Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rita Chertkova | -- | 2017 | 2023-08-26 11:45:49 | | | |
| 2 | Rita Xu | Meta information modification | 2017 | 2023-08-28 04:07:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Semenova, M.A.; Chertkova, R.V.; Kirpichnikov, M.P.; Dolgikh, D.A. Ferrous Neuroglobin and Ferric Cytochrome c. Encyclopedia. Available online: https://encyclopedia.pub/entry/48505 (accessed on 08 February 2026).
Semenova MA, Chertkova RV, Kirpichnikov MP, Dolgikh DA. Ferrous Neuroglobin and Ferric Cytochrome c. Encyclopedia. Available at: https://encyclopedia.pub/entry/48505. Accessed February 08, 2026.
Semenova, Marina A., Rita V. Chertkova, Mikhail P. Kirpichnikov, Dmitry A. Dolgikh. "Ferrous Neuroglobin and Ferric Cytochrome c" Encyclopedia, https://encyclopedia.pub/entry/48505 (accessed February 08, 2026).
Semenova, M.A., Chertkova, R.V., Kirpichnikov, M.P., & Dolgikh, D.A. (2023, August 26). Ferrous Neuroglobin and Ferric Cytochrome c. In Encyclopedia. https://encyclopedia.pub/entry/48505
Semenova, Marina A., et al. "Ferrous Neuroglobin and Ferric Cytochrome c." Encyclopedia. Web. 26 August, 2023.
Copy Citation
Neuroglobin, which is a heme protein from the globin family that is predominantly expressed in nervous tissue, can promote a neuronal survivor. However, the molecular mechanisms underlying the neuroprotective function of Ngb remain poorly understood to this day. The interactions between neuroglobin and mitochondrial cytochrome c may serve as at least one of the mechanisms of neuroglobin-mediated neuroprotection.
neuroglobin
neuroprotection
cytochrome c
heme proteins
1. Introduction
Neuroglobin (Ngb) is a six-coordinate heme protein from the globin family that is predominantly expressed in nervous tissue [1]. Intracellular Ngb concentrations range from 100 μM in the neurons of the hypothalamus and the retinal cells to 1 μM in the other neurons [1][2][3]. Furthermore, there is an average 2-5-fold increase in NGB gene expression in response to various stress signals (e.g., hypoxia or oxidative stress) [4][5]. Ngb is a cytoplasmic protein, although there is evidence for Ngb localization not only in close proximity to mitochondria, but also within mitochondria [6][7][8]. However, Ngb lacks a mitochondrial target sequence, and the exact mechanism regulating Ngb mitochondrial localization is still unclear [9].
Ngb is a monomer with a molecular weight of about 17 kDa, consists of eight α-helices (A-H), and has a typical three-over-three globin fold [1][10][11]. Ngb contains one heme group, and, in the absence of exogenous ligands, the axial coordination positions of the heme are occupied by two histidine residues (His64-Fe-His96) [11][12]. Human Ngb features three cysteine residues (Cys46, Cys55, and Cys120), two of which form an intramolecular disulfide bridge (Cys46–Cys55) [1][10][11]. The formation of other intramolecular disulfide bridges in Ngb involving Cys120 and Cys46 or Cys55 is considered to be sterically impossible [11][13]. The significance of the Cys46–Cys55 disulfide bridge is determined by its effect on the conformation of the CD-loop (the region between α-helices C and D) and modulation of Ngb functionality. Upon cleavage of this disulfide bridge, the bond between the heme iron and His64 is strengthened, thus resulting in a ten-fold decrease of the His64 dissociation rate. As a consequence, the affinity of Ngb for exogenous ligands is reduced because the dissociation of His64 from the heme is a necessary and rate-determining step for the binding of exogenous ligands to Ngb [11][14]. However, it is suggested that, in vivo, more than 88% of Ngb molecules are in the ferrous deoxy form without a disulfide bridge [15][16][17].
Similar to hemoglobin and myoglobin, Ngb reversibly binds O2 and other small gaseous ligands [12][18]. Ngb catalyzes the NO deoxygenation reaction, thus yielding NO3− [19][20], as well as NO generation from NO2− (nitrite reductase activity) [21][22][23].
Ngb has been shown to promote neuronal survival in conditions such as ischemia, hypoxia, Alzheimer’s and Huntington’s diseases, oxidative stress, stroke, spinal cord injury, retinal degeneration, arsenic poisoning, etc., in numerous in vitro and in vivo studies [4][5][9][24][25][26][27][28][29]. However, the molecular mechanisms underlying the neuroprotective function of Ngb remain poorly understood to this day. Because Ngb can bind O2, it was initially assumed that Ngb, like myoglobin, acts as an O2 depot and transporter under hypoxia [1][2][3]. However, relatively low cellular concentrations of Ngb and a very rapid autoxidation contradict this assumption [30].
Recent hypotheses suggest that Ngb-mediated neuroprotection is based on its involvement in various biochemical cascades of the cell [4][9][24][28][29]. Ngb can detoxify reactive oxygen and nitrogen species [31][32][33][34], and there is evidence of Ngb involvement in the Wnt/β-catenin pathway [35][36], as well as in the PI3K/Akt/MAPK signaling pathway [37][38]. Additionally, the protein–protein interactions between Ngb and voltage-dependent anion channels (VDACs) [6][7][39], α-subunits of heterotrimeric G-protein [40][41][42], and mitochondrial cytochrome c (Cyt c) [30][43] may also contribute to Ngb-mediated neuroprotection. This research is focused on the molecular interactions between Ngb and Cyt c.
Mitochondrial Cyt c, a multifunctional heme protein, plays a crucial role as an electron transporter in the respiratory chain. During the activation of apoptosis, Cyt c translocates from the mitochondria into the cytoplasm, where it amplifies the external apoptotic signal or initiates the caspase cascade through the intrinsic pathway (making it Cyt c-dependent) [44][45][46][47]. In addition to physiological role of apoptosis in nervous tissue, it is also associated with pathological conditions, such as neurodegenerative diseases, strokes, ischemia, hypoxia, etc. Furthermore, the initiation of apoptosis occurs under the influence of various stress stimuli, including pathogens, radiation, chemotherapeutic drugs, oxidative stress, etc., which cause mitochondrial dysfunction [48][49][50].
The key event of the intrinsic apoptotic pathway is the outer mitochondrial membrane permeabilization, which leads to the release of various proapoptotic factors, including Cyt c, into the cytoplasm. Furthermore, the activation of the caspase cascade depends on the efficiency of Cyt c binding to apoptotic protease activating factor-1 (Apaf-1), thereby leading to apoptosome assembly. It should be noted that only ferric Cyt c can bind to Apaf-1, thereby activating apoptosome assembly and triggering the caspase cascade [51].
The hypothesis about the interaction between Ngb and Cyt c was proposed based on the kinetic studies of the redox reaction between Ngb(Fe2+) and Cyt c(Fe3+):
This reaction was initially observed by absorbance spectroscopy under anaerobic conditions [43]. According to this hypothesis, Ngb(Fe2+) reacts with Cyt c(Fe3+) when the latter translocates into the cytoplasm during the activation of the intrinsic apoptotic pathway. This reaction prevents Cyt c(Fe3+) from interacting with Apaf-1 and, thus, prevents apoptosome assembly and ultimately blocks the initiation of apoptosis [30][43][52]. Later, it was hypothesized that Ngb can potentially interact with Cyt c(Fe3+), regardless of its redox state [53][54].
Ngb(Fe2+) + Cyt c(Fe3+) → Ngb(Fe3+) + Cyt c(Fe2+).
Therefore, Ngb can protect neurons from apoptosis by reducing the concentration of cytosolic Cyt c(Fe3+), which can increase under conditions that cause mitochondrial dysfunction and during normal mitochondrial function (reducing “accidental” Cyt c release) [30].
2. Electron Transfer between Ferrous Neuroglobin and Ferric Cytochrome c
The investigation of interactions between Ngb and Cyt c began with the observation of the very rapid electron transfer from recombinant murine Ngb(Fe2+) to Cyt c(Fe3+) [43]. The second-order rate constant for the reaction (k = 2 × 107 M−1 s−1) is close to the known physiologically significant Cyt c redox reactions rate constants, e.g., the reduction of cytochrome c oxidase or oxidation of cytochrome b5 [43][46][55]. It should be noted that, unlike human Ngb, murine Ngb lacks the disulfide bridge due to the replacement of Cys46 by Gly [1]. Hence, it can be concluded that the presence of the Ngb disulfide bridge does not affect electron transfer from Ngb(Fe2+) to Cyt c(Fe3+) [43].
However, a recent study [56] has come to somewhat controversial conclusions. The electron transfer between recombinant human Ngb(Fe2+) and Cyt c(Fe3+) was studied using a nanoporous gold electrode. The immobilization of Ngb onto the electrode surface enabled the rapid reduction of the Ngb heme, after which Cyt c(Fe3+) was added to the solution, and its reduction to Cyt c(Fe2+) was observed. In the case of the Ngb C55S mutant without the disulfide bridge (Cys46–Cys55), there were almost no voltammogram changes upon Cyt c(Fe3+) addition. Thus, it was concluded that the disulfide bridge has significance for electron transfer between Ngb(Fe2+) and Cyt c(Fe3+). These results are inconsistent with the previously obtained data on electron transfer between murine Ngb(Fe2+) and Cyt c(Fe3+) [43]. It is assumed that the Ngb disulfide bridge formation under oxidative stress conditions is potentially capable of modulating the functionality of Ngb. For instance, researchers can consider the electron transfer to Cyt c(Fe3+) in response to redox changes in the cell [14][18][56].
Electron transfer between Ngb(Fe2+) and Cyt c(Fe3+) was also detected by stopped-flow spectroscopy [57], while recombinant human Ngb was reduced with an excess of sodium dithionite, which most likely led to the disulfide bridge cleavage as well.
Based on the aforementioned data, two hypotheses regarding the interaction between Ngb(Fe2+) and Cyt c(Fe3+) in vivo were formed [30][43][56]. According to one of them [30][43][52], Ngb(Fe2+) in the ferrous deoxy form, which normally prevails in cells [15][16][17], reduces Cyt c(Fe3+) molecules by leaving the mitochondria into the cytoplasm to Cyt c(Fe2+), thereby preventing apoptosome assembly and apoptosis triggering along the Cyt c-dependent pathway (Figure 1, I). It should be noted that such a mechanism takes place under normal cellular conditions, thus preventing the consequences of the “accidental” Cyt c(Fe3+) release from mitochondria [30], which is a constitutive process associated with transient VDAC pore opening [58][59][60]. Even through “accidental” Cyt c(Fe3+) release is a small-scale process, Cyt c(Fe3+) can bind to the inositol-1,4,5-triphosphate receptors on the endoplasmic reticulum, thus causing Ca2+ release to the cytoplasm. An increase in the level of cytosolic Ca2+ further stimulates Cyt c(Fe3+) release from the mitochondria, thus establishing an ongoing amplification signal of the initial Cyt c(Fe3+) release [30][61]. Therefore, the existence of a resetting mechanism for the threshold level of Cyt c(Fe3+), for instance, via redox reaction with Ngb(Fe2+), is highly probable [30]. This hypothesis also explains the predominant localization of Ngb in neurons and retinal cells at high concentrations [2][3]. These highly specialized and metabolically active cells experience frequent high fluxes of cellular Ca2+ during their normal physiological functioning. As a result, these cell types might have a tendency to undergo programmed cell death as a result of the “accidental” release of Cyt c(Fe3+) in the cytoplasm. Hence, higher cellular concentrations of Ngb are required to protect these cells from apoptosis [30]. This hypothesis is further supported by the data on the Ngb localization in close proximity to mitochondria [7]. It is important to note that, in accordance with this mechanism, Ngb can suppress the triggering of the apoptotic process through the “accidental” release of Cyt c(Fe3+) while still allowing committed programmed cell death to occur under appropriate circumstances [30].
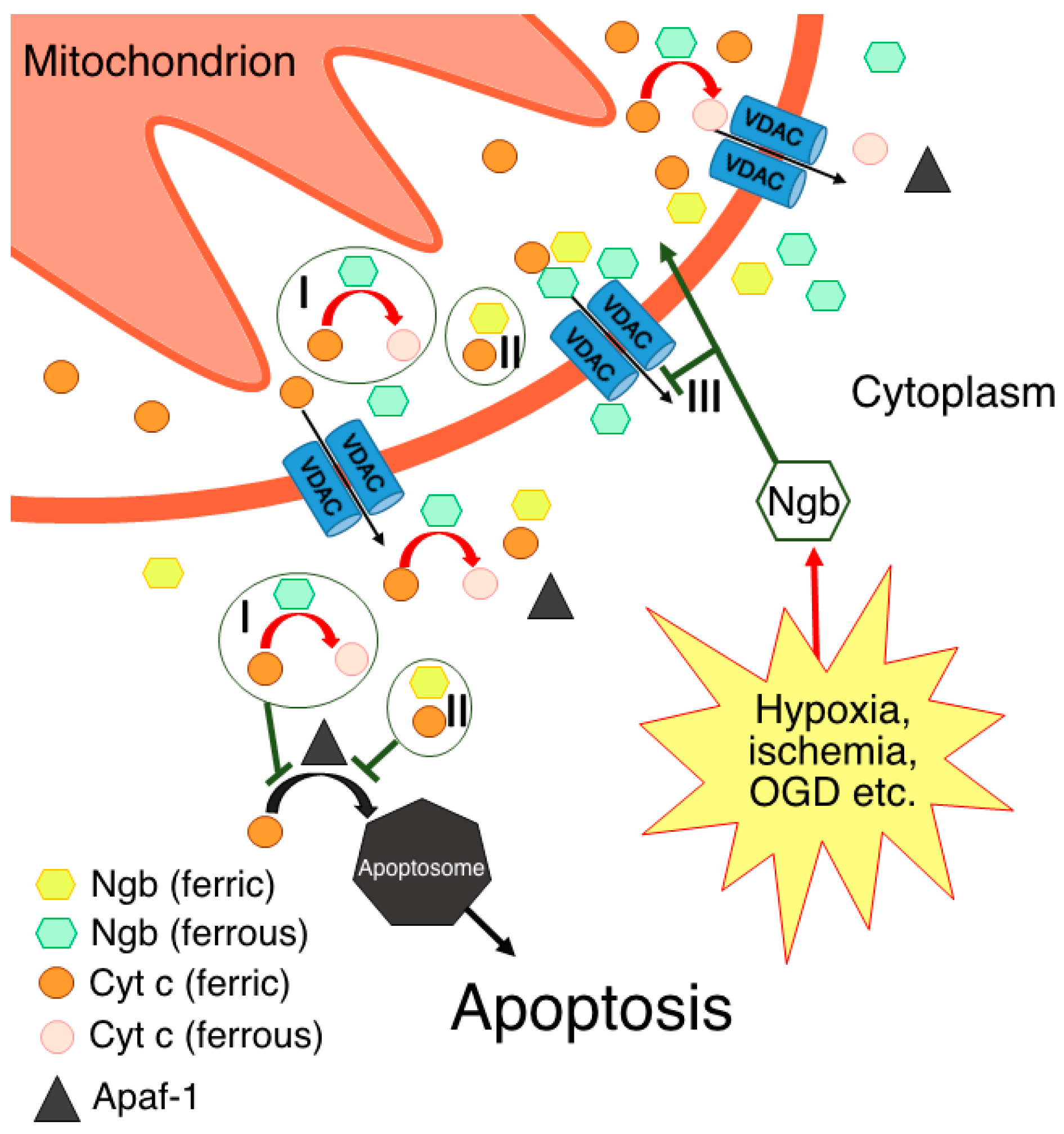
Figure 1. Possible molecular mechanisms of Ngb-mediated neuroprotection through direct or indirect interactions with Cyt c: I—electron transfer reaction, thus resulting in Cyt c being unable to form apoptosome via its interaction with Apaf-1; II—formation of complex between ferric forms of Ngb and Cyt c; III—prevention of Cyt c release in the cytoplasm via Ngb interaction with VDACs.
According to another hypothesis [56], electron transfer from Ngb(Fe2+) to Cyt c(Fe3+) occurs only in the presence of the disulfide bridge in the Ngb structure, which corresponds to the oxidative stress conditions [14][18][56]. In support of this idea, NGB gene expression is upregulated in response to various stress signals [4][5]. Moreover, although Ngb cellular concentration is primarily high only in neurons and retinal cells [2][3], it can increase in other cell types under cellular stress conditions to a level that is sufficient for Ngb-mediated protection against apoptosis [4][5]. This hypothesis contradicts the one described above, but not vice versa. It is quite probable that electron transfer from Ngb(Fe2+) to Cyt c(Fe3+) is possible in both cases: from Ngb(Fe2+) with the disulfide bridge and from Ngb(Fe2+) with reduced cysteines. In this case, the disulfide bridge may play a crucial role in modulating this electron transfer reaction under the corresponding redox conditions. It should be noted that, under oxidative stress conditions, not only cysteine residues, but also the heme of Ngb can be oxidized, which will lead to the inability of the Ngb to act as electron donor for the Cyt c. In addition, a Ngb-reducing system in vivo is still unknown, although several attempts have been made to identify it [43][62][63]. Hence, in the case of Ngb heme oxidation, the interaction with Cyt c is either disrupted or follows other mechanisms without electron transfer [54].
Thus, the studies on electron transfer between Ngb(Fe2+) and Cyt c(Fe3+) are rather inconsistent and limited. This inconsistency arises from the experimental difficulties, such as the preparation of pure ferrous Ngb without a reducing agent as a consequence of Ngb’s high autoxidation rate (0.23 ± 0.03 min−1 [17]). The reducing agent molecules can compete with Ngb(Fe2+) for the reduction of Cyt c(Fe3+) molecules in the solution; thus, the possibility of false positive results originates. Another challenge is the inability to use a classical strategy for the study of the electron transfer between cytochromes and heme globins. This strategy utilizes the difference in CO binding between six-coordinated cytochromes and five-coordinated heme globins, which cannot be used given the six-coordination nature of Ngb [57]. It is evident that further studies of the electron transfer between Ngb(Fe2+) and Cyt c(Fe3+) with alternative approaches are required.
References
- Burmester, T.; Weich, B.; Reinhardt, S.; Hankeln, T. A vertebrate globin expressed in brain. Nature 2000, 407, 520–523.
- Schmidt, M.; Giessl, A.; Laufs, T.; Hankeln, T.; Wolfrum, U.; Burmester, T. How does the eye breath? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J. Biol. Chem. 2003, 278, 1932–1935.
- Bentmann, A.; Schmidt, M.; Reuss, S.; Wolfrum, U.; Hankeln, T.; Burmester, T. Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J. Biol. Chem. 2005, 280, 20660–20665.
- Van Acker, Z.P.; Luyckx, E.; Dewilde, S. Neuroglobin expression in the brain: A story of tissue homeostasis preservation. Mol. Neurobiol. 2019, 56, 2101–2122.
- Fernandez, V.S.; Marino, M.; Fiocchetti, M. Neuroglobin in retinal neurodegeneration: A potential target in therapeutic approaches. Cells 2021, 10, 3200.
- Yu, Z.; Liu, N.; Wang, Y.; Li, X.; Wang, X. Identification of neuroglobin-interacting proteins using yeast two-hybrid screening. Neuroscience 2012, 200, 99–105.
- Yu, Z.; Xu, J.; Liu, N.; Wang, Y.; Li, X.; Pallast, S.; Van Leyen, K.; Wang, X. Mitochondrial distribution of neuroglobin and its response to oxygen–glucose deprivation in primary-cultured mouse cortical neurons. Neuroscience 2012, 218, 235–245.
- De Marinis, E.; Fiocchetti, M.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin upregulation induced by 17β-estradiol sequesters cytocrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells. Cell Death Dis. 2013, 4, e508.
- Fiocchetti, M.; Cracco, P.; Montalesi, E.; Fernandez, V.S.; Stuart, J.A.; Marino, M. Neuroglobin and mitochondria: The impact on neurodegenerarive diseases. Arch. Biochem. Biophys. 2021, 701, 108823.
- Pesce, A.; Dewilde, S.; Nardini, M.; Moens, L.; Ascenzi, P.; Hankeln, T.; Burmester, T.; Bolognes, M. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure 2003, 11, 1087–1095.
- Guimaraes, B.G.; Hamdane, D.; Lechauve, C.; Marden, M.C.; Golinelli-Pimpaneau, B. The crystal structure of wild-type human brain neuroglobin reveals flexibility of the disulfide bond that regulates oxygen affinity. Acta Crystallogr. D 2014, 70, 1005–1014.
- Dewilde, S.; Kiger, L.; Burmester, T.; Hankeln, T.; Baudin-Creuza, V.; Aerts, T.; Marden, M.C.; Caubergs, R.; Moens, L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 2001, 276, 38949–38955.
- Hamdane, D.; Kiger, L.; Dewilde, S.; Green, B.N.; Pesce, A.; Uzan, J.; Burmester, T.; Hankeln, T.; Bolognesi, M.; Moens, L.; et al. Coupling of the heme and an internal disulfide bond in human neuroglobin. Micron 2004, 35, 59–62.
- Bellei, M.; Bortolotti, C.A.; Rocco, G.D.; Borsari, M.; Lancellotti, L.; Ranieri, A.; Sola, M.; Battistuzzi, G. The influence of the Cys46/Cys55 disulfide bond on the redox and spectroscopic properties of human neuroglobin. J. Inorg. Biochem. 2018, 178, 70–86.
- Fago, A.; Hundahl, C.; Dewilde, S.; Gilany, K.; Moens, L.; Weber, R.E. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin: Molecular mechanisms and physiological significance. J. Biol. Chem. 2004, 279, 44417–44426.
- Fago, A.; Hundahl, C.; Malte, H.; Weber, R.E. Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life 2004, 56, 689–696.
- Tejero, J.; Sparacino-Watkins, C.E.; Ragireddy, V.; Frizzell, S.; Gladwin, M.T. Exploring the mechanisms of the reductase activity of neuroglobin by site-directed mutagenesis of the heme distal pocket. Biochemistry 2015, 54, 722–733.
- Hamdane, D.; Kiger, L.; Dewilde, S.; Green, B.N.; Pesce, A.; Uzan, J.; Burmester, T.; Hankeln, T.; Bolognesi, M.; Moens, L.; et al. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 2003, 278, 51713–51721.
- Brunori, M.; Giuffre, A.; Nienhaus, K.; Nienhaus, G.U.; Scandurra, F.M.; Vallone, B. Neuroglobin, nitric oxide, and oxygen: Functional pathways and conformational changes. Proc. Natl. Acad. Sci. USA 2005, 102, 8483–8488.
- Tejero, J.; Gladwin, M.T. The globin superfamily: Functions in nitric oxide formation and decay. Biol. Chem. 2014, 395, 631–639.
- Nicolis, S.; Monzani, E.; Ciaccio, C.; Ascenzi, P.; Moens, L.; Casella, L. Reactivity and endogenous modification by nitrite and hydrogen peroxide: Does human neuroglobin act only as a scavenger? Biochem. J. 2007, 407, 89–99.
- Petersen, M.G.; Dewilde, S.; Fago, A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J. Inorg. Biochem. 2008, 102, 1777–1782.
- Tiso, M.; Tejero, J.; Basu, S.; Azarov, I.; Wang, X.; Simplaceanu, V.; Frizzell, S.; Jayaraman, T.; Geary, L.; Shapiro, C.; et al. Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 2011, 286, 18277–18289.
- Ascenzi, P.; di Masi, A.; Leboffe, L.; Fiocchetti, M.; Nuzzo, M.T.; Brunori, M.; Marino, M. Neuroglobin: From structure to function in health and disease. Mol. Aspects Med. 2016, 52, 1–48.
- Ciccone, L.; Nencetti, S.; Socci, S.; Orlandini, E. Neuroglobin and neuroprotection: The role of natural and synthetic compounds in neuroglobin pharmacological induction. Neural. Regen. Res. 2021, 16, 2353–2358.
- Gorabi, A.M.; Aslani, S.; Barreto, G.E.; Baez-Jurado, E.; Kiaie, N.; Jamialahmadi, T.; Sahebkar, A. The potential of mitochondrial modulation by neuroglobin in treatment of neurological disorders. Free Radic. Biol. Med. 2021, 162, 471–477.
- Barreto, G.E.; McGovern, A.J.; Garcia-Segura, L.M. Role of neuroglobin in the neuroprotective actions of estradiol and estrogenic compounds. Cells 2021, 10, 1907.
- De Simone, G.; Sbardella, D.; Oddone, F.; Pesce, A.; Coletta, M.; Ascenzi, P. Structural and (pseudo-)enzymatic properties of neuroglobin: Its possible role in neuroprotection. Cells 2021, 10, 3366.
- Exertier, C.; Montemiglio, L.C.; Freda, I.; Gugole, E.; Parisi, G.; Savino, C.; Vallone, B. Neuroglobin, clues to function and mechanism. Mol. Aspects Med. 2022, 84, 101055.
- Fago, A.; Mathews, A.J.; Brittain, T. A role for neuroglobin: Resetting the trigger level for apoptosis in neuronal and retinal cells. IUBMB Life 2008, 60, 398–401.
- Cabezas, R.; Vega-Vela, N.E.; Gonzalez-Sanmiguel, J.; Gonzalez, J.; Esquinas, P.; Echeverria, V.; Barreto, G.E. PDGF-BB preserves mitochondrial morphology, attenuates ROS production, and upregulates neuroglobin in an astrocytic model under rotenone insult. Mol. Neurobiol. 2018, 55, 3085–3095.
- Van Acker, Z.P.; Van Raemdonck, G.A.; Logie, E.; Van Acker, S.I.; Baggerman, G.; Vanden Berghe, W.; Ponsaerts, P.; Dewilde, S. Connecting the dots in the neuroglobin-protein interaction network of an unstressed and ferroptotic cell death neuroblastoma model. Cells 2019, 8, 873.
- De Vidania, S.; Palomares-Perez, I.; Frank-García, A.; Saito, T.; Saido, T.C.; Draffin, J.; Szaruga, M.; Chavez-Gutierrez, L.; Calero, M.; Medina, M.; et al. Prodromal Alzheimer’s disease: Constitutive upregulation of neuroglobin prevents the initiation of Alzheimer’s pathology. Front. Neurosci. 2020, 14, 562581.
- Di Rocco, G.; Bernini, F.; Battistuzzi, G.; Ranieri, A.; Bortolotti, C.A.; Borsari, M.; Sola, M. Hydrogen peroxide induces heme degradation and protein aggregation in human neuroglobin: Roles of the disulfide bridge and hydrogen-bonding in the distal heme cavity. FEBS J. 2023, 290, 148–161.
- Xun, Y.; Li, Z.; Tang, Y.; Yang, M.; Long, S.; Shu, P.; Li, J.; Xiao, Y.; Tang, F.; Wei, C.; et al. Neuroglobin regulates Wnt/β-catenin and NFκB signaling pathway through Dvl1. Int. J. Mol. Sci. 2018, 19, 2133.
- Yu, Z.; Cheng, C.; Liu, Y.; Liu, N.; Lo, E.H.; Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018, 9, 945.
- Amri, F.; Ghouili, I.; Amri, M.; Carrier, A.; Masmoudi-Kouki, O. Neuroglobin protects astroglial cells from hydrogen peroxide-induced oxidative stress and apoptotic cell death. J. Neurochem. 2017, 140, 151–169.
- Fiocchetti, M.; Cipolletti, M.; Marino, M. Compensatory role of neuroglobin in nervous and non-nervous cancer cells in response to the nutrient deprivation. PLoS ONE 2017, 12, e0189179.
- Yu, Z.; Liu, N.; Li, Y.; Xu, J.; Wang, X. Neuroglobin overexpression inhibits oxygen-glucose deprivation-induced mitochondrial permeability transition pore opening in primary cultured mouse cortical neurons. Neurobiol. Dis. 2013, 56, 95–103.
- Wakasugi, K.; Nakano, T.; Morishima, I. Oxidized human neuroglobin acts as a heterotrimeric Gα protein guanine nucleotide dissociation inhibitor. J. Biol. Chem. 2003, 278, 36505–36512.
- Kitatsuji, C.; Kurogochi, M.; Nishimura, S.-I.; Ishimori, K.; Wakasugi, K. Molecular basis of guanine nucleotide dissociation inhibitor activity of human neuroglobin by chemical cross-linking and mass spectrometry. J. Mol. Biol. 2007, 368, 150–160.
- Watanabe, S.; Wakasugi, K. Neuroprotective function of human neuroglobin is correlated with its guanine nucleotide dissociation inhibitor activity. Biochem. Biophys. Res. Commun. 2008, 369, 695–700.
- Fago, A.; Mathews, A.J.; Moens, L.; Dewilde, S.; Brittain, T. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006, 580, 4884–4888.
- Ow, Y.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542.
- Kulikov, A.V.; Shilov, E.S.; Mufazalov, I.A.; Gogvadze, V.; Nedospasov, S.A.; Zhivotovsky, B. Cytochrome c: The Achilles’ heel in apoptosis. Cell Mol. Life Sci. 2012, 69, 1787–1797.
- Alvarez-Paggi, D.; Hannibal, L.; Castro, M.A.; Oviedo-Rouco, S.; Demicheli, V.; Tortora, V.; Tomasina, F.; Radi, R.; Murgida, D.H. Multifunctional cytochrome c: Learning new tricks from an old dog. Chem. Rev. 2017, 117, 13382–13460.
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246.
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell death. N. Engl. J. Med. 2009, 361, 1570–1583.
- Chipuk, J.E.; Moldoveanu, T.; Llambi, F.; Parsons, M.J.; Green, D.R. The BCL-2 family reunion. Mol. Cell 2010, 37, 299–310.
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative stress in cell death and cardiovascular diseases. Oxid. Med. Cell Longev. 2019, 2019, 9030563.
- Brown, G.C.; Borutaite, V. Regulation of apoptosis by the redox state of cytochrome c. Biochim. Biophys. Acta 2008, 1777, 877–881.
- Brittain, T.; Skommer, J.; Raychaudhuri, S.; Birch, N. An antiapoptotic neuroprotective role for neuroglobin. Int. J. Mol. Sci. 2010, 11, 2306–2321.
- Raychaudhuri, S.; Skommer, J.; Henty, K.; Birch, N.; Brittain, T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis 2010, 15, 401–411.
- Tiwari, P.B.; Chapagain, P.P.; Üren, A. Investigating molecular interactions between oxidized neuroglobin and cytochrome c. Sci. Rep. 2018, 8, 10557.
- Pérez-Mejías, G.; Díaz-Quintana, A.; Guerra-Castellano, A.; Díaz-Moreno, I.; De la Rosa, M.A. Novel insights into the mechanism of electron transfer in mitochondrial cytochrome c. Coord. Chem. Rev. 2022, 450, 214233.
- Mie, Y.; Takahashi, K.; Itoga, Y.; Sueyoshi, K.; Tsujino, H.; Yamashita, T.; Uno, T. Nanoporous gold based electrodes for electrochemical studies of human neuroglobin. Electrochem. Commun. 2019, 110, 106621.
- Tejero, J. Negative surface charges in neuroglobin modulate the interaction with cytochrome c. Biochem. Biophys. Res. Commun. 2020, 523, 567–572.
- Nesci, S. The mitochondrial permeability transition pore in cell death: A promising drug binding bioarchitecture. Med. Res. Rev. 2020, 40, 811–817.
- Kent, A.C.; El Baradie, K.B.Y.; Hamrick, M.W. Targeting the mitochondrial permeability transition pore to prevent age-associated cell damage and neurodegeneration. Oxid. Med. Cell Longev. 2021, 2021, 6626484.
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The mitochondrial permeability transition pore—Current knowledge of its structure, function, and regulation, and optimized methods for evaluating its functional state. Cells 2022, 12, 1273.
- Boehning, D.; Patterson, R.L.; Sedaghat, L.; Gliebova, N.; Kurosaki, T.; Snyder, S.H. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003, 5, 1051–1061.
- Trandafir, F.; Hoogewijs, D.; Altieri, F.; Rivetti di Val Cervo, P.; Ramser, K.; Van Doorslaer, S.; Vanfleteren, J.R.; Moens, L.; Dewilde, S. Neuroglobin and cytoglobin as potential enzyme or substrate. Gene 2007, 398, 103–113.
- Moschetti, T.; Giuffrè, A.; Ardiccioni, C.; Vallone, B.; Modjtahedi, N.; Kroemer, G.; Brunori, M. Failure of apoptosis-inducing factor to act as neuroglobin reductase. Biochem. Biophys. Res. Commun. 2009, 390, 121–124.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
521
Revisions:
2 times
(View History)
Update Date:
28 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




