Chronic Traumatic Encephalopathy (CTE) is a neurodegenerative disease consistently associated with repetitive traumatic brain injuries (TBIs), which makes multiple professions, such as contact sports athletes and the military, especially susceptible to its onset. There are currently no approved biomarkers to diagnose CTE, thus it can only be confirmed through a post-mortem brain autopsy. Several imaging and cerebrospinal fluid biomarkers have shown promise in the diagnosis. However, blood-based biomarkers can be more easily obtained and quantified, increasing their clinical feasibility and potential for prophylactic use.
- biomarkers
- CTE
- neurodegeneration
- TBI
- miRNA
- exosomes
1. Introduction
2. Pathology of CTE and Rationale for Blood-Based Biomarkers
CTE is a progressive tauopathy characterized by the deposition of neurofibrillary tangles (NFTs) consisting mainly of hyperphosphorylated microtubule-associated protein tau (p-tau) in the perivascular depths of cortical sulci, which increases with the progression of a disease, and is linked to trauma incurred during a TBI [24][25][26][31,32,33]. The aggregation of tau in NFTs induces several neurotoxic mechanisms, including microtubule destabilization, synapse loss, and potential aberrations of intracellular signaling, causing neuronal death [27][34]. This leads to macro-scale changes, such as brain atrophy and a consequent decrease in brain volume, in the advanced stages of the disease [28][29][35,36]. Substantial force impact on the head in a TBI causes a diffuse axonal injury (DAI), which can result in the breakage of axons and a subsequent release of axonal proteins, such as tau, into the interstitial fluid and the CSF [30][31][32][33][37,38,39,40]. On the other hand, TBI also increases the permeability of the blood–brain barrier (BBB) [34][41] leading to a possible efflux of axonal proteins into the systemic circulation (Figure 1). This two-way pathological mechanism allows for the detection and quantification of biomarkers from blood samples [35][42]. An ideal blood-based biomarker should be diagnostically accurate, i.e., be able to correctly discern between patients suffering from CTE and patients who are not; selective towards CTE, i.e., be able to discern CTE from other tauopathies, for example, AD; and feasible, i.e., be easily detectable and quantifiable from blood [36][37][43,44].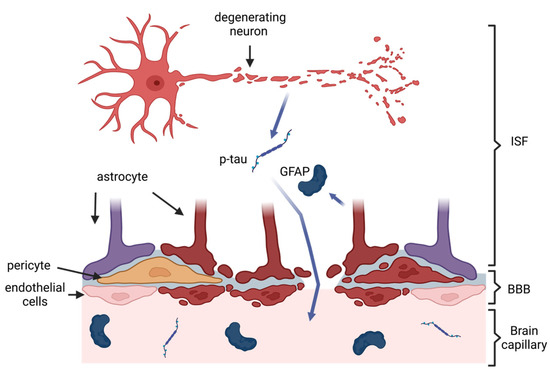
3. Biomarkers of Neurodegeneration in CTE
3.1. Total Tau and Phosphorylated Tau
Tau plays the most prominent role in the pathology of CTE, through the formation of NFTs and consequent neuronal dysfunction and death [27][34]. Numerous studies have shown a significant elevation in the extracellular p-tau deposition in the brains of people with autopsy-confirmed CTE, compared to healthy controls (e.g., Johnson et al. [43][49]). Most studies on the role of tau in the diagnosis of the long-term effects of TBI have focused on t-tau detectable from blood, which corresponds to compromised BBB, and p-tau, in line with signaling compromised BBB, which indicates the presence of DAI and neurotoxic mechanisms [24][25][26][31,32,33]. Thus far, tau has yielded promising results as an imaging biomarker [44][45][50,51], but mixed results as a biomarker from CSF [21][22][46][47][21,22,52,53]. However, both forms were shown to be significantly elevated in the brain and plasma in a mouse model of rTBIs [48][54]. Both t-tau and p-tau concentrations analyzed from plasma have thus far yielded variable results, with many studies failing to discern between people exposed to TBIs and controls [46][49][50][51][52][53][52,55,56,57,58,59]. Nevertheless, Alosco et al. [49][55] reported a relationship between tau levels and RHIs, as well as plasma t-tau levels above 3.56 pg/mL, only in former rugby players despite no significance between the groups. However, others reported no relationship between t-tau and RHI [52][58]. Only Olivera et al. [54][60] observed increased plasma t-tau levels in the military deployed within the last 18 months who self-diagnosed themselves with TBIs, with a greater number of TBIs associated with a more substantial increase in plasma t-tau. As for p-tau, Vasilevskaya et al. [53][59] showed that tau phosphorylated at threonine 181 (p-tau181) was significantly elevated in retired contact sports athletes. While exosomal t-tau and p-tau appear promising as blood-based biomarkers, they so far have not shown substantial specificity to CTE; a significant increase in both plasma and exosomal t-tau and p-tau can be observed, for instance, in AD [55][56][57][75,76,77]. Studies on tau in CTE, thus far, have focused on its diagnostic utility in discerning between people with potential CTE and healthy controls, rather than between different neurodegenerative diseases. However, Turk et al. [22] reported that tau phosphorylated at threonine 231 (p-tau231) from CSF is significantly different between CTE and AD patients, confirmed by brain autopsies. Moreover, p-tau231 was successful in distinguishing AD and CTE diagnoses. There are no studies on the long-term blood levels of p-tau231, but the protein was significantly elevated in the plasma of patients from TBI rehabilitation units with a potential chronic TBI [58][78].3.2. Amyloid Beta
While Amyloid beta (Aβ) plaques are primarily associated with AD, TBI has been shown to increase the concentration of the Amyloid Precursor Protein and Aβ peptides in the brain tissue and CSF. These proteins can foster the formation of plaques [9][59][60][9,82,83], which are toxic to brain cells and trigger neurodegenerative processes [61][84]. The presence of Aβ plaques in cadavers with neuropathologically-diagnosed CTE has been reported, but it is not as universal as the deposition of NFTs in CTE and has been linked to the possession of the Apoϵ4 allele, as well as significantly older age at death, potentially indicating Aβ plaques’ association with old age in CTE [9]. Thus far, the results for the blood-based Aβ peptide have been mixed. From plasma, Lebjman et al. [62][85] reported a significant increase in Aβ40 and a trend for increased Aβ42 in military personnel who experienced TBI, deployed a minimum of 16 months before the investigation, while other studies reported no significant changes in different groups of athletes who experienced TBIs [50][52][56,58]. Exosomal Aβ peptides, in turn, appear more promising, with a significant increase of Aβ42 in groups that experienced TBIs [63][64][70,72], with one study showing no difference [65][74]. While more research is needed to confirm the role of exosomal Aβ as a biomarker, its specificity to CTE is greatly limited.3.3. Neurofilament Light
Neurofilaments are intermediate filaments expressed exclusively in neurons. While their exact function remains to be elucidated, they are thought to play a critical role in axonal stability. Therefore, the efflux of neurofilaments into the CSF and potentially systemic circulation is indicative of neuroaxonal injury and has been suggested as a biomarker of neurological disorders, such as Parkinson’s disease (PD) or AD [66][86]. In the context of TBI, the research has focused on the diagnosis of TBI through plasma NfL, reporting a significant elevation in plasma NfL following a TBI that predicted clinical outcomes [66][67][68][69][70][86,87,88,89,90]. However, knowledge about the long-term relationship between NfL and CTE is scarce. In a rat model, a single blast overpressure exposure was not shown to significantly increase plasma NfL 10 months after a blast simulation, but there was a trend for increased NfL in exposed rats compared to controls [71][91]. The major limitation of NfL is its lack of specificity to CTE. Asken et al. [72][93], analyzing a group of nine cadavers, showed that elevated NfL could be observed in patients with different neuropathologically confirmed neurological disorders, such as Frontotemporal Lobar Degeneration, CTE and AD. Nonetheless, further research into the chronic effects of TBIs and their relationship to NfL is warranted to establish the clinical relevance and selectivity of NfL in the context of CTE.3.4. Other Biomarkers of Neurodegeneration
There have been individual studies investigating several other potential biomarkers of neurodegeneration. Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) is an abundant protein in the brain and is essential to the proper maintenance of axonal integrity. Its dysfunction has been implicated in neurodegeneration, where it can, for example, misfold and constitute NFTs in AD [73][94]. As such, it was found to be significantly elevated in the CSF of AD patients [74][75][95,96]. In potential CTE, CSF UCH-L1 was associated with grey matter abnormalities in long-term TBI survivors, but there was no difference between this group and controls [46][52]. However, no UCH-L1 elevation, as well as no correlation with brain structural changes, was reported in patients with TBIs compared to controls, both from plasma [51][57] and exosomes [64][72], thus far.4. Biomarkers of Neuroinflammation in CTE
4.1. Glial Fibrillary Acidic Protein
GFAP is an intermediate filament protein and a major cytoskeletal component of astrocytes, which maintain synaptic transmission and axonal metabolism [76][106]. Following TBI, astrocytes mediate processes, such as BBB permeability and the inflammatory response [77][107]. Astrocyte immune activation is accompanied by an increase in the expression of GFAP [78][108]. On the other hand, an astrocytic injury could cause the efflux of GFAP [79][109]. Therefore, GFAP could represent both chronic neuroinflammation and neurodegeneration in CTE. Its blood elevation has been shown to relate to structural abnormalities in imaging studies after mild TBI [80][81][82][110,111,112].4.2. Inflammatory Cytokines
The increased activation of microglia, which has been shown to occur following TBIs, upregulates the production of several pro-inflammatory cytokines. These lead to increased permeability of the BBB, elevated secretion of chemokines that cause the migration of peripheral leukocytes into the brain, as well as the production of reactive oxygen species, which altogether foster neuroinflammation and can trigger secondary cell death. Inflammatory cytokines investigated in the context of the long-term consequences of TBI involve IL-6, IL-10, and TNF-α, which can all be secreted by microglia, indicating microgliosis [83][99]. As these can be expressed in all the tissues, the concentration of the cytokines was quantified from neuron-enriched exosomes.5. Micro RNA Biomarkers in CTE
Micro RNAs (miRNAs) are small non-coding RNAs, which regulate a variety of processes at the post-transcriptional level. The expression of miRNAs can change in response to different physiological and pathological states [84][113]. Specifically, several studies identified panels of miRNA biomarkers from saliva [85][114] and blood [86][87][88][89][68,115,116,117] that showed different levels of specific miRNAs between patients following a TBI and controls. As such, they showed great potential in diagnosing TBI. A great advantage of miRNAs over conventional protein panels is their stability; Gilad et al. [84][113] showed that their levels did not change after four hours at room temperature, while two freeze–thaw cycles affected their levels to a small extent. However, only a handful of studies have looked at the expression of miRNAs in potential CTE patients, thus far. Alvia et al. [90][118] compared the expression of different miRNAs previously indicated in the prefrontal cortex of brains donated by people who suffered from either CTE, Amyotrophic Lateral Sclerosis (ALS), or both. While much of the expression of miRNAs overlapped between CTE and ALS, they identified several miRNAs specific to CTE, which were involved in cell growth, apoptotic and inflammatory pathways. As per biological fluids, Ghai et al. [91][119] used next-generation sequencing (NGS) to compare the miRNA profiles of plasma and extracellular vesicles (EV) between veterans with a history of chronic TBI and controls. They detected significant differences in the levels of multiple previously described, as well as novel, miRNAs, which they confirmed using qRT-PCR. They also observed that most miRNAs were circulating freely in plasma, which supports the use of plasma without the need for EVs isolation. Ge et al. [92][120] compared serum and exosome biomarkers between 12 patients with a history of rTBIs and, thus, a different likelihood of CTE and respective controls, distinguishing serum and exosomal miR-1183 and exosomal miR-297 as potential diagnostic miRNA biomarkers of CTE.6. Conclusions
There are currently no approved biomarkers to diagnose CTE, thus it can only be confirmed through a post-mortem brain autopsy. Several imaging and cerebrospinal fluid biomarkers have shown promise in the diagnosis. However, blood-based biomarkers can be more easily obtained and quantified, increasing their clinical feasibility and potential for prophylactic use. This entry comprehensively summarizes the studies into potential blood-based biomarkers of CTE, discussing common themes and limitations, as well as suggesting future research directions. several molecules, such as different phosphorylated tau isoforms, were able to discern CTE from different neurodegenerative diseases. Further, the results from studies on exosomal biomarkers suggest that exosomes are a promising source of biomarkers, reflective of the internal environment of the brain. Nonetheless, more longitudinal studies combining imaging, neurobehavioral, and biochemical approaches are warranted to establish robust biomarkers for CTE.
