Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by William C. Weston and Version 2 by Rita Xu.
Researchers utilize a targeted metabolomics dataset in combination with a reanalysis of past work to investigate the “metabo-bioenergetic” adaptations that occur in White Leghorn laying hens while consuming dietary flaxseed. Results suggest that flaxseed accelerates bioenergetic flux through glycolysis and mitochondrial fatty acid oxidation (FAO) in liver, thereby protecting the animal from obesity, type 2 diabetes, and non-alcoholic fatty liver disease (i.e., primary cancer risk factors).
- flaxseed
- metformin
- cancer
- chicken
- diabetes
- non-alcoholic fatty liver disease
- bioenergetics
1. Obesity, Type 2 Diabetes, and Non-Alcoholic Fatty Liver Disease as Cancer Risk Factors
Since the 1970s, the obesity rate in the United States has more than doubled, and there is no socioeconomic class that protects individuals from obesity [1]. Approximately 33.7% of males and 38% of females in the United States can be classified as obese [2]. After considering genetics, nuclear radiation, and chronological age, the single greatest risk factor for cancer is obesity [3][4][5][6][3,4,5,6]. Obesity enhances cancer risk, in part, by causing chronic, low-grade inflammation that recruits tumor-associated macrophages to the tumor microenvironment [7][8][9][10][11][12][13][14][7,8,9,10,11,12,13,14]. Making matters worse, obesity is a primary risk factor for the development of type 2 diabetes, and most forms of cancer have a greater likelihood of occurrence in type 2 diabetics [15][16][17][18][19][15,16,17,18,19]. Obesity and diabetes also increase the risk of developing non-alcoholic fatty liver disease (NAFLD) [20][21][20,21]. NAFLD, in turn, exacerbates pre-existing diabetic complications [22][23][22,23]. The public needs a simple solution that can mitigate each of these intertwined pathologies.
2. Flaxseed’s Role as an Anti-Cancer Food
Human trials indicate that dietary flaxseed (30 g daily) helps to attenuate BMI [24][25][24,25], blood glucose [26], blood insulin [26], glycated hemoglobin (HbA1c%) [27], metabolic syndrome [28][29][30][28,29,30], and NAFLD [30][31][32][33][30,31,32,33]. These observations illustrate flaxseed’s potential to protect humans from cancer-prone states. However, researchers have not fully clarified the bioenergetic alterations that occur in response to flaxseed consumption. By gaining a better understanding of flaxseed’s effect on animal metabolism, reswearchers can help researchers to appreciate flaxseed’s potential as an anti-cancer food.
TheOur lab specializes in the investigation of flaxseed as an ovarian cancer intervention for White Leghorn laying hens. Laying hens provide a unique model for ovarian cancer research because laying hens develop biologically natural ovarian tumors beginning at two years of age [34][35][36][34,35,36]. Additionally, ovarian cancer’s mutational landscape, histopathology, and causal etiology are similar between humans and laying hens [34][37][38][34,37,38]. TheOur lab’s findings indicate that dietary flaxseed protects laying hens from ovarian cancer by attenuating systemic prostaglandin E2 [36], tumor estrogen receptor expression [39], tumor microRNA-200 expression [40], and tumor survival [41]. RWesearchers recently observed a novel anti-cancer mechanism whereby flaxseed augments one-carbon metabolism in the laying hen [42]. TheOur present work extends that one-carbon metabolic framework by investigating the bioenergetic pathways that are modified when hens consume flaxseed.
3. Vitamin B6, 1-Amino D-Proline, and Flaxseed’s Effect on One-Carbon Metabolism
The term “vitamin B6” refers to six different vitamin B6 isoforms (sometimes called “vitamers”) that are based on three structures: pyridoxine, pyridoxal, and pyridoxamine. The pyridoxal moiety, having a reactive aldehyde at the 4′ carbon, can form a Schiff base with vitamin B6-dependent enzymes [43]. However, pyridoxal 5′-phosphate (PLP) is the cofactor that causes enzyme activation [44]. Over 140 PLP-dependent enzymatic reactions are known in biology, comprising 4% of all enzyme reactions [45][46][45,46]. Related specifically to theour work, PLP is the obligate cofactor for the transsulfuration pathway. In animals, transsulfuration is regulated by two PLP-dependent enzymes: cystathionine beta synthase (CBS) and cystathionase (CSE). CBS oxidizes homocysteine to cystathionine and CSE oxidizes cystathionine to cysteine.
Flaxseed contains a vitamin B6 antagonist “precursory” molecule known as linatine, which in the presence of hydrochloric acid undergoes hydrolysis to yield glutamic acid and 1-amino D-proline (1ADP) [47]. The amino group of 1ADP traps the 4′ carbonyl of pyridoxal or PLP, resulting in the formation of a covalently stable conjugate that cannot support PLP-dependent reactions [47]. Therefore, in nature, 1ADP insults vitamin B6 metabolism by attenuating the bioavailability of vitamin B6 [42][48][49][42,48,49]. In laying hens, reswearchers recently showed that flaxseed attenuates plasma 4-pyridoxic acid and causes an extreme perturbation of the transsulfuration pathway (i.e., 15-fold elevated cystathionine) [42]. Interestingly, homocysteine was not elevated despite such an extreme increase in cystathionine. To account for this, rwesearchers observed evidence that flaxseed accelerates flux through the homocysteine remethylation pathway, culminating in the accelerated biosynthesis of S-adenosylmethionine (SAM) [42]. ResWearchers illustrate these effects in Figure 1.
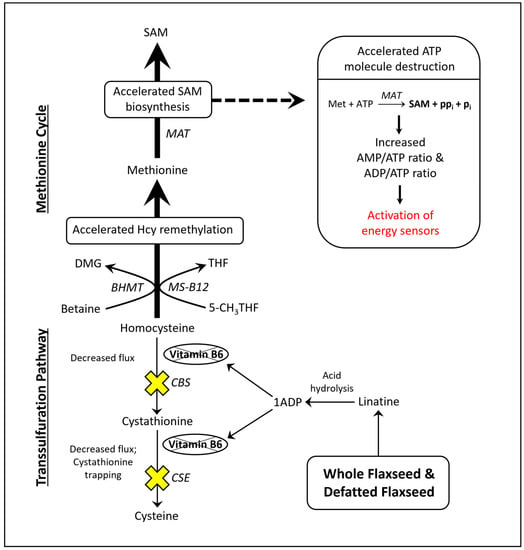
Figure 1. Model illustrating how flaxseed augments one-carbon metabolism in a manner that accelerates SAM biosynthesis, resulting in an elevated AMP/ATP ratio and an elevated ADP/ATP ratio. 1ADP = 1-amino D-proline, 5-CH3THF = 5-methyl tetrahydrofolate, BHMT = betaine homocysteine methyltransferase, CBS = cystathionine beta synthase, CSE = cystathionase, DMG = dimethylglycine, Hcy = homocysteine, MAT = methionine adenosyltransferase, Met = methionine, MS-B12 = methionine synthase complexed with vitamin B12, SAM = S-adenosylmethionine, THF = tetrahydrofolate.
4. SAM Biosynthesis and AMPK Activation
Researchers illustrated that the acceleration of SAM biosynthesis can independently activate AMP-activated protein kinase (AMPK) [50]. SAM biosynthesis is regulated by the ATP-consuming enzyme methionine adenosyltransferase (MAT). MAT is unique because it fully consumes ATP without generating ADP, yielding SAM, pyrophosphate, and inorganic phosphate (Figure 1). By fully consuming ATP, MAT can readily elevate the AMP/ATP ratio and ADP/ATP ratio. Those ratios determine the rate at which AMP and ADP bind to the regulatory subunits of energy sensing proteins like AMPK. AMPK is activated, in part, by the increased binding of AMP and ADP to its γ-regulatory subunits [51][52][51,52]. During states of ATP deficiency, AMPK increases ATP synthesis by accelerating mitochondrial fatty acid oxidation (FAO) or glycolysis [53][54][55][53,54,55]. AMPK can also act to conserve ATP by inhibiting anabolic enzymes like acetyl-CoA carboxylase (ACC) and ornithine decarboxylase (ODC) [56][57][58][56,57,58].
Downstream of glycolysis, the pyruvate dehydrogenase complex (PDC) serves as the primary checkpoint for pyruvate’s entry into the mitochondria. PDC oxidatively decarboxylates pyruvate to form acetyl-CoA, and acetyl-CoA then undergoes a condensation reaction with oxaloacetate to form citrate in the tricarboxylic acid (TCA) cycle [59]. Together, PDC and mitochondrial FAO contribute the vast majority of mitochondrial acetyl-CoA, and the TCA cycle (via the metabolism of acetyl-CoA) serves as the primary generator of mitochondrial NADH. In 1963, Philip Randle first described a critical balancing act between mitochondrial FAO and glycolysis that prevents mitochondrial NADH overload [60]. Specifically, mitochondrial FAO and glycolysis cannot be simultaneously upregulated [60][61][60,61], otherwise mitochondria become overwhelmed by NADH, leading to the toxic shutdown of the tricarboxylic acid (TCA) cycle [62][63][64][62,63,64].
5. Type 2 Diabetes, Vitamin B6 Insufficiency, and Cancer: Human Studies Strongly Indicate That Flaxseed Is Safe in Populations That Are at Risk of Vitamin B6 Insufficiency
Individuals with type 2 diabetes are already predisposed to risk of vitamin B6 insufficiency, because type 2 diabetes (as a condition) shares a bidirectional, causal relationship with low vitamin B6 bioavailability [65]. Type 2 diabetes promotes low vitamin B6 bioavailability because diabetes accelerates the renal clearance of vitamin B6 [66]. By forcing the attenuation of vitamin B6, diabetes contributes to the worsening of the original diabetic condition. Why? Vitamin B6 performs numerous non-canonical roles that inhibit advanced glycation end-product (AGE) formation [67][68][69][67,68,69], hyperglycemia [70], and oxidative stress [71][72][71,72]. However, vitamin B6 cannot perform those biochemical tasks when its bioavailability is low. The result is a worsening of the original diabetic condition, and the vicious cycle between type 2 diabetes and vitamin B6 continues.
A broad sweep across the literature indicates that flaxseed’s anti-vitamin B6 effects are not a threat to at-risk populations (e.g., type 2 diabetics). For example, a meta-analysis of 13 studies on type 2 diabetes suggests that flaxseed is beneficial for HbA1c%, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) [27]. Flaxseed is also beneficial for humans with metabolic syndrome [28][29][30][28,29,30], obesity [24][25][24,25], and NAFLD [30][31][32][33][30,31,32,33]. Does flaxseed hurt individuals who have an exceptionally high risk of vitamin deficiency? A two-year human study of lupus nephritis (a condition where the immune system attacks the nephron and can cause renal failure) suggests that flaxseed consumption decreases serum creatinine and potentially decreases urine microalbumin [73]. Similarly, flaxseed reduces renal injury and attenuates proteinuria in high-risk animal models [74][75][74,75]. Flaxseed also exhibits renal benefits in healthy humans [76]. ResWearchers only found three publications with experimental evidence of increased physiological risk due to flaxseed’s anti-vitamin B6 effects. Those three investigations were designed to test if a vitamin B6 deficient diet predisposes rats to increased risk while consuming flaxseed or 1ADP [48][49][77][48,49,77]. Their conclusion was that a moderate deficiency of vitamin B6 might sensitize humans to the anti-vitamin B6 effects of flaxseed. However, isolated vitamin B6 deficiency is typically rare [78], and flaxseed’s benefits in at-risk human populations are already known (e.g., [24][27][73][24,27,73]).
Regarding cancer, researchers have more recently revealed that type 2 diabetes promotes DNA mutations by enhancing the glycation of nucleotides, histones, and DNA repair proteins, accounting for a portion of diabetic cancer risk [79][80][81][82][79,80,81,82]. AGEs, which commonly occur in type 2 diabetes, promote carcinogenic transformation by increasing tissue oxidative stress and inducing genome instability [80][83][80,83]. Subsequent to carcinogenic transformation, a type 2 diabetic’s elevated blood sugar provides sufficient glucose to satisfy cancer’s common preference for aerobic glycolysis (i.e., Warburg Effect) [84]. Flaxseed’s ability to attenuate diabetic symptoms should help to protect diabetic individuals from these complications.
