.M.; Blennow, K.; Zetterberg, H. Fluid Biomarkers for Chronic Traumatic Encephalopathy. Semin. Neurol. 2020, 40, 411–419.
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42.
- Erturk, A.; Mentz, S.; Stout, E.E.; Hedehus, M.; Dominguez, S.L.; Neumaier, L.; Krammer, F.; Llovera, G.; Srinivasan, K.; Hansen, D.V. Interfering with the Chronic Immune Response Rescues Chronic Degeneration After Traumatic Brain Injury. J. Neurosci. 2016, 36, 9962–9975.
- McKee, A.C. The Neuropathology of Chronic Traumatic Encephalopathy: The Status of the Literature. Semin. Neurol. 2020, 40, 359–369.
- Murray, H.C.; Osterman, C.; Bell, P.; Vinnell, L.; Curtis, M.A. Neuropathology in chronic traumatic encephalopathy: A systematic review of comparative post-mortem histology literature. Acta Neuropathol. Commun. 2022, 10, 108–128.
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread Tau and Amyloid-Beta Pathology Many Years After a Single Traumatic Brain Injury in Humans: Long-Term AD-Like Pathology after Single TBI. Brain Pathol. 2012, 22, 142–149.
- Alosco, M.L.; Su, Y.; Stein, T.D.; Protas, H.; Cherry, J.D.; Adler, C.H.; Balcer, L.J.; Bernick, C.; Pulukuri, S.V.; Abdolmohammadi, B.; et al. Associations between near end-of-life flortaucipir PET and postmortem CTE-related tau neuropathology in six former American football players. Eur. J Nucl. Med. Mol. Imaging 2022, 50, 435–452.
- Mohamed, A.Z.; Cumming, P.; Nasrallah, F.A.; Alzheimer’s Disease Neuroimaging Initiative. Escalation of Tau Accumulation after a Traumatic Brain Injury: Findings from Positron Emission Tomography. Brain Sci. 2022, 12, 876.
- Gorgoraptis, N.; Li, L.M.; Whittington, A.; Zimmerman, K.A.; Maclean, L.M.; McLeod, C.; Ross, E.; Heslegrave, A.; Zetterberg, H.; Passchier, J.; et al. In vivo detection of cerebral tau pathology in long-term survivors of traumatic brain injury. Sci. Transl. Med. 2019, 11, eaaw1993.
- Muraoka, S.; Jedrychowski, M.P.; Tatebe, H.; DeLeo, A.M.; Ikezu, S.; Tokuda, T.; Gygi, S.P.; Stern, R.A.; Ikezu, T. Proteomic Profiling of Extracellular Vesicles Isolated from Cerebrospinal Fluid of Former National Football League Players at Risk for Chronic Traumatic Encephalopathy. Front. Neurosci. 2019, 13, 1059.
- Rubenstein, R.; Sharma, D.R.; Chang, B.; Oumata, N.; Cam, M.; Vaucelle, L.; Lindberg, M.F.; Chiu, A.; Wisniewski, T.; Wang, K.K.W.; et al. Novel Mouse Tauopathy Model for Repetitive Mild Traumatic Brain Injury: Evaluation of Long-Term Effects on Cognition and Biomarker Levels After Therapeutic Inhibition of Tau Phosphorylation. Front. Neurol. 2019, 10, 124.
- Alosco, M.L.; Tripodis, Y.; Jarnagin, J.; Baugh, C.M.; Martin, B.; Chaisson, C.E.; Estochen, N.; Song, L.; Cantu, R.C.; Jeromin, A.; et al. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 33–40.
- Swann, O.J.; Turner, M.; Heslegrave, A.; Zetterberg, H. Fluid biomarkers and risk of neurodegenerative disease in retired athletes with multiple concussions: Results from the International Concussion and Head Injury Research Foundation Brain health in Retired athletes Study of Ageing and Impact-Related Neurodegenerative Disease (ICHIRF-BRAIN study). BMJ Open Sport Exerc. Med. 2022, 8, e001327.
- Shahim, P.; Politis, A.; van der Merwe, A.; Moore, B.; Ekanayake, V.; Lippa, S.M.; Chou, Y.-Y.; Pham, D.L.; Butman, J.A.; Diaz-Arrastia, R.; et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology 2020, 95, e623–e636.
- Shahim, P.; Zetterberg, H.; Simrén, J.; Ashton, N.J.; Norato, G.; Schöll, M.; Tegner, Y.; Diaz-Arrastia, R.; Blennow, K. Association of Plasma Biomarker Levels with Their CSF Concentration and the Number and Severity of Concussions in Professional Athletes. Neurology 2022, 99, e347–e354.
- Vasilevskaya, A.; Taghdiri, F.; Multani, N.; Ozzoude, M.; Tarazi, A.; Khodadadi, M.; Wennberg, R.; Rusjan, P.; Houle, S.; Green, R.; et al. Investigating the use of plasma pTau181 in retired contact sports athletes. J. Neurol. 2022, 269, 5582–5595.
- Olivera, A.; Lejbman, N.; Jeromin, A.; French, L.M.; Kim, H.-S.; Cashion, A.; Mysliwiec, V.; Diaz-Arrasti, R.; Gill, J. Peripheral Total Tau in Military Personnel Who Sustain Traumatic Brain Injuries During Deployment. JAMA Neurol. 2015, 72, 1109.
- Yin, Q.; Ji, X.; Lv, R.; Pei, J.-J.; Du, Y.; Shen, C.; Hou, X. Targetting Exosomes as a New Biomarker and Therapeutic Approach for Alzheimer’s Disease. CIA 2020, 15, 195–205.
- Asken, B.M.; Tanner, J.A.; VandeVrede, L.; Mantyh, W.G.; Casaletto, K.B.; Staffaroni, A.M.; La Joie, R.; Iaccarino, L.; Soleimani-Meigooni, D.; Rojas, J.C. Plasma P-tau181 and P-tau217 in Patients with Traumatic Encephalopathy Syndrome with and without Evidence of Alzheimer Disease Pathology. Neurology 2022, 99, e594–e604.
- Varesi, A.; Carrara, A.; Pires, V.G.; Floris, V.; Pierella, E.; Savioli, G.; Prasad, S.; Esposito, C.; Ricevuti, G.; Chirumbolo, S.; et al. Blood-Based Biomarkers for Alzheimer’s Disease Diagnosis and Progression: An Overview. Cells 2022, 11, 1367.
- Rubenstein, R.; Chang, B.; Yue, J.K.; Chiu, A.; Winkler, E.A.; Puccio, A.M.; Diaz-Arrastia, R.; Yuh, E.L.; Mukherjee, P.; Valadka, A.B.; et al. Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau–Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers. JAMA Neurol. 2017, 74, 1063.
- Smith, D.H.; Chen, X.H.; Iwata, A.; Graham, D.I. Amyloid beta accumulation in axons after traumatic brain injury in humans. J. Neurosurg. 2003, 98, 1072–1077.
- Olsson, A.; Csajbok, L.; Ost, M.; Höglund, K.; Nylén, K.; Rosengren, L.; Nellgård, B.; Blennow, K. Marked increase of beta-amyloid(1–42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J. Neurol. 2004, 251, 870–876.
- Gupta, A.; Goyal, R. Amyloid beta plaque: A culprit for neurodegeneration. Acta Neurol. Belg. 2016, 116, 445–450.
- Lejbman, N.; Olivera, A.; Heinzelmann, M.; Feng, R.; Yun, S.; Kim, H.-S.; Gill, J. Active duty service members who sustain a traumatic brain injury have chronically elevated peripheral concentrations of A β 40 and lower ratios of A β 42/40. Brain Inj. 2016, 30, 1436–1441.
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018, 32, 1359–1366.
- Goetzl, E.J.; Elahi, F.M.; Mustapic, M.; Kapogiannis, D.; Pryhoda, M.; Gilmore, A.; Gorgens, K.A.; Davidson, B.; Granholm, A.; Ledreux, A. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019, 33, 5082–5088.
- Peltz, C.B.; Kenney, K.; Gill, J.; Diaz-Arrastia, R.; Gardner, R.C.; Yaffe, K. Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology 2020, 95, e1126–e1133.
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2008, 14, 577–589.
- Al Nimer, F.; Thelin, E.; Nyström, H.; Dring, A.M.; Svenningsson, A.; Piehl, F.; Nelson, D.W.; Bellander, B.M. Comparative Assessment of the Prognostic Value of Biomarkers in Traumatic Brain Injury Reveals an Independent Role for Serum Levels of Neurofilament Light. PLoS ONE 2015, 10, e0132177.
- Shahim, P.; Gren, M.; Liman, V.; Andreasson, U.; Norgren, N.; Tegner, Y.; Mattsson, N.; Andreasen, N.; Ost, M.; Zetterberg, H.; et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 2016, 6, 36791.
- Boutté, A.M.; Thangavelu, B.; LaValle, C.R.; Nemes, J.; Gilsdorf, J.; Shear, D.A.; Kamimori, G.H. Brain-related proteins as serum biomarkers of acute, subconcussive blast overpressure exposure: A cohort study of military personnel. PLoS ONE 2019, 14, e0221036.
- Gao, W.; Zhang, Z.; Lv, X.; Wu, Q.; Yan, J.; Mao, G.; Xing, W. Neurofilament light chain level in traumatic brain injury: A system review and meta-analysis. Medicine 2020, 99, e22363.
- Dickstein, D.L.; De Gasperi, R.; Gama Sosa, M.A.; Perez-Garcia, G.; Short, J.A.; Sosa, H.; Perez, G.M.; Tschiffely, A.E.; Dams-O’Connor, K.; Pullman, M.Y.; et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol. Psychiatry 2021, 26, 5940–5954.
- Asken, B.M.; Tanner, J.A.; Vande-Vrede, L.; Casaletto, K.B.; Staffaroni, A.M.; Mundada, N.; Fonseca, C.; Iaccarino, L.; La Joie, R.; Tsuei, T.; et al. Multi-Modal Biomarkers of Repetitive Head Impacts and Traumatic Encephalopathy Syndrome: A Clinicopathological Case Series. J. Neurotrauma 2022, 39, 1195–1213.
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462.
- Öhrfelt, A.; Johansson, P.; Wallin, A.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Svensson, J. Increased Cerebrospinal Fluid Levels of Ubiquitin Carboxyl-Terminal Hydrolase L1 in Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 283–294.
- Kulczynska-Przybik, A.; Dulewicz, M.; Mroczko, P.; Borawska, R.; Doroszkiewicz, J.; Litman-Zawadzka, A.; Arslan, D.; Slowik, A. The assessment of ubiquitin C-terminal hydrolase-1 (UCH-L1) in patients with Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, e062156.
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35.
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275, 305–315.
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967.
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172.
- Lumpkins, K.M.; Bochicchio, G.V.; Keledjian, K.; Simard, J.M.; McCunn, M.; Scalea, T. Glial fibrillary acidic protein is highly correlated with brain injury. J. Trauma 2008, 65, 778–784.
- Gill, J.; Latour, L.; Diaz-Arrastia, R.; Motamedi, V.; Turtzo, C.; Shahim, P.; Mondello, S.; DeVoto, C.; Veras, E.; Hanlon, D.; et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018, 91, e1385–e1389.
- Huebschmann, N.A.; Luoto, T.M.; Karr, J.E.; Berghem, K.; Blennow, K.; Zetterberg, H.; Ashton, N.J.; Simrén, J.; Posti, J.P.; Gill, J.M.; et al. Comparing Glial Fibrillary Acidic Protein (GFAP) in Serum and Plasma Following Mild Traumatic Brain Injury in Older Adults. Front. Neurol. 2020, 11, 1054.
- Lozano, D.; Gonzales-Portillo, G.S.; Acosta, S.; de la Pena, I.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 2015, 11, 97–106.
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum MicroRNAs Are Promising Novel Biomarkers. PLoS ONE 2008, 3, e3148.
- Hiskens, M.I.; Mengistu, T.S.; Li, K.M.; Fenning, A.S. Systematic Review of the Diagnostic and Clinical Utility of Salivary microRNAs in Traumatic Brain Injury (TBI). Int. J. Mol. Sci. 2022, 23, 13160.
- Guedes, V.A.; Devoto, C.; Leete, J.; Sass, D.; Acott, J.D.; Mithani, S.; Gill, J.M. Extracellular Vesicle Proteins and MicroRNAs as Biomarkers for Traumatic Brain Injury. Front. Neurol. 2020, 11, 663.
- Bhomia, M.; Balakathiresan, N.S.; Wang, K.K.; Papa, L.; Maheshwari, R.K. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 2016, 6, 28148.
- Redell, J.B.; Moore, A.N.; Ward, N.H.; Hergenroeder, G.W.; Dash, P.K. Human Traumatic Brain Injury Alters Plasma microRNA Levels. J. Neurotrauma 2010, 27, 2147–2156.
- Wyczechowska, D.; Harch, P.G.; Mullenix, S.; Fannin, E.S.; Chiappinelli, B.B.; Jeansonne, D.; Lassak, A.; Bazan, N.G.; Peruzzi, F. Serum microRNAs associated with concussion in football players. Front. Neurol. 2023, 14, 1155479.
- Alvia, M.; Aytan, N.; Spencer, K.R.; Foster, Z.W.; Rauf, N.A.; Guilderson, L.; Robey, I.; Averill, J.G.; Walker, S.E.; Alvarez, V.E.; et al. MicroRNA Alterations in Chronic Traumatic Encephalopathy and Amyotrophic Lateral Sclerosis. Front. Neurosci. 2022, 16, 855096.
- Ghai, V.; Fallen, S.; Baxter, D.; Scherler, K.; Kim, T.-K.; Zhou, Y.; Meabon, J.S.; Logsdon, A.F.; Banks, W.A.; Schindler, A.G.; et al. Alterations in Plasma microRNA and Protein Levels in War Veterans with Chronic Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1418–1430.
- Ge, X.; Guo, M.; Li, M.; Zhang, S.; Qiang, J.; Zhu, L.; Cheng, L.; Li, W.; Wang, Y.; Yu, J.; et al. Potential blood biomarkers for chronic traumatic encephalopathy: The multi-omics landscape of an observational cohort. Front. Aging Neurosci. 2022, 14, 1052765.




