Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tânia Gonçalves Albuquerque | -- | 1751 | 2023-08-28 17:45:16 | | | |
| 2 | Wendy Huang | Meta information modification | 1751 | 2023-08-29 08:46:22 | | | | |
| 3 | Wendy Huang | Meta information modification | 1751 | 2023-08-31 08:41:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Salgado, N.; Silva, M.A.; Figueira, M.E.; Costa, H.S.; Albuquerque, T.G. Health Implications of Oxalates. Encyclopedia. Available online: https://encyclopedia.pub/entry/48550 (accessed on 07 February 2026).
Salgado N, Silva MA, Figueira ME, Costa HS, Albuquerque TG. Health Implications of Oxalates. Encyclopedia. Available at: https://encyclopedia.pub/entry/48550. Accessed February 07, 2026.
Salgado, Neuza, Mafalda Alexandra Silva, Maria Eduardo Figueira, Helena S. Costa, Tânia Gonçalves Albuquerque. "Health Implications of Oxalates" Encyclopedia, https://encyclopedia.pub/entry/48550 (accessed February 07, 2026).
Salgado, N., Silva, M.A., Figueira, M.E., Costa, H.S., & Albuquerque, T.G. (2023, August 28). Health Implications of Oxalates. In Encyclopedia. https://encyclopedia.pub/entry/48550
Salgado, Neuza, et al. "Health Implications of Oxalates." Encyclopedia. Web. 28 August, 2023.
Copy Citation
Oxalate is an antinutrient present in a wide range of foods, with plant products, especially green leafy vegetables, being the main sources of dietary oxalates. This compound has been largely associated with hyperoxaluria, kidney stone formation, and, in more severe cases, systematic oxalosis. Due to its impact on human health, it is extremely important to control the amount of oxalate present in foods, particularly for patients with kidney stone issues.
foods
antinutrient
soluble oxalates
kidney stone formation
hyperoxaluria
gut microbiota
1. Introduction
Oxalate, or oxalic acid, is an antinutrient present, commonly in trace amounts, in fruits, nuts, cereals, fungi, vegetables, aromatic plants, and beverages, with plant-based products being the main sources of dietary oxalates [1]. However, some plants have high quantities of these compounds. In this matter, green leafy vegetables, such as spinach, Swiss chard, and rhubarb, are highlighted [2][3].
Oxalate can form soluble and insoluble salts in water. When binding with sodium, potassium, and ammonium ions, it forms soluble oxalates, whereas with calcium, iron, and magnesium it precipitates, forming insoluble compounds and making these minerals unavailable for absorption. Despite this fact, for example, zinc absorption and metabolism do not appear to be affected. In general, insoluble salts in water can be freely dissolved in acid [4][5][6]. Regarding health, one of the most important insoluble salts is calcium oxalate, having two hydration forms, monohydrates and dihydrates, which impact the shape of its crystals [1].
Depending on the pH of the cell sap, the liquid inside the vacuole of plants where oxalates are mostly found, they can present different chemical structures (Figure 1). On the one hand, when pH is 2, acid oxalate is the main oxalate. On the other hand, when pH is approximately 6, oxalate ion is the majority [5]. At the cytoplasmic pH of 7, oxalic acid also suffers deprotonation and exists as oxalate ion [7].
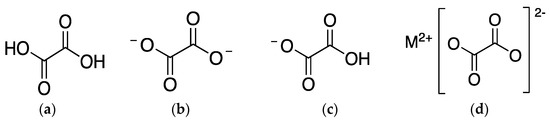
Figure 1. Chemical structure of (a) oxalic acid; (b) oxalate ion; (c) acid oxalate ion; and (d) oxalate salt, being M2+ a metallic cation.
Chemically, oxalic acid is characterized as a dicarboxylic organic acid with low molecular weight, high acidity (pKa1 = 1.25, pKa2 = 4.27), and chelating and reducing abilities. Therefore, in plants, it plays a relevant role in many biological processes such as calcium homeostasis; pH regulation; plant growth, development, and protection; photosynthesis; and detoxification of heavy metals [2][7]. However, when in excess in plants because of a metabolic disorder, this will promote impairment of its functions and, thus, reduction of crop quality [7]. Many factors can influence oxalate accumulation in plants, such as growth, ripeness, variety, season, time of harvest, and cultivation conditions (e.g., use of nitrate fertilizer or soil conditions) [5][8][9][10].
The biosynthesis of oxalate in plants can result from different mechanisms, with glyoxylate, ascorbic acid, and oxaloacetate being the precursors of oxalate, an end product of their metabolisms. Therefore, there are three most-studied pathways: the glycolic acid/glyoxalic acid pathway, the ascorbic acid pathway, and the oxaloacetic acid pathway [5][7].
In addition to photosynthetic organisms, mammals can also produce oxalates in small amounts. In mammals, oxalate produced endogenously is a metabolite of ascorbate, hydroxyproline, glyoxylate, and glycine [2].
2. Antinutritive Effects and Potential Nephrotoxicity of Oxalates
Oxalate has been a concern for human health due to its antinutritive effects and potential nephrotoxicity for a long time [11][12]. In 1989, a fatality from oxalic acid poisoning was reported. A 53-year-old man, with other conditions, had eaten a sorrel soup with 6–8 g of oxalic acid [13]. A lethal dose of oxalic acid for adults was estimated as 10–15 g, although the ingestion of 4–5 g of oxalate was considered the minimum dose able to cause death [5][13]. As antinutrients, oxalates restrict the bioavailability of some nutrients since they can bind to minerals, like calcium, magnesium, or iron, reducing their absorption and use [2][4].
The sources of oxalates in our body can be exogenous or endogenous (Figure 2). Exogenous oxalate sources are mainly plant foods, like vegetables, grains, legumes, and fruits, among others. When these types of foods are ingested, oxalate is absorbed in multiple parts of our gastrointestinal tract, namely the stomach, small intestine, and large intestine. However, the absorption depends on its availability, among other individual features. Insoluble oxalates are excreted in feces since they are less bioavailable and, therefore, pose a lower health risk. In contrast, soluble oxalates are absorbed through the intestines and colon (5–10% of ingested oxalate, under normal conditions), going into the bloodstream.
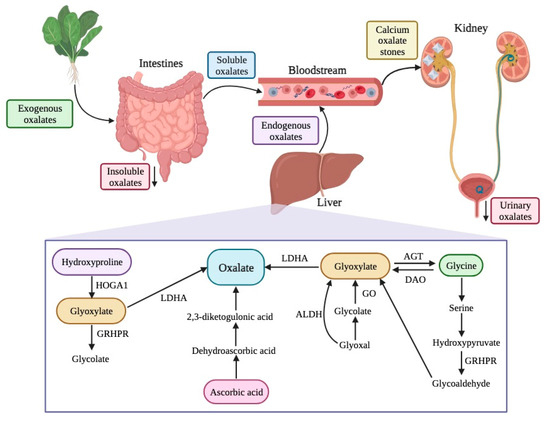
Figure 2. Sources, endogenous pathways, and excretion of oxalates in the human body. HOGA1—4-hydroxy-2-oxoglutarate aldolase type 1; GRHPR—glyoxylate reductase/hydroxypyruvate reductase; LDHA—lactate dehydrogenase A; ALDH—aldehyde dehydrogenase; GO—glycolate oxidase; AGT—alanine/glyoxylate aminotransferase; DAO—D-amino acid oxidase.
Since absorption of oxalates is related to the amount of soluble oxalates, which are more bioavailable, a simultaneous consumption of oxalate with calcium or magnesium can reduce its bioavailability and absorption due to the formation and fecal excretion of insoluble salts, lowering the health risk [1][6][10][14][15]. It has been reported that men with less than 755 mg/day of calcium intake had a higher risk of kidney stone formation, whereas men with a median calcium intake or above had a lower risk [2]. Therefore, dietary calcium intake has been inversely associated with kidney stone formation [2][6][16][17].
Also, it has been observed that intestinal absorption of oxalates in individuals with a history of stone formation was expressly higher than in healthy individuals (9.2% and 6.7%, respectively) [1]. Gastrointestinal health influences oxalate absorption as well, with soluble oxalate being excessively absorbed due to intestinal malfunction.
Despite these facts, oxalate is not typically consumed daily in high concentrations and there are other constituents in foods which have a protective role against kidney stone formation, such as phytate, potassium, calcium, and antioxidant phytochemicals like polyphenols [2]. Also, boiling, steaming, soaking, and processing with calcium sources are some procedures to reduce the content of soluble oxalates, the most harmful oxalates [6][14].
Concerning the endogenous production of oxalates, the liver is the primary source. There are different pathways for oxalate production, including the metabolism of protein (through amino acids, like tyrosine, tryptophan, phenylalanine, and hydroxyproline), ascorbic acid, and precursors of oxalate (such as L-glycerate glycollate and glyoxylate) [18][19]. Glyoxylate is an important intermediary product in several reactions and, for its metabolization into oxalate, enzymes like glycolate oxidase and lactate dehydrogenase are needed.
Free oxalates are delivered to the kidney and can be excreted, increasing urinary oxalates, or can chelate with calcium ions there, resulting in calcium oxalate crystals, which can cause serious health issues like kidney stones, also known as nephrolithiasis or urolithiasis (Figure 2) [2][10][14][17][20][21].
This crystallization in the kidney infiltrates vessel walls and can lead to renal tubular obstruction, vascular necrosis, and hemorrhage, which can cause anuria, uremia, electrolyte disturbances, or even rupture and kidney failure [5][21]. Calcium oxalate and its relationship with kidney stone formation have been amply studied, with calcium oxalate being one of the most common types of human kidney stone reported, followed by calcium phosphate [5][16][20][22][23].
Hyperoxaluria is a metabolic disease that leads to excessive urinary oxalate excretion (>40–45 mg/day) [15][24], being an indicator of possible kidney stone formation [17]. The most reliable way to assess daily oxalate intake is through 24 h urine collection; however, there are also food frequency questionnaires whose credibility is debated [15][17].
Hyperoxaluria can be divided into primary hyperoxaluria (PH1) and secondary hyperoxaluria (PH2). PH1 is a group of rare autosomal recessive diseases that negatively affect key enzymes of oxalate metabolism, leading to an overproduction of oxalates in the liver [25]. When renal function declines and excess oxalate exists in the bloodstream, a phenomenon known as systemic oxalosis occurs and calcium oxalate crystals deposit in various organs, tissues, and bones [24][25][26]. Severe damage in the eyes, joints [27], myocardium, skin [28], oral tissues [24], and bone marrow [29] is reported. Oxalate can also be associated with acute kidney injury, a tubular obstruction due to calcium oxalate crystal deposition, and with chronic kidney disease progression, but further studies are necessary [15].
Patients with hyperoxaluria, especially PH1, from a clinical point of view, frequently present severe bone pain, pathological fractures, and bone deformations. This is frequently associated with the fact that calcium oxalate crystals may deposit within bones, tendons, cartilage, and synovium, causing oxalate arthritis. Then, the calcium oxalate crystals may enter into the synovial fluid, where an inflammatory response will arise, leading to joint effusions and arthralgias [30][31].
PH2 results from increased intestinal absorption of dietary oxalates and can also lead to excessive urinary oxalate [5][21][32]. A high intake of foods rich in oxalate, enteric hyperoxaluria, oxalate-degrading mechanisms, and SLC4 and SLC26 ionic exchangers are linked with PH2. Dietary oxalate plays an important role in PH2, contributing up to 72% of the urinary oxalate excreted [33]. Enteric hyperoxaluria is a form of PH2 that is linked with malabsorption syndromes due to disease or resection of the gastrointestinal tract. In foods, oxalate is usually complexed with calcium, resulting in insoluble oxalate, which is difficult to absorb. Nevertheless, in fat malabsorption conditions, the amount of free oxalates can increase, due to the capacity of free fats to bind calcium. Therefore, PH2 is linked with several conditions that cause fat malabsorption, such as inflammatory bowel disease, celiac disease, short bowel syndrome, and bariatric surgery, among others [30].
The gut microbiome plays an important role since some bacterial species can degrade oxalate to obtain carbon and energy and therefore reduce the concentration of oxalates in blood and urine, minimizing the formation of kidney stones [17][18][19][34][35].
The gut microbiota is usually similar between individuals; however, it can be affected by the age of individuals, by the diet, and by the use of antibiotics, among other factors. Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” and are being abundantly used as preventive therapeutic agents for several diseases, since they have been implicated in the stabilization of gut microbiota and enhancement of immune responses [36].
The best-known oxalate-degrading microorganism is Oxalobacter formigenes, but others are also able to degrade oxalate into carbon dioxide and formate, namely Escherichia coli, Bifidobacterium spp., and Lactobacillus spp. [19][35]. O. formigenes is a Gram-negative anaerobic bacterium isolated from human feces and other animals that utilizes intestinal oxalate as a carbon source, through formyl-CoA transferase and oxalyl-CoA decarboxylase enzymes, and metabolizes the oxalate into carbon dioxide and formate, contributing to regulating oxalate homeostasis [34]. However, its application as a probiotic is limited due to its nutritious requirements, but also because it has less colonization ability and is sensitive to the use of certain antibiotics and drugs [37]. Moreover, the therapeutic use of O. formigenes can be compromised, for example, in patients with PH1 and patients with cystic fibrosis. To date, the best conditions (pH, sugar concentration), as well as the adequate amount of these supplements, are not clear and more research is still needed [19].
References
- Hönow, R.; Hesse, A. Comparison of Extraction Methods for the Determination of Soluble and Total Oxalate in Foods by HPLC-Enzyme-Reactor. Food Chem. 2002, 78, 511–521.
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929.
- Savage, G.P.; Vanhanen, L.; Mason, S.M.; Ross, A.B. Effect of Cooking on the Soluble and Insoluble Oxalate Content of Some New Zealand Foods. J. Food Compos. Anal. 2000, 13, 201–206.
- Natesh, H.N.; Abbey, L.; Asiedu, S.K. An Overview of Nutritional and Anti Nutritional Factors in Green Leafy Vegetables. Hortic. Int. J. 2017, 1, 58–65.
- Noonan, S.C.; Savage, G.P. Oxalate Content of Foods and Its Effect on Humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74.
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89, 104938.
- Li, P.; Liu, C.; Luo, Y.; Shi, H.; Li, Q.; Pinchu, C.; Li, X.; Yang, J.; Fan, W. Oxalate in Plants: Metabolism, Function, Regulation, and Application. J. Agric. Food Chem. 2022, 70, 16037–16049.
- Akhtar, M.S.; Israr, B.; Bhatty, N.; Ali, A. Effect of Cooking on Soluble and Insoluble Oxalate Contents in Selected Pakistani Vegetables and Beans. Int. J. Food Prop. 2011, 14, 241–249.
- Liu, X.X.; Zhou, K.; Hu, Y.; Jin, R.; Lu, L.L.; Jin, C.W.; Lin, X.Y. Oxalate Synthesis in Leaves Is Associated with Root Uptake of Nitrate and Its Assimilation in Spinach (Spinacia oleracea L.) Plants. J. Sci. Food Agric. 2014, 95, 2105–2116.
- Massey, L.K. Food Oxalate: Factors Affecting Measurement, Biological Variation, and Bioavailability. J. Am. Diet. Assoc. 2007, 107, 1191–1194.
- Fatoki, O.S. Determination of Oxalic Acid in Vegetables. In Modern Methods of Plant Analysis: Vegetables and Vegetable Products; Springer Berlin: Heidelberg, Germany, 1994; Volume 16, pp. 161–167.
- Singh, P.P.; Kothari, L.K.; Sharma, D.C.; Saxena, S.N. Nutritional Value of Foods in Relation to Their Oxalic Acid Content. Am. J. Clin. Nutr. 1972, 25, 1147–1152.
- Farré, M.; Xirgu, J.; Salgado, A.; Peracaula, R.; Reig, R.; Sanz, P. Fatal Oxalic Acid Poisoning from Sorrel Soup. Lancet. 1989, 334, 1524.
- Huynh, N.K.; Nguyen, D.H.M.; Nguyen, H.V.H. Effects of Processing on Oxalate Contents in Plant Foods: A Review. J. Food Compos. Anal. 2022, 112, 104685.
- Bargagli, M.; Tio, M.C.; Waikar, S.S.; Ferraro, P.M. Dietary Oxalate Intake and Kidney Outcomes. Nutrients 2020, 12, 2673.
- Taylor, E.N.; Curhan, G.C. Oxalate Intake and the Risk for Nephrolithiasis. J. Am. Soc. Nephrol. 2007, 18, 2198–2204.
- Mitchell, T.; Kumar, P.; Reddy, T.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Dietary Oxalate and Kidney Stone Formation. Am. J. Physiol.-Ren. Physiol. 2019, 316, 409–413.
- Huang, Y.; Zhang, Y.H.; Chi, Z.P.; Huang, R.; Huang, H.; Liu, G.Y.; Zhang, Y.F.; Yang, H.S.; Lin, J.H.; Yang, T.H.; et al. The Handling of Oxalate in the Body and the Origin of Oxalate in Calcium Oxalate Stones. Urol. Int. 2020, 104, 167–176.
- Karamad, D.; Khosravi-Darani, K.; Khaneghah, A.M.; Miller, A.W. Probiotic Oxalate-Degrading Bacteria: New Insight of Environmental Variables and Expression of the Oxc and Frc Genes on Oxalate Degradation Activity. Foods 2022, 11, 2876.
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent Advances on the Mechanisms of Kidney Stone Formation (Review). Int. J. Mol. Med. 2021, 48, 149.
- Witting, C.; Langman, C.B.; Assimos, D.; Baum, M.A.; Kausz, A.; Milliner, D.; Tasian, G.; Worcester, E.; Allain, M.; West, M.; et al. Pathophysiology and Treatment of Enteric Hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2021, 16, 487–495.
- Wang, B.; Wu, B.; Liu, J.; Yao, W.; Xia, D.; Li, L.; Chen, Z.; Ye, Z.; Yu, X. Analysis of Altered MicroRNA Expression Profiles in Proximal Renal Tubular Cells in Response to Calcium Oxalate Monohydrate Crystal Adhesion: Implications for Kidney Stone Disease. PLoS ONE 2014, 9, e0101306.
- Ryall, R.L.; Fleming, D.E.; Doyle, I.R.; Evans, N.A.; Dean, C.J.; Marshall, V.R. Intracrystalline Proteins and the Hidden Ultrastructure of Calcium Oxalate Urinary Crystals: Implications for Kidney Stone Formation. J. Struct. Biol. 2001, 134, 5–14.
- Panis, V.; Tosios, K.I.; Gagari, E.; Griffin, T.J.; Damoulis, P.D. Severe Periodontitis in a Patient With Hyperoxaluria and Oxalosis: A Case Report and Review of the Literature. J. Periodontol. 2010, 81, 1497–1504.
- Fogo, A.B.; Lusco, M.A.; Najafian, B.; Alpers, C.E. AJKD Atlas of Renal Pathology: Oxalosis. Am. J. Kidney Dis. 2017, 69, e13–e14.
- Bacchetta, J.; Farlay, D.; Abelin-Genevois, K.; Lebourg, L.; Cochat, P.; Boivin, G. Bone Impairment in Oxalosis: An Ultrastructural Bone Analysis. Bone 2015, 81, 161–167.
- D’Costa, M.R.; Winkler, N.S.; Milliner, D.S.; Norby, S.M.; Hickson, L.T.J.; Lieske, J.C. Oxalosis Associated With High-Dose Vitamin C Ingestion in a Peritoneal Dialysis Patient. Am. J. Kidney Dis. 2019, 74, 417–420.
- Blackmon, J.A.; Jeffy, B.G.; Malone, J.C.; Knable, A.L., Jr. Oxalosis Involving the Skin: Case Report and Literature Review. Arch. Dermatol. 2011, 147, 1302–1305.
- Sharma, S.; Rao, R.N.; Pani, K.C.; Paul, P. Bone Marrow Oxalosis: An Unusual Cause of Cytopenia in End-Stage Renal Disease; Report of Two Cases. Indian J. Pathol. Microbiol. 2018, 61, 268–270.
- Lorenz, E.C.; Michet, C.J.; Milliner, D.S.; Lieske, J.C. Update on Oxalate Crystal Disease. Curr. Rheumatol. Rep. 2013, 15, 340.
- Bacchetta, J.; Boivin, G.; Cochat, P. Bone Impairment in Primary Hyperoxaluria: A Review. Pediatr. Nephrol. 2016, 31, 1–6.
- Bhasin, B. Primary and Secondary Hyperoxaluria: Understanding the Enigma. World J. Nephrol. 2015, 4, 235–244.
- Holmes, R.P.; Kennedy, M. Estimation of the Oxalate Content of Foods and Daily Oxalate Intake. Kidney Int. 2000, 57, 1662–1667.
- Soliman, N.R.; Effat, B.A.M.; Mehanna, N.S.; Tawfik, N.F.; Ibrahim, M.K. Activity of Probiotics from Food Origin for Oxalate Degradation. Arch. Microbiol. 2021, 203, 5017–5028.
- Ermer, T.; Nazzal, L.; Tio, M.C.; Waikar, S.; Aronson, P.S.; Knauf, F. Oxalate Homeostasis. Nat. Rev. Nephrol. 2023, 19, 123–138.
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006.
- Gomathi, S.; Sasikumar, P.; Anbazhagan, K.; Sasikumar, S.; Kavitha, M.; Selvi, M.S.; Selvam, G.S. Screening of Indigenous Oxalate Degrading Lactic Acid Bacteria from Human Faeces and South Indian Fermented Foods: Assessment of Probiotic Potential. Sci. World J. 2014, 2014, 648059.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
31 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




