Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Kleszcz | -- | 1877 | 2023-08-28 13:37:10 | | | |
| 2 | Dean Liu | -6 word(s) | 1871 | 2023-08-29 02:28:46 | | | | |
| 3 | Dean Liu | Meta information modification | 1871 | 2023-09-06 03:41:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kleszcz, R. Molecularly Targeted HNSCC Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/48538 (accessed on 07 February 2026).
Kleszcz R. Molecularly Targeted HNSCC Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/48538. Accessed February 07, 2026.
Kleszcz, Robert. "Molecularly Targeted HNSCC Therapy" Encyclopedia, https://encyclopedia.pub/entry/48538 (accessed February 07, 2026).
Kleszcz, R. (2023, August 28). Molecularly Targeted HNSCC Therapy. In Encyclopedia. https://encyclopedia.pub/entry/48538
Kleszcz, Robert. "Molecularly Targeted HNSCC Therapy." Encyclopedia. Web. 28 August, 2023.
Copy Citation
Head and Neck Squamous Cell Carcinoma (HNSCC) is a major threat to public health around the world. Its occurrence is linked to genetic events and environmental factors, including Human Papilloma Virus (HPV) infections. Patients with HPV-positive tumors usually have a better prognosis than those with HPV-negative tumors. According to advances in understanding the molecular basis of HNSCC tumors, targeted therapy is thought to improve treatment outcomes.
head and neck cancer
molecular targets
chemotherapy
combinatorial therapy
HPV
EGFR
PI3K
1. Epidermal Growth Factor Receptor (EGFR) Pathway
The most important member of the receptor tyrosine kinases (RTK) family is the epidermal growth factor receptor (EGFR). Cetuximab, a chimeric IgG1 monoclonal antibody against EGFR, was first approved by the U.S. Food and Drug Administration (FDA) in 2004 for patients with irinotecan-resistant colorectal cancer [1], and two years later was authorized for the treatment of locally advanced HNSCC [2]. Even 90% of HNSCC patients have overexpression of the EGFR [3]. Unfortunately, cetuximab has only shown a 20% positive response rate in patients with HPV-negative tumors and only a marginal improvement in combination with radiotherapy and platinum-based chemotherapy [4][5]. Panitumumab is another monoclonal antibody against EGFR [6].
Irreversible EGFR inhibitors act on the intracellular domain, inhibiting the cytoplasmic tyrosine kinase domain. Erlotinib is the most respected representative, and was, for instance, combined with standard docetaxel/cisplatin chemotherapy for recurrent/metastatic (R/M) HNSCC [7]. The combined treatment achieved a response rate of 62% (8% complete response and 54% partial response), which is greater than the previous trial’s response rate of 40% for docetaxel/cisplatin chemotherapy.
Many studies have found that HNSCC and other EGFR-dependent tumors are resistant to EGFR-inhibitory therapy. Yamaoka et al. (2017) summarized four general mechanisms of anti-EGFR antibody and EGFR tyrosine kinase inhibitor resistance in cancer cells: (i) secondary mutations in the EGFR gene; (ii) resistance to apoptotic cell death; (iii) phenotypic transformation (e.g., tumor cells activating stem cell-like characteristics); and finally, (iv) activation of alternative signaling pathways [8].
The active state of EGFR triggers a cascade of intracellular responses. As a result, in many cases of molecular abnormalities, even effective EGFR attenuation cannot influence the downstream activation of altered signal transduction elements. Therefore, there is a recurrence of a tumor that is resistant to previously used therapeutic procedures.
2. Farnesylation of RAS
RAS is a key player in the EGFR signal transduction. The HRAS mutations are called “undruggable”, but advances in the high-resolution understanding of RAS isoform structure provide hope for developing personalized therapies for patients with RAS-dependent cancers [9]. Fortunately, post-transcriptional farnesylation is required for RAS protein to be anchored to the inner side of the cell membrane, which is crucial for EGFR signal transduction. A phase II clinical trial of tipifarnib (inhibitor of farnesyltransferase) involving 30 patients with R/M HNSCC revealed positive response in patients with HRAS mutations [10].
EGFR-dependent RAS activation stimulates two critical intracellular signaling pathways, RAS/RAF/MAPK and PI3K/Akt/mTOR [11].
3. RAS/RAF/MAPK Pathway
In brief, this pathway creates kinase cascades and finally activates extracellular signal-regulated kinases (ERK) [12], which translocate from the cytoplasm to the nucleus to induce specific genes expression [13]. In HNSCC, attempts were made to target this kinase cascade by inhibiting the RAF and MEK proteins. For instance, Sorafenib—a RAF kinase, vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) inhibitor [14]—was evaluated in phase II clinical trial of patients with R/M HNSCC and resulted in a partial response or disease stabilization in 40.7–51% of patients [15][16].
4. PI3K/Akt/mTOR Pathway
Phosphoinositide 3-kinase (PI3K) class IA comprises the p110α/β/δ catalytic subunit and the p85 regulatory subunit. Phosphatidylinositol 3,4,5-trisphosphate, converted from phosphatidylinositol 4,5-bisphosphate, activates downstream signaling factors such as Akt. Another kinase, the mammalian target of rapamycin (mTOR), is the main effector of Akt kinase [17]. Mutations in the PI3K catalytic subunit p110α are the most common genetic abnormality observed in HNSCC. Alpelisib (NVP-BYL719) is the first FDA-approved p110α inhibitor for the treatment of hormone receptor-positive, HER2-negative, PI3K catalytic subunit alpha (PIK3CA)-mutated, advanced or metastatic breast cancer, and it may be useful in HNSCC as well [18][19].
Akt phosphorylates a variety of targets, including tuberous sclerosis complex 2 (TCS2), which, along with TCS1, inhibits the activity of the mTOR complex (mTORC) [20]. Based on the U.S. National Library of Medicine online (https://clinicaltrials.gov) database of clinical studies, Akt inhibitors-ipatasertib (GDC-0068) and capivasertib (AZD5363), are tested for R/M HNSCC in mono-treatment (NCT02465060 and NCT02465060, respectively), followed by ipatasertib in combination with cisplatin and radiotherapy (NCT05172245).
Finally, mTOR inhibition may be used to target this pathway, e.g., by everolimus. A meta-analysis of studies involving mTOR inhibition confirms that monotherapy cannot improve the prognosis of HNSCC patients but can accelerate partial tumor response when combined with other anticancer agents [21].
5. Other Receptor Tyrosine Kinases and Their Downstream Signaling Pathways
Other RTK, in addition to EGFR, may be promising pharmacological targets in HNSCC. In some tumors, the fibroblast growth factor receptor (FGFR) is overexpressed and partially amplified [22] and was linked to poor overall survival and disease-free survival in HPV-negative patients. The small molecule, AZD4547, is a potential FGFR inhibitor, which was found to decrease the growth of HNSCC cells in vitro [23].
The VEGFR signaling orchestrates neovascularization of growing tumors [24]. Because RAS, PI3K, and STAT3 proteins are downstream effectors of VEGFR [25], its simulation promotes many other tumor-promoting features controlled by those pathways. Bevacizumab, a humanized monoclonal antibody against VEGF, is frequently examined in clinical trials; for instance, it was combined with EGF-receptor-targeted therapy based on cetuximab [26] or erlotinib [27], which benefits patients.
The PDGFR signaling, among others, influences Akt-dependent activation of pro-oxidative NF-κB signaling [28]. Overexpression of PDGF and its receptor has been associated with neck lymph node metastasis, advanced TNM stage, and poor survival in HNSCC patients [29]. Multifunctional kinase inhibitors are currently being used to target this receptor along with other RTKs. Imatinib, a PDGF(R) and VEGF(R) inhibitor suppressed their expression synergistically in vitro [30].
In HNSCC, the hepatocyte growth factor/mesenchymal-epithelial-transition factor (HGF/c-MET) pathway promotes PI3K/Akt, RAS/MAPK, STAT3, and Src/NF-κB intracellular signaling, resulting in cancer cell proliferation and apoptosis avoidance, followed by extensive growth and metastasis [31][32]. Wang et al. (2021) used three c-Met inhibitors (crizotinib, tivantinib, and cabozantinib) in combination with the pan-HER inhibitor afatinib. In HNSCC cell lines, xenografts, and patient-derived xenograft animal models, the drugs’ combination exceeds monotherapy regarding anticancer efficacy, confirming the significance of further clinical trials [33].
STAT canonical signaling can be activated by RTK, resulting in neovascularization, increased cell proliferation, survival, and even immune response evasion [34]. The nuclear accumulation of phosphorylated STAT3 has been identified as a prognostic marker in the early premalignant stages of HNSCC [35]. STAT5 inhibitor 573108, in combination with radiotherapy, was found to improve cell survival in a panel of HNSCC cell lines [36].
6. Cancer Stem Cell-Related Signaling Pathways
Cancer stem cells (CSC) are a subpopulation of cells that express specific extracellular and molecular markers and can self-renew [37][38][39]. After temporary tumor bulk reduction, conventional anticancer therapy that does not affect CSC leads to tumor recurrence with an enriched, therapy-resistant CSC population.
The NOTCH pathway regulates body pattern formation, cell fate, and proliferation during embryogenesis, and stem cell activity in both early and adult organisms [40]. The global mutation rate of NOTCH1 is approximately 15%, making this gene one of the most frequently mutated in HNSCC [41]. The NOTCH1 gene was thought to be a tumor suppressor due to the high percentage of mutations in HNSCC [42], but this pathway can be induced in tumors as well [43]. The NOTCH pathway promotes the self-renewal capacity of HNSCC cells, as evidenced by increased expression of Oct4, Sox2, and CD44 stemness markers [44].
The Wnt/β-catenin signaling is essential for cell differentiation and proliferation during embryogenesis and in proliferative tissues in adulthood, including the stem cell subpopulation [45][46]. This signaling is extensively activated in colorectal cancers, but its dysregulation at various levels of signal transduction is also critical for the development of HNSCC [47]. In particular, the porcupine inhibitor (IWP-2) and the inhibitor of the interaction between β-catenin and the CREB binding protein (PRI-724) effectively inhibited HNSCC cell lines [48].
The Hedgehog (Hh) canonical pathway is activated by the Sonic Hedgehog (SHh) ligand and is present in various tissues/organs during development and in the adult organism [49][50]. The significance of the Hh pathway in the development of basal cell carcinoma of the head and neck was practically confirmed by the FDA’s approval of the Hh signaling inhibitor, vismodegib, in 2012 [51]. Several studies [52][53][54][55] have identified active Hh signaling as a negative prognostic marker for HNSCC patients and multi-drug resistance. Furthermore, in HNSCC, Hh signaling is strongly linked to CSC markers [56].
NOTCH signaling activation can upregulate components of the Wnt and Hh pathways, and further crosstalk between those signaling pathways supports the maintenance and development of HNSCC by promoting the activity of CSC [57]. In addition, it is possible that also the Hippo pathway, which is involved in organ development, regeneration, and stemness, could be used as a target for HNSCC combinatorial therapy, while its crosstalk with NOTCH, Wnt, and Hh signaling has been demonstrated [58][59]. Finally, because transforming growth factor-β (TGF-β) is a regulatory cytokine involved in the control of CSC and immune cells [60][61], it is a good target for innovative combinatorial HNSCC treatment.
7. Defective Immune Response, Dysregulated Energy Metabolism, and Other Targets for HNSCC Therapy
The use of two monoclonal antibodies against programmed cell death 1 (PD-1) was a practical success in overcoming an abnormal immune response of HNSCC cells. Cancer cells produce excessive PD-1 ligands (PD-L1/2), which binds to PD-1 receptors on the surface of T-cells. As a result, T-cell activity, proliferation, cytokine secretion, and overall survival are all affected [62][63]. Pembrolizumab is an FDA-approved IgG4-κ humanized monoclonal antibody against PD-1, activating the immune response [64][65]. Another IgG4 antibody, nivolumab, was also approved to treat HNSCC [66].
Otto Warburg observed specific energy metabolism in cancer cells using glycolysis and fermentation, despite access to oxygen [67]. Nowadays, researchers have a much better understanding of the so-called Warburg effect. Glycolysis, glutaminolysis, NAD synthesis, tricarboxylic acid cycle, mitochondrial activity, changes in intra- and extracellular pH, lipid and amino acid metabolism, and control of master regulators of energy metabolism such as c-Myc, HIF-1α, Akt, or sirtuins are examples of metabolic targets [68][69][70]. Some commonly used chemotherapeutics target metabolism (e.g., methotrexate - folic acid metabolism) are registered for non-cancer purposes and used in antitumor procedures (e.g., metformin related with glucose metabolism), or are in clinical trials (e.g., AZD-3965 inhibiting lactate transporter MCT1) [71]. The reorganization of cancer cells’ metabolism cooperates with other molecular abnormalities and should be considered an adjuvant therapy in most cases.
Figure 1 represents the targets, enriched with other possible targets of HNSCC therapy [72][73][74][75][76][77][78].
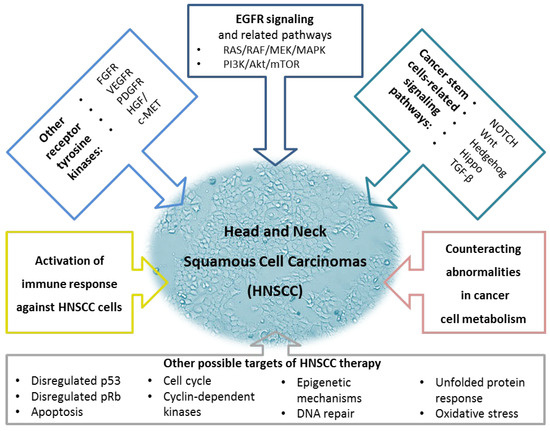
Figure 1. A summary of possible therapeutic targets for Head and Neck Squamous Carcinomas. The upper part of the figure represents essential signaling pathways. The two major new features of cancer cells, changes in immune system response and energy metabolism, are mentioned in the middle. The lower part of the figure demonstrates other possible targets of HNSCC therapy. The figure was created using information from the references given (Molecular Targeted Therapy of HNSCC). EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; HGF/c-MET, hepatocyte growth factor/mesenchymal-epithelial-transition factor; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; TGF-β, transforming growth factor-β; VEGFR, vascular endothelial growth factor receptor.
References
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 351, 337–345.
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578.
- Kalyankrishna, S.; Grandis, J.R. Epidermal Growth Factor Receptor Biology in Head and Neck Cancer. J. Clin. Oncol. 2006, 24, 2666–2672.
- Rehmani, H.S.; Issaeva, N. EGFR in head and neck squamous cell carcinoma: Exploring possibilities of novel drug combinations. Ann. Transl. Med. 2020, 8, 813.
- Vermorken, J.; Specenier, P. Cetuximab: Its unique place in head and neck cancer treatment. Biol. Targets Ther. 2013, 2013, 77–90.
- Saltz, L.; Easley, C.; Kirkpatrick, P. Panitumumab. Nat. Rev. Drug Discov. 2006, 5, 987–988.
- William, W.N., Jr.; Tsao, A.S.; Feng, L.; Ginsberg, L.E.; Lee, J.J.; Kies, M.S.; Glisson, B.S.; Kim, E.S. Single Arm, Phase II Study of Cisplatin, Docetaxel, and Erlotinib in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinomas. Oncologist 2018, 23, e526–e549.
- Yamaoka, T.; Ohba, M.; Ohmori, T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017, 18, 2420.
- Parker, J.A.; Mattos, C. The K-Ras, N-Ras, and H-Ras Isoforms: Unique Conformational Preferences and Implications for Targeting Oncogenic Mutants. Cold Spring Harb. Perspect. Med. 2017, 8, a031427.
- Ho, A.L.; Brana, I.; Haddad, R.; Bauman, J.; Bible, K.; Oosting, S.; Wong, D.J.; Ahn, M.-J.; Boni, V.; Even, C.; et al. Tipifarnib in Head and Neck Squamous Cell Carcinoma with HRAS Mutations. J. Clin. Oncol. 2021, 39, 1856–1864.
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341.
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198.
- Eblen, S.T. Extracellular-Regulated Kinases: Signaling from Ras to ERK Substrates to Control Biological Outcomes. In Advances in Cancer Research; Elsevier: New York, NY, USA, 2018; Volume 138, pp. 99–142.
- Mousa, A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J. Gastroenterol. 2008, 14, 40–42.
- Elser, C.; Siu, L.L.; Winquist, E.; Agulnik, M.; Pond, G.R.; Chin, S.F.; Francis, P.; Cheiken, R.; Elting, J.; McNabola, A.; et al. Phase II Trial of Sorafenib in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck or Nasopharyngeal Carcinoma. J. Clin. Oncol. 2007, 25, 3766–3773.
- Williamson, S.K.; Moon, J.; Huang, C.H.; Guaglianone, P.P.; LeBlanc, M.; Wolf, G.T.; Urba, S.G. Phase II Evaluation of Sorafenib in Advanced and Metastatic Squamous Cell Carcinoma of the Head and Neck: Southwest Oncology Group Study S0420. J. Clin. Oncol. 2010, 28, 3330–3335.
- Dilmaghani, N.A.; Safaroghli-Azar, A.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/Akt/mTORC signaling axis in head and neck squamous cell carcinoma: Possibilities for therapeutic interventions either as single agents or in combination with conventional therapies. IUBMB Life 2021, 73, 618–642.
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R.; et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 3741–3748.
- Narayan, P.; Prowell, T.M.; Gao, J.J.; Fernandes, L.L.; Li, E.; Jiang, X.; Qiu, J.; Fan, J.; Song, P.; Yu, J.; et al. FDA Approval Summary: Alpelisib Plus Fulvestrant for Patients with HR-positive, HER2-negative, PIK3CA-mutated, Advanced or Metastatic Breast Cancer. Clin. Cancer Res. 2020, 27, 1842–1849.
- Dan, H.C.; Baldwin, A.S. Differential Involvement of IκB Kinases α and β in Cytokine- and Insulin-Induced Mammalian Target of Rapamycin Activation Determined by Akt. J. Immunol. 2008, 180, 7582–7589.
- Patel, J.; Nguyen, S.A.; Ogretmen, B.; Gutkind, J.S.; Nathan, C.; Day, T. mTOR inhibitor use in head and neck squamous cell carcinoma: A meta-analysis on survival, tumor response, and toxicity. Laryngoscope Investig. Otolaryngol. 2020, 5, 243–255.
- Freier, K.; Schwaenen, C.; Sticht, C.; Flechtenmacher, C.; Mühling, J.; Hofele, C.; Radlwimmer, B.; Lichter, P.; Joos, S. Recurrent FGFR1 amplification and high FGFR1 protein expression in oral squamous cell carcinoma (OSCC). Oral Oncol. 2007, 43, 60–66.
- Koole, K.; Brunen, D.; van Kempen, P.M.; Noorlag, R.; de Bree, R.; Lieftink, C.; van Es, R.J.; Bernards, R.; Willems, S.M. FGFR1 Is a Potential Prognostic Biomarker and Therapeutic Target in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 3884–3893.
- Brands, R.C.; Knierim, L.M.; De Donno, F.; Steinacker, V.; Hartmann, S.; Seher, A.; Kübler, A.C.; Müller-Richter, U.D. Targeting VEGFR and FGFR in head and neck squamous cell carcinoma in vitro. Oncol. Rep. 2017, 38, 1877–1885.
- Hsu, H.-W.; Wall, N.R.; Hsueh, C.-T.; Kim, S.; Ferris, R.L.; Chen, C.-S.; Mirshahidi, S. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014, 50, 19–26.
- Argiris, A.; Kotsakis, A.P.; Hoang, T.; Worden, F.P.; Savvides, P.; Gibson, M.K.; Gyanchandani, R.; Blumenschein, G.R., Jr.; Chen, H.X.; Grandis, J.R.; et al. Cetuximab and bevacizumab: Preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann. Oncol. 2013, 24, 220–225.
- Cohen, E.E.; Davis, D.W.; Karrison, T.G.; Seiwert, T.Y.; Wong, S.J.; Nattam, S.; Kozloff, M.F.; Clark, J.I.; Yan, D.-H.; Liu, W.; et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: A phase I/II study. Lancet Oncol. 2009, 10, 247–257.
- Ying, H.-Z.; Chen, Q.; Zhang, W.-Y.; Zhang, H.-H.; Ma, Y.; Zhang, S.-Z.; Fang, J.; Yu, C.-H. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics. Mol. Med. Rep. 2017, 16, 7879–7889.
- Lin, L.-H.; Lin, J.-S.; Yang, C.-C.; Cheng, H.-W.; Chang, K.-W.; Liu, C.-J. Overexpression of Platelet-Derived Growth Factor and Its Receptor Are Correlated with Oral Tumorigenesis and Poor Prognosis in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 2360.
- Schultz, J.D.; Rotunno, S.; Riedel, F.; Anders, C.; Erben, P.; Hofheinz, R.D.; Faber, A.; Thorn, C.; Sommer, J.U.; Hörmann, A.; et al. Synergistic effects of imatinib and carboplatin on VEGF, PDGF and PDGF-Rα/ß expression in squamous cell carcinoma of the head and neck in vitro. Int. J. Oncol. 2011, 38, 1001–1012.
- Raj, S.; Kesari, K.K.; Kumar, A.; Rathi, B.; Sharma, A.; Gupta, P.K.; Jha, S.K.; Jha, N.K.; Slama, P.; Roychoudhury, S.; et al. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol. Cancer 2022, 21, 31.
- Ariyawutyakorn, W.; Saichaemchan, S.; Varella-Garcia, M. Understanding and Targeting MET Signaling in Solid Tumors—Are We There Yet? J. Cancer 2016, 7, 633–649.
- Wang, D.; Lu, Y.; Nannapaneni, S.; Griffith, C.C.; Steuer, C.; Qian, G.; Wang, X.; Chen, Z.; Patel, M.; El-Deiry, M.; et al. Combinatorial approaches targeting the EGFR family and c-Met in SCCHN. Oral Oncol. 2020, 112, 105074.
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 1–33.
- Macha, M.A.; Matta, A.; Kaur, J.; Chauhan, S.S.; Thakar, A.; Shukla, N.K.; Gupta, S.D.; Ralhan, R. Prognostic significance of nuclear pSTAT3 in oral cancer. Head Neck 2011, 33, 482–489.
- Stegeman, H.; Kaanders, J.H.; Verheijen, M.M.; Peeters, W.J.; Wheeler, D.L.; Iida, M.; Grénman, R.; van der Kogel, A.J.; Span, P.N.; Bussink, J. Combining radiotherapy with MEK1/2, STAT5 or STAT6 inhibition reduces survival of head and neck cancer lines. Mol. Cancer 2013, 12, 133.
- Prince, M.E.; Ailles, L.E. Cancer Stem Cells in Head and Neck Squamous Cell Cancer. J. Clin. Oncol. 2008, 26, 2871–2875.
- Xiao, M.; Liu, L.; Zhang, S.; Yang, X.; Wang, Y. Cancer stem cell biomarkers for head and neck squamous cell carcinoma: A bioinformatic analysis. Oncol. Rep. 2018, 40, 3843–3851.
- Gunduz, M.; Gunduz, E.; Tamagawa, S.; Enomoto, K.; Hotomi, M. Cancer Stem Cells in Oropharyngeal Cancer. Cancers 2021, 13, 3878.
- Leong, K.G.; Karsan, A. Recent insights into the role of Notch signaling in tumorigenesis. Blood 2006, 107, 2223–2233.
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.-X.; Zhang, J.; Wang, J.; et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science 2011, 333, 1154–1157.
- Nyman, P.E.; Buehler, D.; Lambert, P.F. Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis. Clin. Cancer Res. 2018, 24, 6308–6318.
- Sun, W.; Gaykalova, D.A.; Ochs, M.F.; Mambo, E.; Arnaoutakis, D.; Liu, Y.; Loyo, M.; Agrawal, N.; Howard, J.; Li, R.; et al. Activation of the NOTCH Pathway in Head and Neck Cancer. Cancer Res. 2014, 74, 1091–1104.
- Lee, S.H.; Do, I.S.; Lee, H.J.; Kang, H.J.; Koo, B.S.; Lim, Y.C. Notch1 signaling contributes to stemness in head and neck squamous cell carcinoma. Lab. Investig. 2016, 96, 508–516.
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2016, 36, 1461–1473.
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3.
- Paluszczak, J. The Significance of the Dysregulation of Canonical Wnt Signaling in Head and Neck Squamous Cell Carcinomas. Cells 2020, 9, 723.
- Kleszcz, R.; Szymańska, A.; Krajka-Kuźniak, V.; Baer-Dubowska, W.; Paluszczak, J. Inhibition of CBP/β-catenin and porcupine attenuates Wnt signaling and induces apoptosis in head and neck carcinoma cells. Cell. Oncol. 2019, 42, 505–520.
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp Genes Are Coexpressed at Many Diverse Sites of Cell–Cell Interaction in the Mouse Embryo. Dev. Biol. 1995, 172, 126–138.
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 1996, 383, 407–413.
- Rudin, C.M. Vismodegib. Clin. Cancer Res. 2012, 18, 3218–3222.
- Cierpikowski, P.; Lis-Nawara, A.; Bar, J. Sonic Hedgehog is a novel prognostic biomarker in patients with oral squamous cell carcinoma. Neoplasma 2021, 68, 867–874.
- Noman, A.S.M.; Parag, R.R.; Rashid, M.I.; Rahman, M.Z.; Chowdhury, A.A.; Sultana, A.; Jerin, C.; Siddiqua, A.; Rahman, L.; Shirin, A.; et al. Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920911229.
- Enzenhofer, E.; Parzefall, T.; Haymerle, G.; Schneider, S.; Kadletz, L.; Heiduschka, G.; Pammer, J.; Oberndorfer, F.; Wrba, F.; Loader, B.; et al. Impact of Sonic Hedgehog Pathway Expression on Outcome in HPV Negative Head and Neck Carcinoma Patients after Surgery and Adjuvant Radiotherapy. PLoS ONE 2016, 11, e0167665.
- Lu, X.; Wang, Z.; Huang, H.; Wang, H. Hedgehog signaling promotes multidrug resistance by regulation of ABC transporters in oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 897–906.
- Cierpikowski, P.; Lis-Nawara, A.; BAR, J. SHH Expression Is Significantly Associated with Cancer Stem Cell Markers in Oral Squamous Cell Carcinoma. Anticancer Res. 2021, 41, 5405–5413.
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—Clinical implications. Cell. Oncol. 2021, 44, 473–494.
- Fu, V.; Plouffe, S.W.; Guan, K.-L. The Hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 2017, 49, 99–107.
- Nishio, M.; Otsubo, K.; Maehama, T.; Mimori, K.; Suzuki, A. Capturing the mammalian Hippo: Elucidating its role in cancer. Cancer Sci. 2013, 104, 1271–1277.
- Oshimori, N. Cancer stem cells and their niche in the progression of squamous cell carcinoma. Cancer Sci. 2020, 111, 3985–3992.
- Pang, X.; Tang, Y.L.; Liang, X.H. Transforming growth factor-β signaling in head and neck squamous cell carcinoma: Insights into cellular responses (Review). Oncol. Lett. 2018, 16, 4799–4806.
- Chen, Y.; Ding, X.; Bai, X.; Zhou, Z.; Liu, Y.; Zhang, X.; Yu, J.; Hu, M. The current advances and future directions of PD-1/PD-L1 blockade in head and neck squamous cell carcinoma (HNSCC) in the era of immunotherapy. Int. Immunopharmacol. 2023, 120, 110329.
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742.
- Longoria, T.C.; Tewari, K.S. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1247–1253.
- Larkins, E.; Blumenthal, G.M.; Yuan, W.; He, K.; Sridhara, R.; Subramaniam, S.; Zhao, H.; Liu, C.; Yu, J.; Goldberg, K.B.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma with Disease Progression on or After Platinum-Containing Chemotherapy. Oncologist 2017, 22, 873–878.
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867.
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314.
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819.
- Yamamoto, M.; Inohara, H.; Nakagawa, T. Targeting metabolic pathways for head and neck cancers therapeutics. Cancer Metastasis Rev. 2017, 36, 503–514.
- Hsieh, Y.-T.; Chen, Y.-F.; Lin, S.-C.; Chang, K.-W.; Li, W.-C. Targeting Cellular Metabolism Modulates Head and Neck Oncogenesis. Int. J. Mol. Sci. 2019, 20, 3960.
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2021, 21, 141–162.
- Xu, C.; Nikolova, O.; Basom, R.S.; Mitchell, R.M.; Shaw, R.; Moser, R.D.; Park, H.; Gurley, K.E.; Kao, M.C.; Green, C.L.; et al. Functional Precision Medicine Identifies Novel Druggable Targets and Therapeutic Options in Head and Neck Cancer. Clin. Cancer Res. 2018, 24, 2828–2843.
- Cole, D.W.; Svider, P.F.; Shenouda, K.G.; Lee, P.B.; Yoo, N.G.; McLeod, T.M.; Mutchnick, S.A.; Yoo, G.H.; Kaufman, R.J.; Callaghan, M.U.; et al. Targeting the unfolded protein response in head and neck and oral cavity cancers. Exp. Cell Res. 2019, 382, 111386.
- Gaździcka, J.; Gołąbek, K.; Strzelczyk, J.K.; Ostrowska, Z. Epigenetic Modifications in Head and Neck Cancer. Biochem. Genet. 2019, 58, 213–244.
- Kordbacheh, F.; Farah, C.S. Current and Emerging Molecular Therapies for Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5471.
- Van Harten, A.M.; Brakenhoff, R.H. Targeted Treatment of Head and Neck (Pre)Cancer: Preclinical Target Identification and Development of Novel Therapeutic Applications. Cancers 2021, 13, 2774.
- Romanowska, K.; Sobecka, A.; Rawłuszko-Wieczorek, A.A.; Suchorska, W.M.; Golusiński, W. Head and Neck Squamous Cell Carcinoma: Epigenetic Landscape. Diagnostics 2020, 11, 34.
- Ghosh, S.; Shah, P.A.; Johnson, F.M. Novel Systemic Treatment Modalities Including Immunotherapy and Molecular Targeted Therapy for Recurrent and Metastatic Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 7889.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
581
Revisions:
3 times
(View History)
Update Date:
06 Sep 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




