Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jinxin Liu | -- | 2920 | 2023-08-29 09:02:45 | | | |
| 2 | Fanny Huang | Meta information modification | 2920 | 2023-08-30 07:59:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, J.; Chen, H.; Li, X.; Song, C.; Wang, L.; Wang, D. Effects of Natural Products on Lipid Metabolism Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/48573 (accessed on 16 January 2026).
Liu J, Chen H, Li X, Song C, Wang L, Wang D. Effects of Natural Products on Lipid Metabolism Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/48573. Accessed January 16, 2026.
Liu, Jinxin, Huanwen Chen, Xiaoli Li, Chunmei Song, Li Wang, Deguo Wang. "Effects of Natural Products on Lipid Metabolism Disorders" Encyclopedia, https://encyclopedia.pub/entry/48573 (accessed January 16, 2026).
Liu, J., Chen, H., Li, X., Song, C., Wang, L., & Wang, D. (2023, August 29). Effects of Natural Products on Lipid Metabolism Disorders. In Encyclopedia. https://encyclopedia.pub/entry/48573
Liu, Jinxin, et al. "Effects of Natural Products on Lipid Metabolism Disorders." Encyclopedia. Web. 29 August, 2023.
Copy Citation
Natural products that are extracted from the source and from concentrated, fractionated, and purified yielding, which are generally defined as bioactive compounds, have the ability to modulate lipid metabolism, improve insulin signaling, and protect against cardiovascular damage.
natural products
miRNAs
lipid metabolism disorders
1. Introduction
Metabolic diseases, which encompass a variety of risk factors highly associated with obesity, diabetes, and cardiovascular diseases, have come to be regarded as public health challenges [1][2][3][4]. Due to their complex mechanisms of action, effective comprehensive treatments are still lacking. Even worse, the side effects of some curative drugs have been a major concern for their therapeutic usage [5][6][7]. Therefore, it is imperative to provide an effective treatment approach to overcome the aforementioned diseases.
Natural products that are extracted from the source and from concentrated, fractionated, and purified yielding, which are generally defined as bioactive compounds [8][9], have the ability to modulate lipid metabolism, improve insulin signaling, and protect against cardiovascular damage [10][11]. More importantly, natural products are widely distributed and readily available in nature [12]. To date, extensive studies have shown that plentiful drugs are derived from structural modification based on natural products [13]. MicroRNAs (miRNAs) and small noncoding RNAs are characterized by binding to the regulatory sites of 3′UTR of target mRNA, resulting in the inhibition of transcription or the promotion of degradation, accompanied by decreased protein synthesis [14][15]. Natural products could also ameliorate metabolic diseases by targeting abundant miRNAs [16][17][18]. Thus, the possibility for natural products to modify the abnormal patterns of these diseases is, at least in part, possible through a newly defined mechanism: the miRNAs cascade.
2. Effects of Natural Products on Lipid Metabolism Disorders
Lipid metabolism is a crucial and complex biochemical reaction in the body, and diseases caused by lipid metabolism disorders are common in modern society, such as obesity and hyperlipidemia [19]. Lipids are known to be important substances in energy storage and energy supply. Hence, the proper amount of adipose tissue is necessary for the human body. In general, however, patients have difficulty sticking to a long-term diet and physical activity regimen to combat these metabolic disorders. Therefore, food components that ameliorate the risk factors associated with these diseases can facilitate dietary-based therapies [16]. Dietary natural products have long been of great interest for improving lipid metabolism by modulating miRNA expression.
2.1. Regulatory Effects on Fatty Acid Synthesis and Decomposition
It is well known that fatty acids are the simplest type of lipids and are the building blocks of many more complex fats. Furthermore, they are also perceived as one of the main sources of energy on account of releasing a lot of energy during oxidation into CO2 and H2O in the case of a sufficient oxygen supply. Therefore, the role of fatty acids in the processes of lipogenesis and lipodieresis cannot be ignored. Recently, investigators have examined the regulatory effects of natural products on lipogenesis and lipodieresis, resulting in improved lipid metabolism through the management of diverse miRNAs (Table 1 and Table 2).
Table 1. The effects of natural products (extracts) on lipid metabolism disorders.
| Natural Products (Extracts) | Relevant miRNAs | Dose | Administration Methods |
Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
| Averrhoa carambola free phenolic extract | miR-33↓ miR-34a↓ |
10, 20, 30 g/kg/d for 8 weeks | Gavage | db/db mice | / | ● Reduced liver TG; ● Inhibited the signal transduction of hepatic lipogenesis; ● Exhibited a potent hepatic steatosis-relieving effect. |
[20] |
| Cerasus humilis polyphenol extract | miR-7a/b↓ | 40 μg/mL for 48 h; 250 g/kg/day for 12 weeks |
Cell culture; gavage |
3T3-L1 pre-adipocyte cells; obese mice | Sirt1, Prdm16 | ● Reduced body weight; ● Improved abnormal serum lipid and glucose levels; ● Inhibited adipocyte differentiation; ● Reduced fat accumulation by mitigating fat deposition, inflammation, and oxidation. |
[21] |
| Citrus peel flavonoids | miR-33↓ miR-122↓ |
10 μg/mL for 0.5, 1, 3 and 6 h | Cell culture | Oleic acid-treated HepG2 cells | FAS, CPT1a | ● Attenuated intracellular lipid accumulation. | [22] |
| Coffee polyphenols | miR-122↑ | 2.5 × 10−4%; diet containing 0.5% or 1.0% coffee polyphenols for 15 weeks |
Cell culture; diet |
Hepa 1-6 cells; HFD-fed mice |
SREBP1c | ● Activated AMPK; ● Enhanced energy metabolism; ● Reduced lipogenesis; ● Reduced body weight gain, abdominal and liver fat accumulation. |
[23] |
| Ginger extract | miR-21↓ miR-132↓ |
Diet containing 0.8% ginger extract for 10 weeks | Diet | HFD-fed rats | / | ● Lowered body weight and white adipose tissue mass; ● Reduced serum and hepatic lipid levels; ● Enhanced AMPK activity; ● Ameliorated obesity and inflammation. |
[24] |
| Grape seed proanthocyanidins extract | miR-33a↓ miR-122↓ |
5, 25, 50 mg/kg for 3 weeks | Gavage | HFD–induced obese rats | ABCA1; FAS, PPARβ/δ |
● Hypolipidemic; ● Decreased total liver fat. |
[16] |
| miR-33a↓ miR-122↓ |
5, 15, 25, 50 mg/kg for 3 weeks | Gavage | Healthy Wistar rats | ABCA1; FAS |
● Improved postprandial hyperlipemia; ● Increased liver cholesterol efflux to HDL formation; ● Reduced fatty acid synthesis. |
[25] | |
| miR-33↓ miR-122↓ |
10, 25, 50, or 100 mg/L for 0.5, 1, 3, or 5 h; 250 mg/kg for 1 or 3 h |
Cell culture; gavage |
FAO cells; Wistar rats |
ABCA1; FAS |
● Hypolipidemic; ● Reduced lipogenesis; ● Increased liver cholesterol efflux to HDL formation. |
[26] | |
| miR-33a↓ miR-122↓ |
25 mg/kg for 3 weeks | Gavage | Dyslipidemic obese rats | ABCA1, CPT1a; FAS, PPARβ/δ |
● Improved dyslipidemia; ● Decreased total liver fat. |
[27] | |
| miR-96↓ | 200 mg/kg/day for 180 days | Diet | HFD-fed mice | mTOR, FOXO1 | ● Decreased the weight gain, serum levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol but increased high-density lipoprotein cholesterol; ● Clearance of lipid accumulation. |
[28] | |
| miR-33↓ miR-122↓ |
250 mg/kg once | Gavage | HFD-fed grass carp | / | ● Decreased TG accumulation by reducing de novo lipogenesis and enhancing lipolysis and β-oxidation. | [29] | |
| Green tea extract | miR-34a↓ miR-194↑ |
500 mg/kg for 12 weeks (5 days/week) | Gavage | HFD-fed mice | Sirt1, PPARα, INSIG2; HMGCS, APOA5 | ● Protected against NAFLD development by altering lipid metabolism, increasing gene expression involved in triglycerides and fatty acid catabolism, and decreasing uptake and lipid accumulation. | [30] |
| miR-335↓ | 500 mg/kg for 12 weeks (5 days/week) | Gavage | HFD-fed mice | FOXO1, GSK3β | ● Reduced weight gain, adiposity and inflammation; ● Increased energy expenditure; ● Improved insulin sensitivity. |
[31] | |
| Guarana extract | miR-27b↓ miR-34b↓ miR-760↓ |
150 µg/mL for 48 h | Cell culture | 3T3-L1 pre-adipocyte cells | Wnt3a, Wnt1, Wnt10b | ● Anti-adipogenic effect. | [32] |
| Lychee pulp phenolics | miR-33↓ miR-122↓ |
500 mg/kg for 10 weeks | Gavage | HFD-fed mice | ABCA1, ABCG1, NPC1; FAS, ACC1, ACC2, SCD1, ACLY |
● Hypolipidemic; ● Repressed fatty acid synthesis and promoting fatty acid β-oxidation and cholesterol efflux in the liver; ● Decreased body fat accumulation; ● Ameliorated lipid metabolism. |
[33] |
| Mulberry fruit extract | miR-33↓ | Diet containing 0.4% mulberry fruit extract for 4 weeks | Diet | High cholesterol/cholic acid diet-fed rats | / | ● Promoted serum high-density lipoprotein cholesterol levels; ● Decreased serum and hepatic cholesterol, serum low-density lipoprotein cholesterol, and fecal bile acid levels. |
[34] |
| Mulberry leaf extract | miR-34a↓ | 3 mg/mL for 24 h | Cell culture | Glucolipotoxicity-induced HepG2 cells | Sirt1 | ● Reduced liver fat accumulation; ● Decreased inflammatory responses and steatohepatitis; ● Exerted anti-glucolipotoxicity effects. |
[35] |
| Moringa oleifera leaf extract | miR-21a↓ miR-103↓ miR-122↓ miR-34a↓ |
9.375 mg/d for 8 weeks | Gavage | HFD-fed mice | / | ● Improved ITT and decreased SREBP1c hepatic protein, while Sirt1 increased; ● Reduced insulin resistance, de novo lipogenesis, hepatic inflammation, and ER stress; ● Prevented progression of liver damage in a model of NASH. |
[36] |
| Portulaca oleracea extract | miR-122↓ | 25, 50, 100 mg/kg/d for 7 days | Gavage | Acute alcoholic liver injury rats | / | ● Reduced the ethanol-elevated serum level of ALT, AST, ALP, and TG; ● Enhanced activities of SOD and GSH-Px; ● Decreased content of NO and MDA; ● Increased antioxidant capacity; ● Relieved the inflammatory injury; ● Improved the lipid metabolism disorder. |
[37] |
| miR-33↓ miR-34a↓ |
Diet containing 0.8% portulaca oleracea L. extract for 4 weeks | Diet | High-cholesterol diet-fed rats | / | ● Improved serum, liver, and fecal lipid profiles; ● Promoted cholesterol efflux and bile acid synthesis; ● Enhanced hepatic AMPK activity. |
[38] | |
| Rosmarinus officinalis extract | miR let-7f-1↑ | 30 μg/mL for 35 days | Cell culture | Human primary omental pre-adipocytes and adipocytes | / | ● Decreased triglyceride accumulation; ● Increased glycerol release; ● Stimulated lipolytic activity in differentiating pre-adipocytes and mature adipocytes; ● Modulated the adipocyte life cycle at different levels. |
[39] |
The up arrow means an increase, and the down arrow means a decrease.
Table 2. The effects of natural products (compounds) on lipid metabolism disorders.
| Natural Products (Compounds) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
A-type ECG and EGCG dimers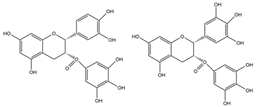 |
miR-7a/b↑ | ECG dimer: 20 μg/mL for 1–8 days; ECGG dimer: 60 μg/mL for 1–8 days |
Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ | ● Inhibited pre-adipocyte differentiation; ● Reduced intracellular lipid accumulation; ● Blocked MCE process; ● Decreased the fluidity and hydrophobicity and increased the permeability of membrane. |
[40] |
Curcumin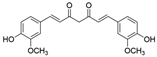 |
miR-17↓ | 2 μM or 10 μM for 6 h; | Cell culture | 3T3-L1 pre-adipocyte cells; HFD-fed mice |
TCF7L2 | ● Inhibited adipocyte differentiation and adipogenesis; ● Stimulated the Wnt signaling pathway. |
[41] |
Grape seed procyanidin B2 |
miR-483↓ | 150 μg/mL for 48 h | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ | ● Inhibited pre-adipocyte differentiation; ● Reduced intracellular lipid accumulation. |
[42] |
EGCG |
miR-143↑ | 50 μM for 24 h | Cell culture | 3T3-L1 pre-adipocyte cells | MAPK7 | ● Inhibited 3T3-L1 cell growth. | [43] |
Lycopene |
miR-21↑ | 50 μM for 24 h; diet containing 0.05% lycopene for 8 weeks |
Cell culture; gavage |
Hepa 1–6 cells; HFD-fed mice |
FABP7 | ● Lowered body weight; ● Inhibited intracellular lipid accumulation; ● Protected against HFD-induced hepatic steatosis. |
[44] |
Nonivamide |
miR let-7d↑ | 1 μM for 12 days | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ | ● Impaired adipogenesis; ● Reduced mean lipid accumulation; ● Activated TRPV1. |
[45] |
Oleanolic acid |
miR-98↑ | 10 mM for 6, 12, 24 h; 20 mg/kg for 4 weeks |
Cell culture | HFD-fed mice; db/db mice |
PGC1β | ● Hypolipidemic. | [46] |
Persimmon tannin |
miR-27↑ | 20, 40, or 60 μg/mL for 1–8 days | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ, C/EBPα | ● Inhibited pre-adipocyte differentiation; ● Reduced intracellular lipid accumulation; ● Delayed MCE process. |
[47] |
Pseudoprotodioscin |
miR-33a/b↓ | 25 μM for 24 h | Cell culture | Human HepG2 cells and THP-1 monocytic cells | SREBP1c, SREBP2 | ● Promoted the cholesterol effluxion. | [48] |
Resveratrol |
miR-103↓ miR-107↓ miR-122↓ |
30 mg/kg for 6 weeks | Diet | Obesogenic diet-fed rats | SREBP1; SREBP1, CPT1a; FAS |
● Reduced obesogenic diet-induced hepatic steatosis; ● Activated AMPK. |
[49] |
| miR-539↑ | 30 mg/kg for 6 weeks | Diet | Obesogenic diet-fed rats | SP1 | ● Inhibited de novo lipogenesis. | [50] | |
| miR-155↑ | 25 μM for 1–8 days | Cell culture | 3T3-L1 pre-adipocyte cells | CEBP/α | ● Inhibited adipogenesis. | [51] | |
Zerumbone |
miR-46b↓ | 25 μM for 48 h; diet containing 0.025% zerumbone for 8 weeks |
Cell culture; diet |
3T3-L1 fibroblasts; HFD-fed mice |
Sirt1 | ● Induced AMPK activation and phosphorylation of acetyl-CoA carboxylase; ● Ameliorated diet-induced obesity and inhibited adipogenesis. |
[52] |
The up arrow means an increase, and the down arrow means a decrease.
Specifically, miR-122 and miR-33 are two of the best-studied miRNAs involved in the regulation of lipid metabolism [53]. As is shown in Table 1 and Table 2, numerous pieces of evidence have revealed that grape seed proanthocyanidin extract treatments reduced fatty acid synthesis and de novo lipogenesis, increased liver cholesterol efflux to high-density lipoprotein (HDL) formation by decreasing the expression of miR-122 and miR-33, which could regulate several genes that control fatty acid and transcriptional regulatory factors, such as fatty acid synthase (FAS) and peroxisome proliferator-activated receptor beta/delta (PPARβ/δ), as well as genes that regulate fatty acid β-oxidation, such as ATP-binding cassette transporter A1 (ABCA1) and carnitine palmitoyltransferase 1a (CPT1a), respectively [16][25][26][27][29][54]. Further detection revealed that the levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) were reduced while the level of high-density lipoproteins cholesterol (HDL-C) was enhanced in a dose-dependent manner [16][25][26][27]. Averrhoa carambola-free phenolic extract, citrus peel flavonoids, lychee pulp phenolics, mulberry fruit extract, and portulaca oleracea extract treatments could also improve lipid metabolism in in vitro and in vivo studies; the underlying mechanism was miR-33 or miR-122-mediated changes in the signaling pathways [20][22][33][34][37][38]. However, the opposite expression of miR-122 was reflected in a natural product experiment using coffee polyphenols, which could enhance energy metabolism and reduce lipogenesis by targeting sterol regulatory element binding protein (SREBP) 1c mediated by miR-122 [23]. Accountably, SREBP1c, one of the three isoforms of SREBPs, comes into play in fatty acid synthesis and metabolism [55][56]. It potentially illustrates the point that the same type of miRNAs can act on a variety of target genes with different expressions. Similarly, the same target gene may also be regulated by multiple miRNAs. Recent studies have shown that miR-103 and miR-107 reduced obesogenic diet-induced hepatic steatosis via decreasing the protein expression of SREBP1 in resveratrol-treated rats [49], and pseudoprotodioscin promoted cholesterol effluxion through targeting SREBP1c and SREBP2 mediated by miR-33a/b in an in vitro experiment [48]. In addition, distinctively, the overexpression of hepatic miR-98 induced by oleanolic acid, an active component of the traditional Chinese herb olea europaea, increased the degradation of peroxisome proliferator-activated receptor gamma coactivator-1beta (PGC1β), known as a transcriptional co-activator of SREBP-1 and the master regulator of hepatic lipogenesis [46][57].
Intuitively, both FAS and SREBP1 are involved in the process of fatty acid synthesis, and the connection between them is found in the following experiments. SP1 transcription factor (SP1), an important member of the ubiquitously expressed SP/KLF transcription factor family, acts together with SREBP1 to synergistically activate the promoter of the FAS gene and is involved in de novo lipogenesis [58]. Hence, resveratrol reduced the expression of SP1 through upregulating miR-539, along with decreasing the expression of the SREBP1 protein and FAS gene in vivo and in vitro [50]. Nevertheless, the correlation between them still needs to be systematically and intensively investigated beyond all doubt.
Lipid metabolism is a complex process, and natural products can regulate lipogenesis and lipodieresis in a variety of ways. Zerumbone is a cyclic sesquiterpene isolated from the wild ginger Zingiber zerumbet smith. It has been proved that zerumbone could improve lipid metabolism disorder by reducing lipogenesis and increasing fatty acid oxidation [52]. For one thing, zerumbone acted as a miR-146b inhibitor and downregulated miR-146b, leading to the activation of sirtuin type 1 (Sirt1), which induced the de-acetylation of forkhead box O1 (FOXO1) and peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC1α); for another, zerumbone induced the phosphorylation of AMP-activated protein kinase (AMPK), which could limit fatty acid efflux from adipocytes and favor fatty acid oxidation, as well as decrease de novo fatty acid synthesis through the phosphorylation-mediated inhibition of acetyl-CoA carboxylase (ACC) [59][60][61] and also activated Sirt1 indirectly [52]. With these similar natural product experiments, miR-27a/b, miR-96, miR-34a, miR-194, and miR-355 also participated in the process of lipid metabolism by targeting Sirt1 or FOXO1, which could both increase energy expenditure and the clearance of lipid accumulation [21][28][30][31][35][36]. And ginger extract could enhance AMPK activity and ameliorate obesity and inflammation by regulating miRNAs expressions in high-fat diet (HFD)-fed rats [24].
Based on the present studies, regulating miRNAs is potentially becoming a dominant feature in terms of natural products regulating lipid metabolism (Figure 1). On the one hand, it can inhibit fatty synthesis by reducing fatty acid synthesis and increasing fatty acids mobilization. On the other hand, it can also accelerate lipodieresis by enhancing the oxidation and phosphorylation of fatty acids.

Figure 1. Schematic illustration of the main mechanisms by which natural products improve lipid metabolism disorders mediated by miRNAs. The red arrow means an increase, and the green arrow means a decrease.
2.2. Inhibitory Effects on Adipocyte Differentiation and Accumulation
From the perspective of the cellular level, however, the growth of adipose tissue is the result of an increase in the number of adipocytes and the volume of individual cells [51]. The former contributes to promoting pre-adipocyte differentiation into mature adipocytes, whereas the latter is due to lipid accumulation. The functional role of natural products in this regard, as well as their potential mechanisms of action was summarized (Figure 1 and Table 1 and Table 2).
Adipocyte differentiation is a highly precisely regulated cellular process. Ahead of terminal differentiation, the mitotic clonal expansion (MCE) of stimulated pre-adipocytes is an essential procedure in adipocyte differentiation. Moreover, the transcriptional activation of adipocyte-specific functional genes is closely related to their differentiation [62]. 3T3-L1 pre-adipocytes have long been considered as the “gold standard” for investigating pre-adipocyte differentiation in vitro [63][64]. There has been evidence that the MCE process could be delayed by persimmon tannin by enhancing the expression of miR-27 in 3T3-L1 pre-adipocytes [47]. Furthermore, multiple transcriptional factors, including peroxisome proliferator-activated receptor-gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα) were also attenuated by miR-27, resulting in a decrease in adipocyte-specific genes, such as adipocyte fatty acid binding protein (aP2) and lipoprotein lipase (LPL). Similarly, the MCE process was blocked by miR-27a/b in the study of a-type ECG and EGCG dimers [40]. Lipids are important structural components in cell membranes [65]. Notably, with different molecular structures, a-type ECG and EGCG dimers strongly disturbed the structures of cell membranes by decreasing fluidity and hydrophobicity and increasing the permeability of the membrane of 3T3-L1 pre-adipocyte cells, thus displaying significant inhibition on differentiation [40]. EGCG also suppressed 3T3-L1 cell growth via miR-143/MAPK7 pathways [43]. Nonivamide-induced reduction in lipid accumulation was mediated by transient receptor potential cation channel subfamily V member 1 (TRPV1) activation [45]. Although miRNAs are involved in the adipocyte differentiation process, whether they affect membrane structure remains to be intensively studied in natural product therapy.
The activation of C/EBPα and PPARγ is not only necessary for adipocyte differentiation in the early stage but is also crucial for terminal adipocyte differentiation [66]. The evidence suggests that grape seed procyanidin B2 could inhibit pre-adipocyte differentiation and reduce intracellular lipid accumulation by modulating the miR-483/PPARγ axis [42]. Resveratrol reduced the expression of CEBP/α by boosting miR-155, resulting in decreasing lipogenesis [51]. Consistent with these, as shown in Table 2, similar results were also obtained in the research of lycopene by regulating the expression of miR-21 [44]. What is noteworthy is that accompanied with the involvement of multiple miRNAs, Rosmarinus officinalis extract significantly reduced triglyceride incorporation during pre-adipocyte maturation in a dose-dependent manner and decreased the expression of cell cycle genes, such as cyclin-dependent kinase 4, cyclin D1, and cyclin-dependent kinase inhibitor 1A [39]. The final and most studied phase of adipocyte differentiation involves terminal differentiation and the induction of a signaling cascade to promote the expression of the genes necessary for adipocyte function [67][68]. The canonical Wnt signaling cascade is an effective approach to suppress adipogenesis [69][70][71]. Recently, investigators found that curcumin repressed 3T3-L1 pre-adipocyte cell adipogenic differentiation by inhibiting the expression of miR-17 and stimulating transcription factor 7-like 2 (TCF7L2), which is the Wnt signaling pathway effector and a direct downstream target of miR-17 [41]. And guarana extract also exerted an anti-adipogenic effect by regulating the Wnt signaling pathway, mediated by miRNAs [32]. In summary, natural products may inhibit adipocyte differentiation and accumulation by regulating miRNAs, which play a crucial role in the process of lipogenesis.
References
- Cawley, J.; Wen, K. Policies to Prevent Obesity and Promote Healthier Diets: A Critical Selective Review. Clin. Chem. 2018, 64, 163–172.
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442.
- Kan, J.; Velliquette, R.A.; Grann, K.; Burns, C.R.; Scholten, J.; Tian, F.; Zhang, Q.; Gui, M. A novel botanical formula prevents diabetes by improving insulin resistance. BMC Complement. Altern. Med. 2017, 17, 352.
- Smilowitz, N.R.; Gupta, N.; Guo, Y.; Beckman, J.A.; Bangalore, S.; Berger, J.S. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart 2018, 104, 1180–1186.
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471.
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25.
- Dietrich, M.O.; Horvath, T.L. Limitations in anti-obesity drug development: The critical role of hunger-promoting neurons. Nat. Rev. Drug Discov. 2012, 11, 675–691.
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244.
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220.
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160.
- Shukla, S.K.; Gupta, S.; Ojha, S.K.; Sharma, S.B. Cardiovascular friendly natural products: A promising approach in the management of CVD. Nat. Prod. Res. 2010, 24, 873–898.
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695.
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140.
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T.; et al. A MicroRNA targeting dicer for metastasis control. Cell 2010, 141, 1195–1207.
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297.
- Baselga-Escudero, L.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A.; Blade, C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015, 35, 337–345.
- Zhang, L.; He, S.; Yang, F.; Yu, H.; Xie, W.; Dai, Q.; Zhang, D.; Liu, X.; Zhou, S.; Zhang, K. Hyperoside ameliorates glomerulosclerosis in diabetic nephropathy by downregulating miR-21. Can. J. Physiol. Pharmacol. 2016, 94, 1249–1256.
- Liu, L.; Ning, B.; Cui, J.; Zhang, T.; Chen, Y. miR-29c is implicated in the cardioprotective activity of Panax notoginseng saponins against isoproterenol-induced myocardial fibrogenesis. J. Ethnopharmacol. 2017, 198, 1–4.
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir Peptides Prevent Hyperlipidemia and Obesity in High-Fat-Diet-Induced Obese Rats via Lipid Metabolism Modulation. Mol. Nutr. Food Res. 2017, 62, 1700505.
- Pang, D.; You, L.; Zhou, L.; Li, T.; Zheng, B.; Liu, R.H. Averrhoa carambola free phenolic extract ameliorates nonalcoholic hepatic steatosis by modulating mircoRNA-34a, mircoRNA-33 and AMPK pathways in leptin receptor-deficient db/db mice. Food Funct. 2017, 8, 4496–4507.
- Liu, S.; Chang, X.; Yu, J.; Xu, W. Cerasus humilis Cherry Polyphenol Reduces High-Fat Diet-Induced Obesity in C57BL/6 Mice by Mitigating Fat Deposition, Inflammation, and Oxidation. J. Agric. Food Chem. 2020, 68, 4424–4436.
- Su, D.; Liu, H.; Qi, X.; Dong, L.; Zhang, R.; Zhang, J. Citrus peel flavonoids improve lipid metabolism by inhibiting miR-33 and miR-122 expression in HepG2 cells. Biosci. Biotechnol. Biochem. 2019, 83, 1747–1755.
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133.
- Kim, S.; Lee, M.S.; Jung, S.; Son, H.Y.; Park, S.; Kang, B.; Kim, S.Y.; Kim, I.H.; Kim, C.T.; Kim, Y. Ginger Extract Ameliorates Obesity and Inflammation via Regulating MicroRNA-21/132 Expression and AMPK Activation in White Adipose Tissue. Nutrients 2018, 10, 1567.
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Chronic supplementation of proanthocyanidins reduces postprandial lipemia and liver miR-33a and miR-122 levels in a dose-dependent manner in healthy rats. J. Nutr. Biochem. 2014, 25, 151–156.
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012, 56, 1636–1646.
- Baselga-Escudero, L.; Arola-Arnal, A.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Blade, C. Chronic administration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS ONE 2013, 8, e69817.
- Shi, Y.; Jia, M.; Xu, L.; Fang, Z.; Wu, W.; Zhang, Q.; Chung, P.; Lin, Y.; Wang, S.; Zhang, Y. miR-96 and autophagy are involved in the beneficial effect of grape seed proanthocyanidins against high-fat-diet-induced dyslipidemia in mice. Phytother. Res. 2019, 33, 1222–1232.
- Lu, R.H.; Qin, C.B.; Yang, F.; Zhang, W.Y.; Zhang, Y.R.; Yang, G.K.; Yang, L.P.; Meng, X.L.; Yan, X.; Nie, G.X. Grape seed proanthocyanidin extract ameliorates hepatic lipid accumulation and inflammation in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2020, 46, 1665–1677.
- Torres, L.F.; Cogliati, B.; Otton, R. Green Tea Prevents NAFLD by Modulation of miR-34a and miR-194 Expression in a High-Fat Diet Mouse Model. Oxid. Med. Cell. Longev. 2019, 2019, 4168380.
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179.
- Lima, N.D.S.; Numata, E.P.; Mesquita, L.M.S.; Dias, P.H.; Vilegas, W.; Gambero, A.; Ribeiro, M.L. Modulatory Effects of Guarana (Paullinia cupana) on Adipogenesis. Nutrients 2017, 9, 635.
- Su, D.; Zhang, R.; Hou, F.; Chi, J.; Huang, F.; Yan, S.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, M. Lychee pulp phenolics ameliorate hepatic lipid accumulation by reducing miR-33 and miR-122 expression in mice fed a high-fat diet. Food Funct. 2017, 8, 808–815.
- Lee, S.; Lee, M.S.; Chang, E.; Lee, Y.; Lee, J.; Kim, J.; Kim, C.T.; Kim, I.H.; Kim, Y. Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet. Nutrients 2020, 12, 1499.
- Yang, T.Y.; Yu, M.H.; Wu, Y.L.; Hong, C.C.; Chen, C.S.; Chan, K.C.; Wang, C.J. Mulberry Leaf (Morus alba L.) Extracts and Its Chlorogenic Acid Isomer Component Improve Glucolipotoxicity-Induced Hepatic Lipid Accumulation via Downregulating miR-34a and Decreased Inflammation. Nutrients 2022, 14, 4808.
- Monraz-Méndez, C.A.; Escutia-Gutiérrez, R.; Rodriguez-Sanabria, J.S.; Galicia-Moreno, M.; Monroy-Ramírez, H.C.; Sánchez-Orozco, L.; García-Bañuelos, J.; de la Rosa-Bibiano, R.; Santos, A.; Armendáriz-Borunda, J.; et al. Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications. Nutrients 2022, 14, 4225.
- Qiao, J.Y.; Li, H.W.; Liu, F.G.; Li, Y.C.; Tian, S.; Cao, L.H.; Hu, K.; Wu, X.X.; Miao, M.S. Effects of Portulaca Oleracea Extract on Acute Alcoholic Liver Injury of Rats. Molecules 2019, 24, 2887.
- Jang, S.; Lee, M.S.; Kang, S.A.; Kim, C.T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14, 3330.
- Stefanon, B.; Pomari, E.; Colitti, M. Effects of Rosmarinus officinalis extract on human primary omental preadipocytes and adipocytes. Exp. Biol. Med. 2015, 240, 884–895.
- Zhu, W.; Zou, B.; Nie, R.; Zhang, Y.; Li, C.M. A-type ECG and EGCG dimers disturb the structure of 3T3-L1 cell membrane and strongly inhibit its differentiation by targeting peroxisome proliferator-activated receptor gamma with miR-27 involved mechanism. J. Nutr. Biochem. 2015, 26, 1124–1135.
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017, 8, e2559.
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor gamma with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017, 86, 292–296.
- Chen, C.P.; Su, T.C.; Yang, M.J.; Chen, W.T.; Siao, A.C.; Huang, L.R.; Lin, Y.Y.; Kuo, Y.C.; Chung, J.F.; Cheng, C.F.; et al. Green tea epigallocatechin gallate suppresses 3T3-L1 cell growth via microRNA-143/MAPK7 pathways. Exp. Biol. Med. 2022, 247, 1670–1679.
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 56, 1665–1674.
- Rohm, B.; Holik, A.K.; Kretschy, N.; Somoza, M.M.; Ley, J.P.; Widder, S.; Krammer, G.E.; Marko, D.; Somoza, V. Nonivamide enhances miRNA let-7d expression and decreases adipogenesis PPARgamma expression in 3T3-L1 cells. J. Cell. Biochem. 2015, 116, 1153–1163.
- Chen, S.; Wen, X.; Zhang, W.; Wang, C.; Liu, J.; Liu, C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1beta axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 2017, 31, 1085–1096.
- Zou, B.; Ge, Z.; Zhu, W.; Xu, Z.; Li, C. Persimmon tannin represses 3T3-L1 preadipocyte differentiation via up-regulating expression of miR-27 and down-regulating expression of peroxisome proliferator-activated receptor-gamma in the early phase of adipogenesis. Eur. J. Nutr. 2015, 54, 1333–1343.
- Gai, Y.; Li, Y.; Xu, Z.; Chen, J. Pseudoprotodioscin inhibits SREBPs and microRNA 33a/b levels and reduces the gene expression regarding the synthesis of cholesterol and triglycerides. Fitoterapia 2019, 139, 104393.
- Gracia, A.; Fernandez-Quintela, A.; Miranda, J.; Eseberri, I.; Gonzalez, M.; Portillo, M.P. Are miRNA-103, miRNA-107 and miRNA-122 Involved in the Prevention of Liver Steatosis Induced by Resveratrol? Nutrients 2017, 9, 360.
- Gracia, A.; Miranda, J.; Fernandez-Quintela, A.; Eseberri, I.; Garcia-Lacarte, M.; Milagro, F.I.; Martinez, J.A.; Aguirre, L.; Portillo, M.P. Involvement of miR-539-5p in the inhibition of de novo lipogenesis induced by resveratrol in white adipose tissue. Food Funct. 2016, 7, 1680–1688.
- Eseberri, I.; Lasa, A.; Miranda, J.; Gracia, A.; Portillo, M.P. Potential miRNA involvement in the anti-adipogenic effect of resveratrol and its metabolites. PLoS ONE 2017, 12, e0184875.
- Ahn, J.; Lee, H.; Jung, C.H.; Choi, W.H.; Ha, T.Y. Zerumbone ameliorates high-fat diet-induced adiposity by restoring AMPK-regulated lipogenesis and microRNA-146b/SIRT1-mediated adipogenesis. Oncotarget 2017, 8, 36984–36995.
- Rottiers, V.; Naar, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250.
- Moore, K.J.; Rayner, K.J.; Suarez, Y.; Fernandez-Hernando, C. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annu. Rev. Nutr. 2011, 31, 49–63.
- Qiu, C.P.; Lv, Q.T.; Dongol, S.; Wang, C.; Jiang, J. Single nucleotide polymorphism of SREBF-1 gene associated with an increased risk of endometrial cancer in Chinese women. PLoS ONE 2014, 9, e90491.
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131.
- Nagai, Y.; Yonemitsu, S.; Erion, D.M.; Iwasaki, T.; Stark, R.; Weismann, D.; Dong, J.; Zhang, D.; Jurczak, M.J.; Loffler, M.G.; et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009, 9, 252–264.
- Bennett, M.K.; Lopez, J.M.; Sanchez, H.B.; Osborne, T.F. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J. Biol. Chem. 1995, 270, 25578–25583.
- Sullivan, J.E.; Brocklehurst, K.J.; Marley, A.E.; Carey, F.; Carling, D.; Beri, R.K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994, 353, 33–36.
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62.
- Saha, A.K.; Schwarsin, A.J.; Roduit, R.; Masse, F.; Kaushik, V.; Tornheim, K.; Prentki, M.; Ruderman, N.B. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J. Biol. Chem. 2000, 275, 24279–24283.
- Bengestrate, L.; Virtue, S.; Campbell, M.; Vidal-Puig, A.; Hadaschik, D.; Hahn, P.; Bielke, W. Genome-wide profiling of microRNAs in adipose mesenchymal stem cell differentiation and mouse models of obesity. PLoS ONE 2011, 6, e21305.
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736.
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809.
- Nickels, J.D.; Chatterjee, S.; Stanley, C.B.; Qian, S.; Cheng, X.; Myles, D.A.A.; Standaert, R.F.; Elkins, J.G.; Katsaras, J. The in vivo structure of biological membranes and evidence for lipid domains. PLoS Biol. 2017, 15, e2002214.
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896.
- Price, N.L.; Fernandez-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta 2016, 1861, 2104–2110.
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139.
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953.
- Dogan, A.; Demirci, S.; Apdik, H.; Bayrak, O.F.; Gulluoglu, S.; Tuysuz, E.C.; Gusev, O.; Rizvanov, A.A.; Nikerel, E.; Sahin, F. A new hope for obesity management: Boron inhibits adipogenesis in progenitor cells through the Wnt/beta-catenin pathway. Metabolism 2017, 69, 130–142.
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
670
Revisions:
2 times
(View History)
Update Date:
30 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




