
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dong-Ho Nahm | -- | 2998 | 2023-08-29 05:02:43 | | | |
| 2 | Peter Tang | Meta information modification | 2998 | 2023-08-29 05:15:18 | | | | |
| 3 | Peter Tang | Meta information modification | 2998 | 2023-08-30 11:02:45 | | | | |
| 4 | Peter Tang | Meta information modification | 2998 | 2023-08-31 02:53:11 | | | | |
| 5 | Peter Tang | Meta information modification | 2998 | 2023-08-31 02:56:03 | | | | |
| 6 | Peter Tang | Meta information modification | 2998 | 2023-09-01 03:55:59 | | | | |
| 7 | Peter Tang | -185 word(s) | 2813 | 2023-09-01 03:59:42 | | |
Video Upload Options
Patients with atopic dermatitis (AD) are seeking a permanent cure. This entry provides three working hypotheses and perspectives for the cure of AD by restoring immune homeostasis (immune tolerance state) through activation of regulatory T (Treg) cells as follows. (1) A decreased number or function of Treg cells is a critical event leading to the development and maintenance of AD. (2) Activation of Treg cells is an effective therapeutic approach for long-term clinical improvement of AD. (3) Many different immunomodulatory strategies activating Treg cells can provide a long-term treatment-free clinical remission (cure) of AD by induction of immune tolerance state. Currently available Treg cell-targeted immunomodulatory therapies for AD include allergen immunotherapy, microbiota, vitamin D, polyvalent human immunoglobulin G, and monoclonal antibodies to the surface antigens of T cell or antigen-presenting cell.
1. Introduction
2. Unmet Needs of Patients with AD
3. Hypothesis on the Pathogenesis of AD
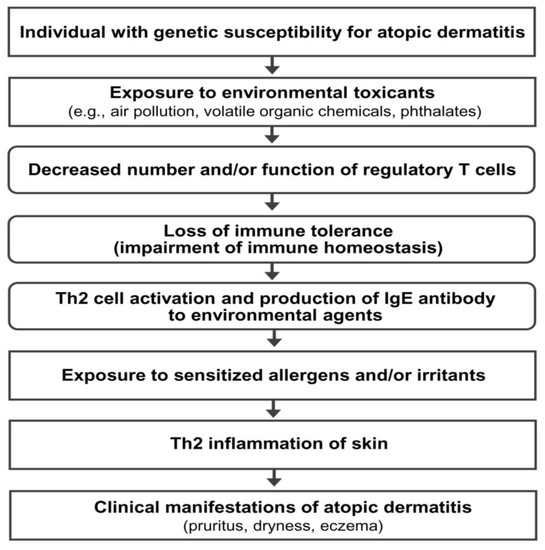
4. Previous Reports on the Long-Term Clinical Improvement of AD
5. Five Questions and Three Hypotheses on the Regulatory T Cell-Targeted Immunomodulatory Strategies to Achieve a Long-Term Clinical Improvement of AD
|
1. What is the mechanism of natural induction of a long-term clinical improvement of atopic dermatitis in children? 2. Is the induction of immune tolerance responsible for a natural long-term clinical improvement of atopic dermatitis in children? 3. Is the activation of type 1 regulatory T cells a key mechanism inducing a natural long-term clinical improvement of atopic dermatitis? 4. Is the activation of type 1 regulatory T cells a common mechanism inducing a long-term clinical improvement of atopic dermatitis by allergen immunotherapy and a natural long-term clinical improvement in children with atopic dermatitis? 5. Can various kinds of immunomodulatory strategies activating regulatory T cells provide a long-term clinical improvement in patients with atopic dermatitis? |
|
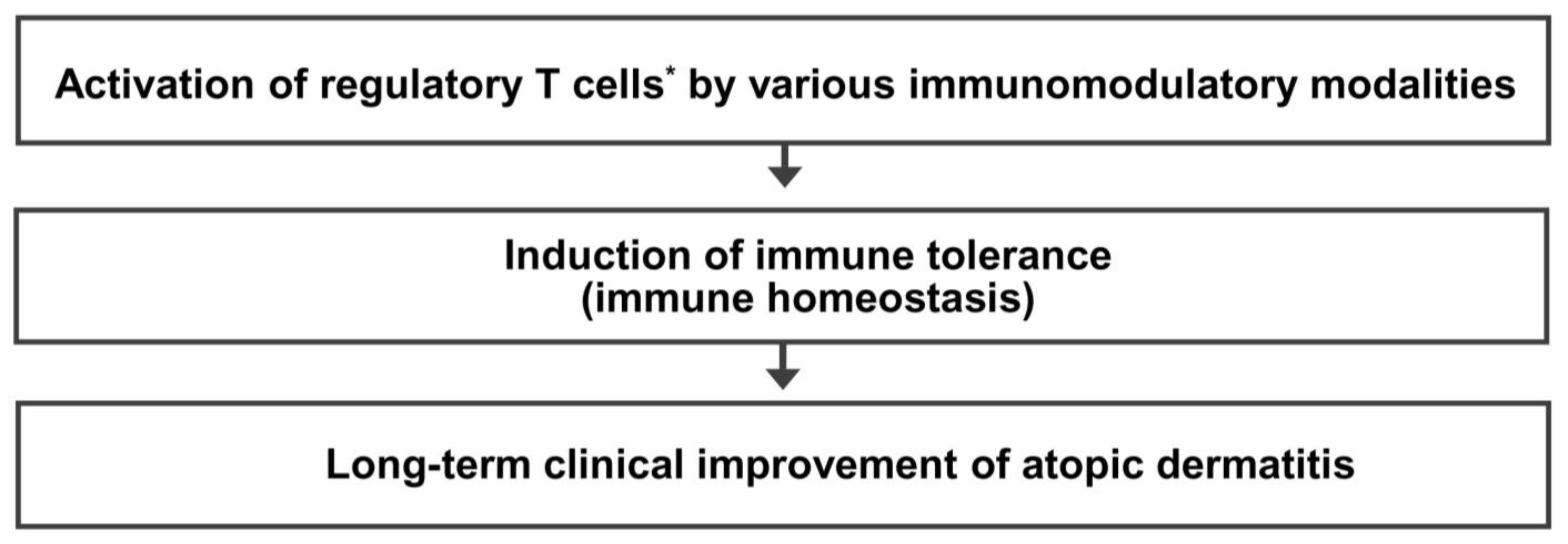
1. A decreased number or function of regulatory T cells is a critical event that causes the activation of Th2 cells, leading to the development and maintenance of atopic dermatitis. 2. Activation of regulatory T cells * is an effective therapeutic approach to achieve a long-term clinical improvement of atopic dermatitis. 3. Many different immunomodulatory strategies activating regulatory T cells can provide a long-term clinical improvement of atopic dermatitis by induction of immune tolerance. |
* Activation of regulatory T cells means an increase in the number and/or function of regulatory T cells. Th2 cells, T-helper type 2 cells.
6. Mechanism of Immune Tolerance and Rationale of Regulatory T Cell-Targeted Immunomodulatory Therapy for AD
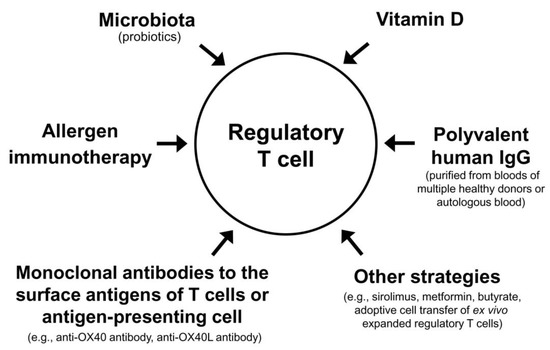
7. Immunomodulatory Strategies Activating Regulatory T Cells for AD: In Vivo Activation
|
Strategies with proven clinical efficacy by at least one randomized clinical trial |
|
(1) Allergen immunotherapy (2) Microbial therapy (probiotics) (3) Vitamin D (4) Subcutaneous or intramuscular injection of polyvalent human IgG from multiple healthy blood donors (5) Intramuscular injection of autologous total IgG (6) Monoclonal antibody to antigen on the surface of T cells (anti-OX40 antibody) |
|
Strategies without proven clinical efficacy in patients with atopic dermatitis by a clinical trial |
|
(1) Sirolimus (also called rapamycin) (2) Metformin (3) Butyrate (4) Adoptive cell therapy with ex vivo expanded regulatory T cells |
IgG, immunoglobulin G.
8. Combinations of Different Modalities Activating Regulatory T Cells
9. Conclusions
References
- Wollenberg, A.; Christen-Zach, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744.
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065.
- Nahm, D.H. Personalized Immunomodulatory Therapy for Atopic Dermatitis: An Allergist’s View. Ann. Dermatol. 2015, 27, 355–363.
- Wang, F.P.; Tang, X.J.; Wei, C.Q.; Xu, L.R.; Mao, H.; Luo, F.M. Dupilumab treatment in moderate-to-severe atopic dermatitis: A systematic review and meta-analysis. J. Dermatol. Sci. 2018, 90, 190–198.
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348.
- Li, C.; Sun, X.; Zhao, K.; Meng, F.; Li, L.; Mu, Z.; Han, X. Efficacy and Safety of Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis. Dermatology 2022, 238, 725–735.
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaci, D.; Chu, C.Y.; Hong, H.C.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021, 397, 2151–2168.
- Verhagen, J.; Akdis, M.; Traidl-Hoffmann, C.; Schmid-Grendelmeier, P.; Hijnen, D.; Knol, E.F.; Behrendt, H.; Blaser, K.; Akdis, C.A. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J. Allergy Clin. Immunol. 2006, 117, 176–183.
- Fyhrquist, N.; Lehtimaki, S.; Lahl, K.; Savinko, T.; Lappetelainen, A.M.; Sparwasser, T.; Wolff, H.; Lauerma, A.; Alenius, H. Foxp3+ cells control Th2 responses in a murine model of atopic dermatitis. J. Investig. Dermatol. 2012, 132, 1672–1680.
- Vieira, B.L.; Lim, N.R.; Lohman, M.E.; Lio, P.A. Complementary and Alternative Medicine for Atopic Dermatitis: An Evidence-Based Review. Am. J. Clin. Dermatol. 2016, 17, 557–581.
- Genuis, S.J. Sensitivity-related illness: The escalating pandemic of allergy, food intolerance and chemical sensitivity. Sci. Total Env. 2010, 408, 6047–6061.
- Lee, J.H.; Lee, H.S.; Park, M.R.; Lee, S.W.; Kim, E.H.; Cho, J.B.; Kim, J.; Han, Y.; Jung, K.; Cheong, H.K. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol. Res. 2014, 6, 517–524.
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999; discussion 1000.
- Kim, E.H.; Jeon, B.H.; Kim, J.; Kim, Y.M.; Han, Y.; Ahn, K.; Cheong, H.K. Exposure to phthalates and bisphenol A are associated with atopic dermatitis symptoms in children: A time-series analysis. Environ. Health 2017, 16, 24.
- Byremo, G.; Rod, G.; Carlsen, K.H. Effect of climatic change in children with atopic eczema. Allergy 2006, 61, 1403–1410.
- Vahavihu, K.; Ylianttila, L.; Salmelin, R.; Lamberg-Allardt, C.; Viljakainen, H.; Tuohimaa, P.; Reunala, T.; Snellman, E. Heliotherapy improves vitamin D balance and atopic dermatitis. Br. J. Dermatol. 2008, 158, 1323–1328.
- Lee, J.; Lee, H.; Noh, S.; Bae, B.G.; Shin, J.U.; Park, C.O.; Lee, K.H. Retrospective Analysis on the Effects of House Dust Mite Specific Immunotherapy for More Than 3 Years in Atopic Dermatitis. Yonsei Med. J. 2016, 57, 393–398.
- Chait, I.; Allkins, V. Remission of life-long atopic dermatitis after hyposensitisation to house dust mite. Practitioner 1985, 229, 609–612.
- Leroy, B.P.; Lachapelle, J.-M.; Somville, M.; Jacquemin, M.; Saint-Remy, J. Injection of allergen-antibody complexes is an effective treatment of atopic dermatitis. Dermatology 1991, 182, 98–106.
- Tuft, L. Studies in atopic dermatitis. V. Problems in inhalant hyposensitization and results of treatment. J. Allergy 1960, 31, 1–11.
- Hua, T.C.; Hwang, C.Y.; Chen, Y.J.; Chu, S.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; Liu, H.N. The natural course of early-onset atopic dermatitis in Taiwan: A population-based cohort study. Br. J. Dermatol. 2014, 170, 130–135.
- Sykes, M. Immune tolerance: Mechanisms and application in clinical transplantation. J. Intern. Med. 2007, 262, 288–310.
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787.
- Sharkey, P.; Thomas, R. Immune tolerance therapies for autoimmune diseases: Shifting the goalpost to cure. Curr. Opin. Pharmacol. 2022, 65, 102242.
- Akdis, M.; Akdis, C.A. Therapeutic manipulation of immune tolerance in allergic disease. Nat. Rev. Drug Discov. 2009, 8, 645–660.
- Ray, S.K.; Putterman, C.; Diamond, B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: A paradigm for autoimmune disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2019–2024.
- Mohamed Khosroshahi, L.; Rezaei, N. Dysregulation of the immune response in coronavirus disease 2019. Cell Biol. Int. 2021, 45, 702–707.
- O’Garra, A.; Vieira, P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004, 10, 801–805.
- Miyara, M.; Sakaguchi, S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol. Med. 2007, 13, 108–116.
- Miyara, M.; Wing, K.; Sakaguchi, S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J. Allergy Clin. Immunol. 2009, 123, 749–755; quiz 756–757.
- Schmitt, E.G.; Williams, C.B. Generation and function of induced regulatory T cells. Front. Immunol. 2013, 4, 152.
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566.
- Ferreira, L.M.R.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019, 18, 749–769.
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336.
- Riemann, M.; Andreas, N.; Fedoseeva, M.; Meier, E.; Weih, D.; Freytag, H.; Schmidt-Ullrich, R.; Klein, U.; Wang, Z.-Q.; Weih, F. Central immune tolerance depends on crosstalk between the classical and alternative NF-κB pathways in medullary thymic epithelial cells. J. Autoimmun. 2017, 81, 56–67.
- Xing, Y.; Hogquist, K.A. T-cell tolerance: Central and peripheral. Cold Spring Harb. Perspect. Biol. 2012, 4, a006957.
- Akdis, C.A.; Akdis, M.; Blesken, T.; Wymann, D.; Alkan, S.S.; Muller, U.; Blaser, K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J. Clin. Investig. 1996, 98, 1676–1683.
- Akdis, C.A.; Blesken, T.; Akdis, M.; Wuthrich, B.; Blaser, K. Role of interleukin 10 in specific immunotherapy. J. Clin. Investig. 1998, 102, 98–106.
- Francis, J.N.; Till, S.J.; Durham, S.R. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 111, 1255–1261.
- Jutel, M.; Akdis, M.; Budak, F.; Aebischer-Casaulta, C.; Wrzyszcz, M.; Blaser, K.; Akdis, C.A. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 2003, 33, 1205–1214.
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 2006, 18, 738–744.
- Akdis, M.; Verhagen, J.; Taylor, A.; Karamloo, F.; Karagiannidis, C.; Crameri, R.; Thunberg, S.; Deniz, G.; Valenta, R.; Fiebig, H. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004, 199, 1567–1575.
- Vaseghi-Shanjani, M.; Smith, K.L.; Sara, R.J.; Modi, B.P.; Branch, A.; Sharma, M.; Lu, H.Y.; James, E.L.; Hildebrand, K.J.; Biggs, C.M.; et al. Inborn errors of immunity manifesting as atopic disorders. J. Allergy Clin. Immunol. 2021, 148, 1130–1139.
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507.
- Halabi-Tawil, M.; Ruemmele, F.M.; Fraitag, S.; Rieux-Laucat, F.; Neven, B.; Brousse, N.; De Prost, Y.; Fischer, A.; Goulet, O.; Bodemer, C. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br. J. Dermatol. 2009, 160, 645–651.
- Adriani, M.; Jones, K.; Kirby, M.; Anderson, S.; Silvin, C.; Wincowitch, S.; Candotti, F. Defects of Regulatory T Cell function In The Wiskott-Aldrich Syndrome. (49.25). J. Immunol. 2010, 184, 49.25.
- Saurat, J.H. Eczema in primary immune-deficiencies. Clues to the pathogenesis of atopic dermatitis with special reference to the Wiskott-Aldrich syndrome. Acta Derm. -Venereol. Suppl. 1985, 114, 125–128.
- Hesse, L.; van Ieperen, N.; Petersen, A.H.; Elberink, J.; van Oosterhout, A.J.M.; Nawijn, M.C. High dose vitamin D(3) empowers effects of subcutaneous immunotherapy in a grass pollen-driven mouse model of asthma. Sci. Rep. 2020, 10, 20876.
- Jerzynska, J.; Stelmach, W.; Rychlik, B.; Lechanska, J.; Podlecka, D.; Stelmach, I. The clinical effect of vitamin D supplementation combined with grass-specific sublingual immunotherapy in children with allergic rhinitis. Allergy Asthma Proc. 2016, 37, 105–114.
- Chiewchalermsri, C.; Sangkanjanavanich, S.; Pradubpongsa, P.; Mitthamsiri, W.; Jaisupa, N.; Jindarat, S.; Buranapraditkun, S.; Jacquet, A.; Sangasapaviliya, A.; Boonpiyathad, T. Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin D Supplementation in the Build-up Phase of House Dust Mite-Specific Immunotherapy. Allergy Asthma Immunol. Res. 2023, 15, 336–347.
- Leroy, B.P.; Boden, G.; Lachapelle, J.M.; Jacquemin, M.G.; Saint-Remy, J.M. A novel therapy for atopic dermatitis with allergen-antibody complexes: A double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 1993, 28, 232–239.




