Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | William C. Weston | -- | 1459 | 2023-08-26 02:54:52 | | | |
| 2 | Rita Xu | Meta information modification | 1459 | 2023-08-28 04:04:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Weston, W.C.; Hales, K.H.; Hales, D.B. Flaxseed Reduces Cancer Risk. Encyclopedia. Available online: https://encyclopedia.pub/entry/48502 (accessed on 07 February 2026).
Weston WC, Hales KH, Hales DB. Flaxseed Reduces Cancer Risk. Encyclopedia. Available at: https://encyclopedia.pub/entry/48502. Accessed February 07, 2026.
Weston, William C., Karen H. Hales, Dale B. Hales. "Flaxseed Reduces Cancer Risk" Encyclopedia, https://encyclopedia.pub/entry/48502 (accessed February 07, 2026).
Weston, W.C., Hales, K.H., & Hales, D.B. (2023, August 26). Flaxseed Reduces Cancer Risk. In Encyclopedia. https://encyclopedia.pub/entry/48502
Weston, William C., et al. "Flaxseed Reduces Cancer Risk." Encyclopedia. Web. 26 August, 2023.
Copy Citation
Researchers utilize a targeted metabolomics dataset in combination with a reanalysis of past work to investigate the “metabo-bioenergetic” adaptations that occur in White Leghorn laying hens while consuming dietary flaxseed. Results suggest that flaxseed accelerates bioenergetic flux through glycolysis and mitochondrial fatty acid oxidation (FAO) in liver, thereby protecting the animal from obesity, type 2 diabetes, and non-alcoholic fatty liver disease (i.e., primary cancer risk factors).
flaxseed
metformin
cancer
chicken
diabetes
non-alcoholic fatty liver disease
bioenergetics
1. Obesity, Type 2 Diabetes, and Non-Alcoholic Fatty Liver Disease as Cancer Risk Factors
Since the 1970s, the obesity rate in the United States has more than doubled, and there is no socioeconomic class that protects individuals from obesity [1]. Approximately 33.7% of males and 38% of females in the United States can be classified as obese [2]. After considering genetics, nuclear radiation, and chronological age, the single greatest risk factor for cancer is obesity [3][4][5][6]. Obesity enhances cancer risk, in part, by causing chronic, low-grade inflammation that recruits tumor-associated macrophages to the tumor microenvironment [7][8][9][10][11][12][13][14]. Making matters worse, obesity is a primary risk factor for the development of type 2 diabetes, and most forms of cancer have a greater likelihood of occurrence in type 2 diabetics [15][16][17][18][19]. Obesity and diabetes also increase the risk of developing non-alcoholic fatty liver disease (NAFLD) [20][21]. NAFLD, in turn, exacerbates pre-existing diabetic complications [22][23]. The public needs a simple solution that can mitigate each of these intertwined pathologies.
2. Flaxseed’s Role as an Anti-Cancer Food
Human trials indicate that dietary flaxseed (30 g daily) helps to attenuate BMI [24][25], blood glucose [26], blood insulin [26], glycated hemoglobin (HbA1c%) [27], metabolic syndrome [28][29][30], and NAFLD [30][31][32][33]. These observations illustrate flaxseed’s potential to protect humans from cancer-prone states. However, researchers have not fully clarified the bioenergetic alterations that occur in response to flaxseed consumption. By gaining a better understanding of flaxseed’s effect on animal metabolism, researchers can help researchers to appreciate flaxseed’s potential as an anti-cancer food.
The lab specializes in the investigation of flaxseed as an ovarian cancer intervention for White Leghorn laying hens. Laying hens provide a unique model for ovarian cancer research because laying hens develop biologically natural ovarian tumors beginning at two years of age [34][35][36]. Additionally, ovarian cancer’s mutational landscape, histopathology, and causal etiology are similar between humans and laying hens [34][37][38]. The lab’s findings indicate that dietary flaxseed protects laying hens from ovarian cancer by attenuating systemic prostaglandin E2 [36], tumor estrogen receptor expression [39], tumor microRNA-200 expression [40], and tumor survival [41]. Researchers recently observed a novel anti-cancer mechanism whereby flaxseed augments one-carbon metabolism in the laying hen [42]. The present work extends that one-carbon metabolic framework by investigating the bioenergetic pathways that are modified when hens consume flaxseed.
3. Vitamin B6, 1-Amino D-Proline, and Flaxseed’s Effect on One-Carbon Metabolism
The term “vitamin B6” refers to six different vitamin B6 isoforms (sometimes called “vitamers”) that are based on three structures: pyridoxine, pyridoxal, and pyridoxamine. The pyridoxal moiety, having a reactive aldehyde at the 4′ carbon, can form a Schiff base with vitamin B6-dependent enzymes [43]. However, pyridoxal 5′-phosphate (PLP) is the cofactor that causes enzyme activation [44]. Over 140 PLP-dependent enzymatic reactions are known in biology, comprising 4% of all enzyme reactions [45][46]. Related specifically to the work, PLP is the obligate cofactor for the transsulfuration pathway. In animals, transsulfuration is regulated by two PLP-dependent enzymes: cystathionine beta synthase (CBS) and cystathionase (CSE). CBS oxidizes homocysteine to cystathionine and CSE oxidizes cystathionine to cysteine.
Flaxseed contains a vitamin B6 antagonist “precursory” molecule known as linatine, which in the presence of hydrochloric acid undergoes hydrolysis to yield glutamic acid and 1-amino D-proline (1ADP) [47]. The amino group of 1ADP traps the 4′ carbonyl of pyridoxal or PLP, resulting in the formation of a covalently stable conjugate that cannot support PLP-dependent reactions [47]. Therefore, in nature, 1ADP insults vitamin B6 metabolism by attenuating the bioavailability of vitamin B6 [42][48][49]. In laying hens, researchers recently showed that flaxseed attenuates plasma 4-pyridoxic acid and causes an extreme perturbation of the transsulfuration pathway (i.e., 15-fold elevated cystathionine) [42]. Interestingly, homocysteine was not elevated despite such an extreme increase in cystathionine. To account for this, researchers observed evidence that flaxseed accelerates flux through the homocysteine remethylation pathway, culminating in the accelerated biosynthesis of S-adenosylmethionine (SAM) [42]. Researchers illustrate these effects in Figure 1.
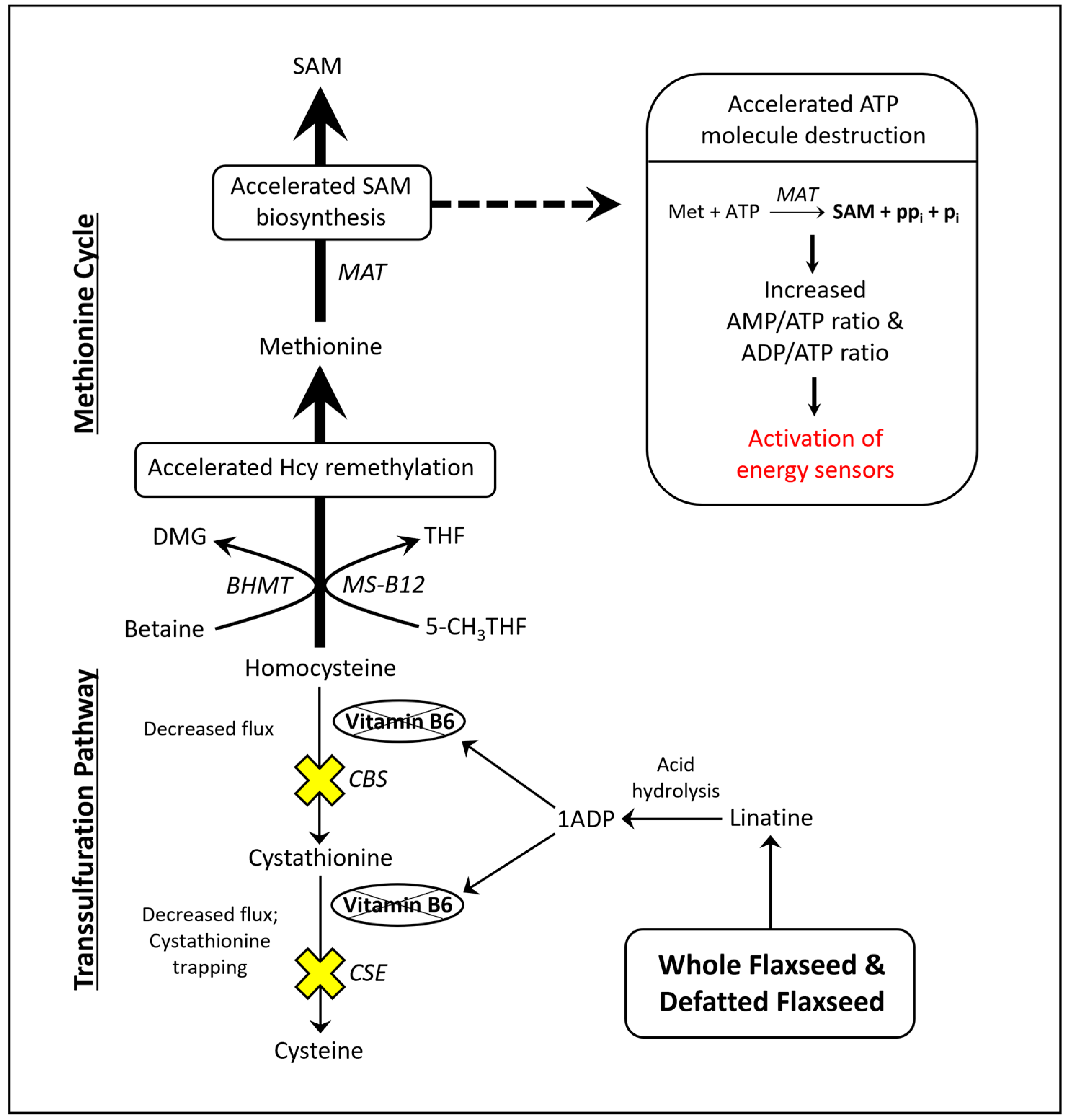
Figure 1. Model illustrating how flaxseed augments one-carbon metabolism in a manner that accelerates SAM biosynthesis, resulting in an elevated AMP/ATP ratio and an elevated ADP/ATP ratio. 1ADP = 1-amino D-proline, 5-CH3THF = 5-methyl tetrahydrofolate, BHMT = betaine homocysteine methyltransferase, CBS = cystathionine beta synthase, CSE = cystathionase, DMG = dimethylglycine, Hcy = homocysteine, MAT = methionine adenosyltransferase, Met = methionine, MS-B12 = methionine synthase complexed with vitamin B12, SAM = S-adenosylmethionine, THF = tetrahydrofolate.
4. SAM Biosynthesis and AMPK Activation
Researchers illustrated that the acceleration of SAM biosynthesis can independently activate AMP-activated protein kinase (AMPK) [50]. SAM biosynthesis is regulated by the ATP-consuming enzyme methionine adenosyltransferase (MAT). MAT is unique because it fully consumes ATP without generating ADP, yielding SAM, pyrophosphate, and inorganic phosphate (Figure 1). By fully consuming ATP, MAT can readily elevate the AMP/ATP ratio and ADP/ATP ratio. Those ratios determine the rate at which AMP and ADP bind to the regulatory subunits of energy sensing proteins like AMPK. AMPK is activated, in part, by the increased binding of AMP and ADP to its γ-regulatory subunits [51][52]. During states of ATP deficiency, AMPK increases ATP synthesis by accelerating mitochondrial fatty acid oxidation (FAO) or glycolysis [53][54][55]. AMPK can also act to conserve ATP by inhibiting anabolic enzymes like acetyl-CoA carboxylase (ACC) and ornithine decarboxylase (ODC) [56][57][58].
Downstream of glycolysis, the pyruvate dehydrogenase complex (PDC) serves as the primary checkpoint for pyruvate’s entry into the mitochondria. PDC oxidatively decarboxylates pyruvate to form acetyl-CoA, and acetyl-CoA then undergoes a condensation reaction with oxaloacetate to form citrate in the tricarboxylic acid (TCA) cycle [59]. Together, PDC and mitochondrial FAO contribute the vast majority of mitochondrial acetyl-CoA, and the TCA cycle (via the metabolism of acetyl-CoA) serves as the primary generator of mitochondrial NADH. In 1963, Philip Randle first described a critical balancing act between mitochondrial FAO and glycolysis that prevents mitochondrial NADH overload [60]. Specifically, mitochondrial FAO and glycolysis cannot be simultaneously upregulated [60][61], otherwise mitochondria become overwhelmed by NADH, leading to the toxic shutdown of the tricarboxylic acid (TCA) cycle [62][63][64].
5. Type 2 Diabetes, Vitamin B6 Insufficiency, and Cancer: Human Studies Strongly Indicate That Flaxseed Is Safe in Populations That Are at Risk of Vitamin B6 Insufficiency
Individuals with type 2 diabetes are already predisposed to risk of vitamin B6 insufficiency, because type 2 diabetes (as a condition) shares a bidirectional, causal relationship with low vitamin B6 bioavailability [65]. Type 2 diabetes promotes low vitamin B6 bioavailability because diabetes accelerates the renal clearance of vitamin B6 [66]. By forcing the attenuation of vitamin B6, diabetes contributes to the worsening of the original diabetic condition. Why? Vitamin B6 performs numerous non-canonical roles that inhibit advanced glycation end-product (AGE) formation [67][68][69], hyperglycemia [70], and oxidative stress [71][72]. However, vitamin B6 cannot perform those biochemical tasks when its bioavailability is low. The result is a worsening of the original diabetic condition, and the vicious cycle between type 2 diabetes and vitamin B6 continues.
A broad sweep across the literature indicates that flaxseed’s anti-vitamin B6 effects are not a threat to at-risk populations (e.g., type 2 diabetics). For example, a meta-analysis of 13 studies on type 2 diabetes suggests that flaxseed is beneficial for HbA1c%, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) [27]. Flaxseed is also beneficial for humans with metabolic syndrome [28][29][30], obesity [24][25], and NAFLD [30][31][32][33]. Does flaxseed hurt individuals who have an exceptionally high risk of vitamin deficiency? A two-year human study of lupus nephritis (a condition where the immune system attacks the nephron and can cause renal failure) suggests that flaxseed consumption decreases serum creatinine and potentially decreases urine microalbumin [73]. Similarly, flaxseed reduces renal injury and attenuates proteinuria in high-risk animal models [74][75]. Flaxseed also exhibits renal benefits in healthy humans [76]. Researchers only found three publications with experimental evidence of increased physiological risk due to flaxseed’s anti-vitamin B6 effects. Those three investigations were designed to test if a vitamin B6 deficient diet predisposes rats to increased risk while consuming flaxseed or 1ADP [48][49][77]. Their conclusion was that a moderate deficiency of vitamin B6 might sensitize humans to the anti-vitamin B6 effects of flaxseed. However, isolated vitamin B6 deficiency is typically rare [78], and flaxseed’s benefits in at-risk human populations are already known (e.g., [24][27][73]).
Regarding cancer, researchers have more recently revealed that type 2 diabetes promotes DNA mutations by enhancing the glycation of nucleotides, histones, and DNA repair proteins, accounting for a portion of diabetic cancer risk [79][80][81][82]. AGEs, which commonly occur in type 2 diabetes, promote carcinogenic transformation by increasing tissue oxidative stress and inducing genome instability [80][83]. Subsequent to carcinogenic transformation, a type 2 diabetic’s elevated blood sugar provides sufficient glucose to satisfy cancer’s common preference for aerobic glycolysis (i.e., Warburg Effect) [84]. Flaxseed’s ability to attenuate diabetic symptoms should help to protect diabetic individuals from these complications.
References
- Mitchell, N.S.; Catenacci, V.A.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an Epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732.
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the Prevalence of Overweight, Obesity and Central Obesity Levelled off in the United States? Trends, Patterns, Disparities, and Future Projections for the Obesity Epidemic. Int. J. Epidemiol. 2020, 49, 810–823.
- De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 291546.
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135.
- Mehrgou, A.; Akouchekian, M. The Importance of BRCA1 and BRCA2 Genes Mutations in Breast Cancer Development. Med. J. Islam. Repub. Iran 2016, 30, 369.
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and Cancer Risk: A Potentially Modifiable Relationship. Am. J. Prev. Med. 2014, 46, S7–S15.
- Aronson, D.; Bartha, P.; Zinder, O.; Kerner, A.; Markiewicz, W.; Avizohar, O.; Brook, G.J.; Levy, Y. Obesity Is the Major Determinant of Elevated C-Reactive Protein in Subjects with the Metabolic Syndrome. Int. J. Obes. Relat. Metab. Disord 2004, 28, 674–679.
- El-Mikkawy, D.M.E.; EL-Sadek, M.A.; EL-Badawy, M.A.; Samaha, D. Circulating Level of Interleukin-6 in Relation to Body Mass Indices and Lipid Profile in Egyptian Adults with Overweight and Obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7.
- Xu, L.; Kitade, H.; Ni, Y.; Ota, T. Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules 2015, 5, 1563–1579.
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor Necrosis Factor Alpha: A Key Component of the Obesity-Diabetes Link. Diabetes 1994, 43, 1271–1278.
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes Promote Ovarian Cancer Metastasis and Provide Energy for Rapid Tumor Growth. Nat. Med. 2011, 17, 1498–1503.
- Koca, T.T. Does Obesity Cause Chronic Inflammation? The Association between Complete Blood Parameters with Body Mass Index and Fasting Glucose. Pak. J. Med. Sci. 2017, 33, 65–69.
- Maeda, H.; Akaike, T. Nitric Oxide and Oxygen Radicals in Infection, Inflammation, and Cancer. Biochemistry 1998, 63, 854–865.
- Lin, Y.; Xu, J.; Lan, H. Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol. 2019, 12, 76.
- Ling, S.; Brown, K.; Miksza, J.K.; Howells, L.; Morrison, A.; Issa, E.; Yates, T.; Khunti, K.; Davies, M.J.; Zaccardi, F. Association of Type 2 Diabetes With Cancer: A Meta-Analysis With Bias Analysis for Unmeasured Confounding in 151 Cohorts Comprising 32 Million People. Diabetes Care 2020, 43, 2313–2322.
- González, N.; Prieto, I.; Del Puerto-Nevado, L.; Portal-Nuñez, S.; Ardura, J.A.; Corton, M.; Fernández-Fernández, B.; Aguilera, O.; Gomez-Guerrero, C.; Mas, S.; et al. 2017 Update on the Relationship between Diabetes and Colorectal Cancer: Epidemiology, Potential Molecular Mechanisms and Therapeutic Implications. Oncotarget 2017, 8, 18456–18485.
- Lee, J.Y.; Jeon, I.; Kim, J.W.; Song, Y.-S.; Yoon, J.-M.; Park, S.M. Diabetes Mellitus and Ovarian Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Gynecol. Cancer 2013, 23, 402–412.
- Barnes, A.S. The Epidemic of Obesity and Diabetes: Trends and Treatments. Tex. Heart Inst. J. 2011, 38, 142–144.
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2020, 10, 615375.
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97.
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The Complex Link between NAFLD and Type 2 Diabetes Mellitus—Mechanisms and Treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612.
- Tomah, S.; Alkhouri, N.; Hamdy, O. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes: Where Do Diabetologists Stand? Clin. Diabetes Endocrinol. 2020, 6, 9.
- Cho, H.J.; Hwang, S.; Park, J.I.; Yang, M.J.; Hwang, J.C.; Yoo, B.M.; Lee, K.M.; Shin, S.J.; Lee, K.J.; Kim, J.H.; et al. Improvement of Nonalcoholic Fatty Liver Disease Reduces the Risk of Type 2 Diabetes Mellitus. Gut Liver 2019, 13, 440–449.
- Mohammadi-Sartang, M.; Mazloom, Z.; Raeisi-Dehkordi, H.; Barati-Boldaji, R.; Bellissimo, N.; Totosy de Zepetnek, J.O. The Effect of Flaxseed Supplementation on Body Weight and Body Composition: A Systematic Review and Meta-Analysis of 45 Randomized Placebo-Controlled Trials. Obes. Rev. 2017, 18, 1096–1107.
- Ahmadniay Motlagh, H.; Aalipanah, E.; Mazidi, M.; Faghih, S. Effect of Flaxseed Consumption on Central Obesity, Serum Lipids, and Adiponectin Level in Overweight or Obese Women: A Randomised Controlled Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14592.
- Mohammadi-Sartang, M.; Sohrabi, Z.; Barati-Boldaji, R.; Raeisi-Dehkordi, H.; Mazloom, Z. Flaxseed Supplementation on Glucose Control and Insulin Sensitivity: A Systematic Review and Meta-Analysis of 25 Randomized, Placebo-Controlled Trials. Nutr. Rev. 2018, 76, 125–139.
- Xi, H.; Zhou, W.; Sohaib, M.; Niu, Y.; Zhu, R.; Guo, Y.; Wang, S.; Mao, J.; Wang, X.; Guo, L. Flaxseed Supplementation Significantly Reduces Hemoglobin A1c in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutr. Res. 2023, 110, 23–32.
- Haidari, F.; Banaei-Jahromi, N.; Zakerkish, M.; Ahmadi, K. The Effects of Flaxseed Supplementation on Metabolic Status in Women with Polycystic Ovary Syndrome: A Randomized Open-Labeled Controlled Clinical Trial. Nutr. J. 2020, 19, 8.
- Morshedzadeh, N.; Rahimlou, M.; Shahrokh, S.; Karimi, S.; Mirmiran, P.; Zali, M.R. The Effects of Flaxseed Supplementation on Metabolic Syndrome Parameters, Insulin Resistance and Inflammation in Ulcerative Colitis Patients: An Open-Labeled Randomized Controlled Trial. Phytother. Res. 2021, 35, 3781–3791.
- Yari, Z.; Cheraghpour, M.; Hekmatdoost, A. Flaxseed and/or Hesperidin Supplementation in Metabolic Syndrome: An Open-Labeled Randomized Controlled Trial. Eur. J. Nutr. 2021, 60, 287–298.
- Yari, Z.; Rahimlou, M.; Eslamparast, T.; Ebrahimi-Daryani, N.; Poustchi, H.; Hekmatdoost, A. Flaxseed Supplementation in Non-Alcoholic Fatty Liver Disease: A Pilot Randomized, Open Labeled, Controlled Study. Int. J. Food Sci. Nutr. 2016, 67, 461–469.
- Kristensen, M.; Jensen, M.G.; Aarestrup, J.; Petersen, K.E.; Søndergaard, L.; Mikkelsen, M.S.; Astrup, A. Flaxseed Dietary Fibers Lower Cholesterol and Increase Fecal Fat Excretion, but Magnitude of Effect Depend on Food Type. Nutr. Metab. 2012, 9, 8.
- Edel, A.L.; Rodriguez-Leyva, D.; Maddaford, T.G.; Caligiuri, S.P.B.; Austria, J.A.; Weighell, W.; Guzman, R.; Aliani, M.; Pierce, G.N. Dietary Flaxseed Independently Lowers Circulating Cholesterol and Lowers It beyond the Effects of Cholesterol-Lowering Medications Alone in Patients with Peripheral Artery Disease. J. Nutr. 2015, 145, 749–757.
- Johnson, P.A.; Giles, J.R. The Hen as a Model of Ovarian Cancer. Nat. Rev. Cancer 2013, 13, 432–436.
- Ansenberger, K.; Richards, C.; Zhuge, Y.; Barua, A.; Bahr, J.M.; Luborsky, J.L.; Hales, D.B. Decreased Severity of Ovarian Cancer and Increased Survival in Hens Fed a Flaxseed-Enriched Diet for 1 Year. Gynecol. Oncol. 2010, 117, 341–347.
- Eilati, E.; Bahr, J.M.; Hales, D.B. Long Term Consumption of Flaxseed Enriched Diet Decreased Ovarian Cancer Incidence and Prostaglandin E2in Hens. Gynecol. Oncol. 2013, 130, 620–628.
- Hakim, A.A.; Barry, C.P.; Barnes, H.J.; Anderson, K.E.; Petitte, J.; Whitaker, R.; Lancaster, J.M.; Wenham, R.M.; Carver, D.K.; Turbov, J.; et al. Ovarian Adenocarcinomas in the Laying Hen and Women Share Similar Alterations in P53, Ras, and HER-2/Neu. Cancer Prev. Res. 2009, 2, 114–121.
- Barua, A.; Bitterman, P.; Abramowicz, J.S.; Dirks, A.L.; Bahr, J.M.; Hales, D.B.; Bradaric, M.J.; Edassery, S.L.; Rotmensch, J.; Luborsky, J.L. Histopathology of Ovarian Tumors in Laying Hens: A Preclinical Model of Human Ovarian Cancer. Int. J. Gynecol. Cancer 2009, 19, 531–539.
- Dikshit, A.; Hales, K.; Hales, D.B. Whole Flaxseed Diet Alters Estrogen Metabolism to Promote 2-Methoxtestradiol-Induced Apoptosis in Hen Ovarian Cancer. J. Nutr. Biochem. 2017, 42, 117–125.
- Hales, K.H.; Speckman, S.C.; Kurrey, N.K.; Hales, D.B. Uncovering Molecular Events Associated with the Chemosuppressive Effects of Flaxseed: A Microarray Analysis of the Laying Hen Model of Ovarian Cancer. BMC Genom. 2014, 15, 709.
- Pal, P.; Hales, K.; Petrik, J.; Hales, D.B. Pro-Apoptotic and Anti-Angiogenic Actions of 2-Methoxyestradiol and Docosahexaenoic Acid, the Biologically Derived Active Compounds from Flaxseed Diet, in Preventing Ovarian Cancer. J. Ovarian Res. 2019, 12, 49.
- Weston, W.C.; Hales, K.H.; Hales, D.B. Flaxseed Increases Animal Lifespan and Reduces Ovarian Cancer Severity by Toxically Augmenting One-Carbon Metabolism. Molecules 2021, 26, 5674.
- Liang, J.; Han, Q.; Tan, Y.; Ding, H.; Li, J. Current Advances on Structure-Function Relationships of Pyridoxal 5′-Phosphate-Dependent Enzymes. Front. Mol. Biosci. 2019, 6, 4.
- Cellini, B.; Zelante, T.; Dindo, M.; Bellet, M.M.; Renga, G.; Romani, L.; Costantini, C. Pyridoxal 5′-Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism. Int. J. Mol. Sci. 2020, 21, 5823.
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84.
- Mooney, S.; Leuendorf, J.-E.; Hendrickson, C.; Hellmann, H. Vitamin B6: A Long Known Compound of Surprising Complexity. Molecules 2009, 14, 329–351.
- Klosterman, H.J.; Lamoureux, G.L.; Parsons, J.L. Isolation, Characterization, and Synthesis of Linatine. A Vitamin B6 Antagonist from Flaxseed (Linum Usitatissimum). Biochemistry 1967, 6, 170–177.
- Mayengbam, S.; Raposo, S.; House, J. Effect of Vitamin B6-Antagonist from Flaxseed on Amino Acid Metabolism in Moderately Vitamin B6-Deficient Rats. FASEB J. 2015, 29, 134.6.
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. Oral Exposure to the Anti-Pyridoxine Compound 1-Amino d-Proline Further Perturbs Homocysteine Metabolism through the Transsulfuration Pathway in Moderately Vitamin B6 Deficient Rats. J. Nutr. Biochem. 2015, 26, 241–249.
- Takafumi, O.; Ryohei, T.; Muneyoshi, K.; Tetsuya, K.; Tsutomu, F.; Haruyuki, I.; Tomoyoshi, S.; Kazunori, K.; Tokichi, M.; Dai, H.; et al. Stimulating S-Adenosyl-l-Methionine Synthesis Extends Lifespan via Activation of AMPK. Proc. Natl. Acad. Sci. USA 2016, 113, 11913–11918.
- Ross, F.A.; Jensen, T.E.; Hardie, D.G. Differential Regulation by AMP and ADP of AMPK Complexes Containing Different γ Subunit Isoforms. Biochem. J. 2016, 473, 189–199.
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP Is a True Physiological Regulator of AMP-Activated Protein Kinase by Both Allosteric Activation and Enhancing Net Phosphorylation. Cell Metab. 2013, 18, 556–566.
- Cai, Z.; Li, C.-F.; Han, F.; Liu, C.; Zhang, A.; Hsu, C.-C.; Peng, D.; Zhang, X.; Jin, G.; Rezaeian, A.-H.; et al. Phosphorylation of PDHA by AMPK Drives TCA Cycle to Promote Cancer Metastasis. Mol. Cell 2020, 80, 263–278.e7.
- Zammit, V.A.; Arduini, A. The AMPK-Malonyl-CoA-CPT1 Axis in the Control of Hypothalamic Neuronal Function. Cell Metab. 2008, 8, 175.
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135.
- Lee, M.; Katerelos, M.; Gleich, K.; Galic, S.; Kemp, B.E.; Mount, P.F.; Power, D.A. Phosphorylation of Acetyl-CoA Carboxylase by AMPK Reduces Renal Fibrosis and Is Essential for the Anti-Fibrotic Effect of Metformin. J. Am. Soc. Nephrol. 2018, 29, 2326–2336.
- Zhang, T.; Hu, L.; Tang, J.-F.; Xu, H.; Tian, K.; Wu, M.-N.; Huang, S.-Y.; Du, Y.-M.; Zhou, P.; Lu, R.-J.; et al. Metformin Inhibits the Urea Cycle and Reduces Putrescine Generation in Colorectal Cancer Cell Lines. Molecules 2021, 26, 1990.
- Passariello, C.L.; Gottardi, D.; Cetrullo, S.; Zini, M.; Campana, G.; Tantini, B.; Pignatti, C.; Flamigni, F.; Guarnieri, C.; Caldarera, C.M.; et al. Evidence That AMP-Activated Protein Kinase Can Negatively Modulate Ornithine Decarboxylase Activity in Cardiac Myoblasts. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 800–807.
- Lazzarino, G.; Amorini, A.M.; Signoretti, S.; Musumeci, G.; Lazzarino, G.; Caruso, G.; Pastore, F.S.; Di Pietro, V.; Tavazzi, B.; Belli, A. Pyruvate Dehydrogenase and Tricarboxylic Acid Cycle Enzymes Are Sensitive Targets of Traumatic Brain Injury Induced Metabolic Derangement. Int. J. Mol. Sci. 2019, 20, 5774.
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The Glucose Fatty-Acid Cycle its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet 1963, 281, 785–789.
- Hue, L.; Taegtmeyer, H. The Randle Cycle Revisited: A New Head for an Old Hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591.
- Alabduladhem, T.O.; Bordoni, B. Physiology, Krebs Cycle; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551.
- Maynard, A.G.; Kanarek, N. NADH Ties One-Carbon Metabolism to Cellular Respiration. Cell Metab. 2020, 31, 660–662.
- Mascolo, E.; Vernì, F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 3669.
- Iwakawa, H.; Nakamura, Y.; Fukui, T.; Fukuwatari, T.; Ugi, S.; Maegawa, H.; Doi, Y.; Shibata, K. Concentrations of Water-Soluble Vitamins in Blood and Urinary Excretion in Patients with Diabetes Mellitus. Nutr. Metab. Insights 2016, 9, NMI.S40595.
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants 2019, 8, 344.
- Booth, A.A.; Khalifah, R.G.; Todd, P.; Hudson, B.G. Kinetic Studies of Formation of Antigenic Advanced Glycation End Products (AGEs): Novel inhibition of post-amadori glycation pathways. J. Biol. Chem. 1997, 272, 5430–5437.
- Higuchi, O.; Nakagawa, K.; Tsuzuki, T.; Suzuki, T.; Oikawa, S.; Miyazawa, T. Aminophospholipid Glycation and Its Inhibitor Screening System: A New Role of Pyridoxal 5′-Phosphate as the Inhibitor. J. Lipid. Res. 2006, 47, 964–974.
- Kim, H.H.; Kang, Y.-R.; Lee, J.-Y.; Chang, H.-B.; Lee, K.W.; Apostolidis, E.; Kwon, Y.-I. The Postprandial Anti-Hyperglycemic Effect of Pyridoxine and Its Derivatives Using In Vitro and In Vivo Animal Models. Nutrients 2018, 10, 285.
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 Deficient Plants Display Increased Sensitivity to High Light and Photo-Oxidative Stress. BMC Plant Biol. 2009, 9, 130.
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (Pyridoxine) and Its Derivatives Are Efficient Singlet Oxygen Quenchers and Potential Fungal Antioxidants. Photochem. Photobiol. 2000, 71, 129–134.
- Clark, W.F.; Kortas, C.; Heidenheim, A.P.; Garland, J.; Spanner, E.; Parbtani, A. Flaxseed in Lupus Nephritis: A Two-Year Nonplacebo-Controlled Crossover Study. J. Am. Coll. Nutr. 2001, 20, 143–148.
- Al Za’abi, M.; Ali, H.; Ali, B.H. Effect of Flaxseed on Systemic Inflammation and Oxidative Stress in Diabetic Rats with or without Chronic Kidney Disease. PLoS ONE 2021, 16, e0258800.
- Velasquez, M.T.; Bhathena, S.A.M.J.; Ranich, T.; Schwartz, A.M.; Kardon, D.E.; Ali, A.L.I.A.; Haudenschild, C.C.; Hansen, C.T. Dietary Flaxseed Meal Reduces Proteinuria and Ameliorates Nephropathy in an Animal Model of Type II Diabetes Mellitus. Kidney Int. 2003, 64, 2100–2107.
- Stuglin, C.; Prasad, K. Effect of Flaxseed Consumption on Blood Pressure, Serum Lipids, Hemopoietic System and Liver and Kidney Enzymes in Healthy Humans. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 23–27.
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. A Vitamin B-6 Antagonist from Flaxseed Perturbs Amino Acid Metabolism in Moderately Vitamin B-6–Deficient Male Rats. J. Nutr. 2015, 146, 14–20.
- Brown, M.J.; Ameer, M.A.; Beier, K. Vitamin B6 Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Mir, A.R.; Habib, S.; Uddin, M. Recent Advances in Histone Glycation: Emerging Role in Diabetes and Cancer. Glycobiology 2021, 31, 1072–1079.
- Deo, P.; McCullough, C.L.; Almond, T.; Jaunay, E.L.; Donnellan, L.; Dhillon, V.S.; Fenech, M. Dietary Sugars and Related Endogenous Advanced Glycation End-Products Increase Chromosomal DNA Damage in WIL2-NS Cells, Measured Using Cytokinesis-Block Micronucleus Cytome Assay. Mutagenesis 2020, 35, 169–177.
- Thornalley, P.J. Protein and Nucleotide Damage by Glyoxal and Methylglyoxal in Physiological Systems—Role in Ageing and Disease. Drug Metab. Drug Interact. 2008, 23, 125–150.
- Ciminera, A.K.; Shuck, S.C.; Termini, J. Elevated Glucose Increases Genomic Instability by Inhibiting Nucleotide Excision Repair. Life Sci. Alliance 2021, 4, e202101159.
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222.
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
656
Revisions:
2 times
(View History)
Update Date:
28 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




